Method for preparing (E)-4-(benzenesulfonyl) butyl-3-olefine acid
A technology of benzenesulfonyl and crotonic acid, which is applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve the problem of highly toxic organotin reagents, irritating odor of toluenesulfonyl chloride, and selection of regions that need to be regulated. It can reduce the environmental impact and save the production cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042]A synthetic method of (E)-4-(benzenesulfonyl) but-3-enoic acid, comprising the following steps:
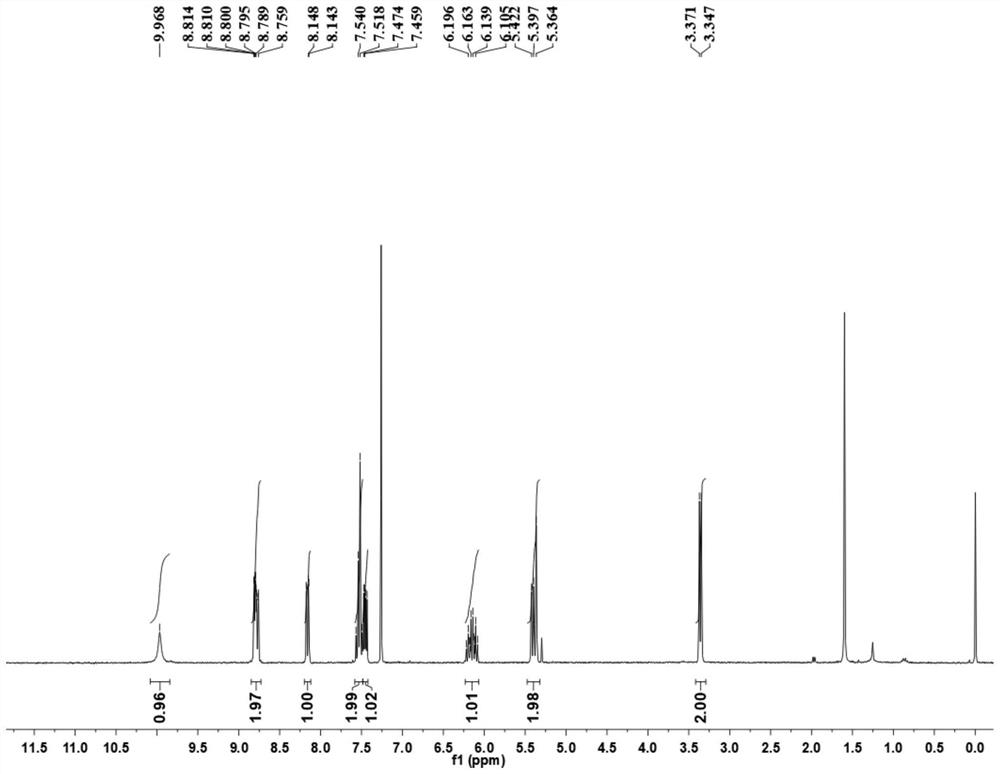
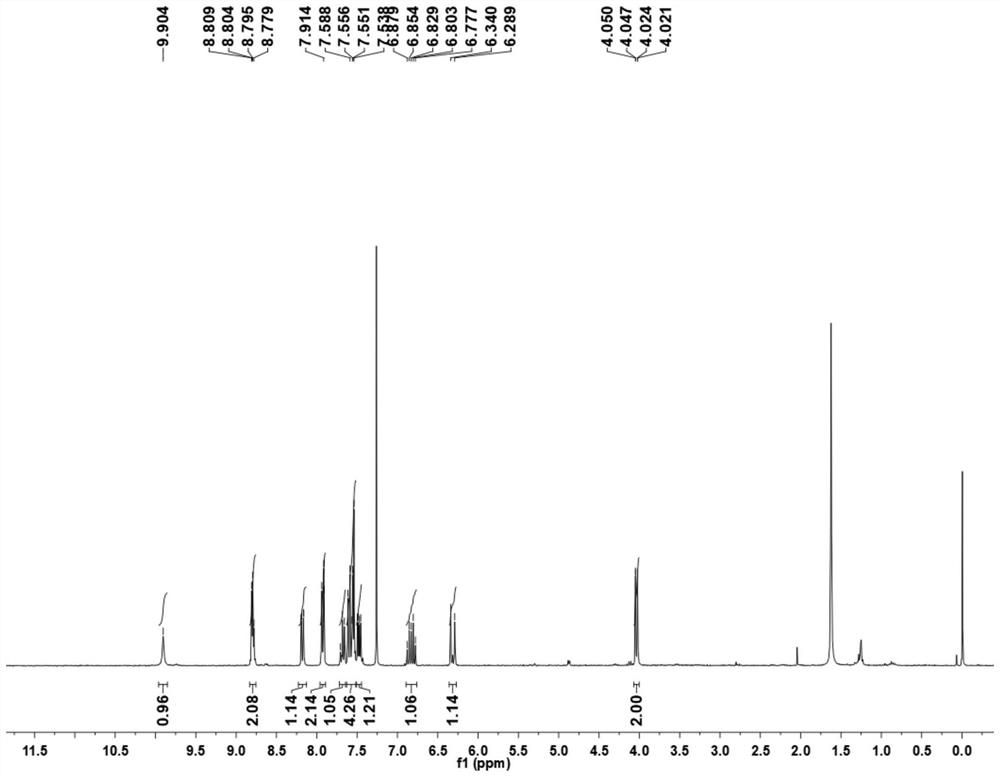
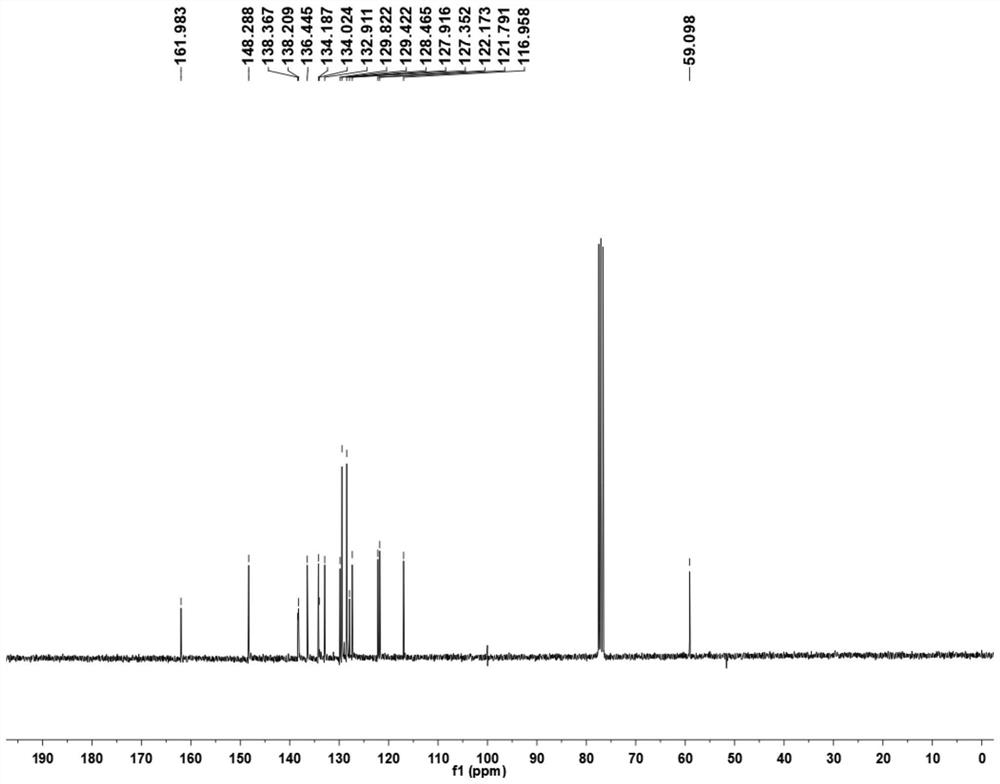
[0043] (1) 1.44g (10.00mmol) of 8-aminoquinoline (compound 1) was added to a 250mL round bottom flask filled with dichloromethane (30.0mL, DCM), followed by 4.94g (13.00mmol) of HATU (2-(7-azobenzotriazole)-N,N,N',N'-tetramethylurea hexafluorophosphate), 1.12g (13.00mmol) 3-butenoic acid (compound 2) and 2.42 g (20.00 mmol) of 2,4,6-collidine, and stirred at room temperature for 16 h. The reaction was quenched with ethyl acetate (200 mL), the system was transferred to a separatory funnel, the organic phase was washed with 100 mL of saturated sodium bicarbonate solution and 100 mL of saturated brine successively, and then dried over anhydrous sodium sulfate. Filtration and concentration, the obtained mixture was separated by column chromatography (eluent petroleum ether: ethyl acetate (v / v)=25~20:1) to obtain the target product (compound 3) (1.70 g, yield 80.1%) . The H NM...
Embodiment 2
[0051] A synthetic method of (E)-4-(benzenesulfonyl) but-3-enoic acid, comprising the following steps:
[0052] (1) 1.44g (10.00mmol) of 8-aminoquinoline (compound 1) was added to a 250mL round-bottomed flask filled with dichloromethane (30.0mL), followed by 5.32g (14.00mmol) of HATU (2) -(7-Azobenzotriazole)-N,N,N',N'-tetramethylurea hexafluorophosphate), 1.21 g (14.00 mmol) 3-butenoic acid (compound 2) and 2.67 g g (22.00 mmol) 2,4,6-collidine, and stirred at room temperature for 16 h. The reaction was quenched with ethyl acetate (200 mL), the system was transferred to a separatory funnel, the organic phase was washed successively with 100 mL of saturated sodium bicarbonate solution and 100 mL of saturated brine, and then dried over anhydrous sodium sulfate. Filtration and concentration, the obtained mixture was separated by column chromatography (eluent petroleum ether: ethyl acetate (v / v)=25~20:1) to obtain the target product (compound 3) (1.74 g, yield 82.0%) .
[0053...
Embodiment 3
[0057] A synthetic method of (E)-4-(benzenesulfonyl) but-3-enoic acid, comprising the following steps:
[0058] (1) 1.44g (10.00mmol) of 8-aminoquinoline (compound 1) was added to a 250mL round-bottomed flask filled with dichloromethane (30.0mL), followed by 5.70g (15.00mmol) of HATU (2) -(7-Azobenzotriazole)-N,N,N',N'-tetramethylurea hexafluorophosphate), 1.29g (15.00mmol) 3-butenoic acid (compound 2) and 3.03 g g (25.00 mmol) 2,4,6-collidine, stirred at room temperature for 16 h. The reaction was quenched with ethyl acetate (200 mL), the system was transferred to a separatory funnel, the organic phase was washed with 100 mL of saturated sodium bicarbonate solution and 100 mL of saturated brine successively, and then dried over anhydrous sodium sulfate. Filtration and concentration, the obtained mixture was separated by column chromatography (eluent petroleum ether: ethyl acetate (v / v)=25~20:1) to obtain the target product (compound 3) (1.73 g, yield 81.5%) .
[0059] (2) ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com