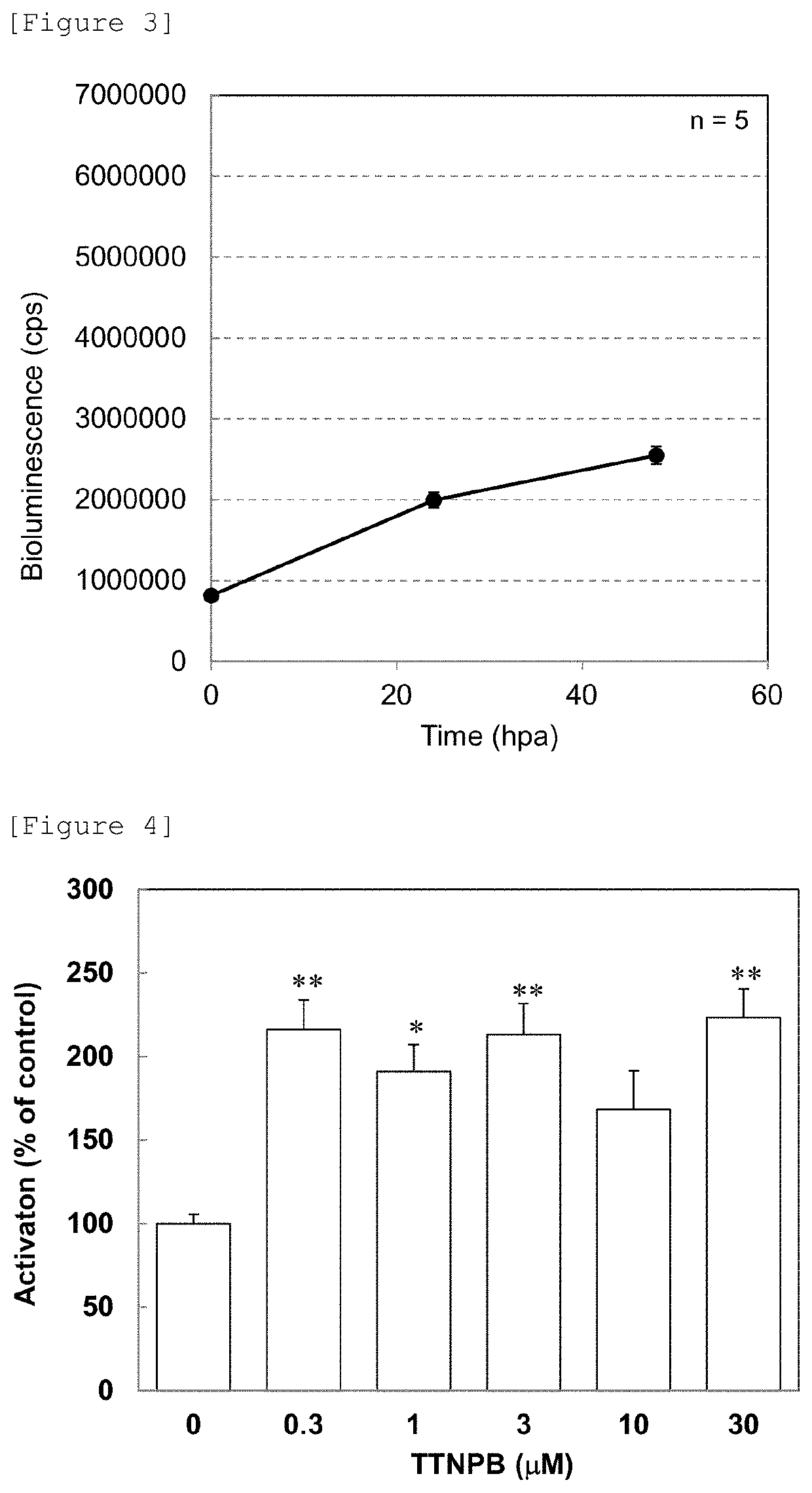
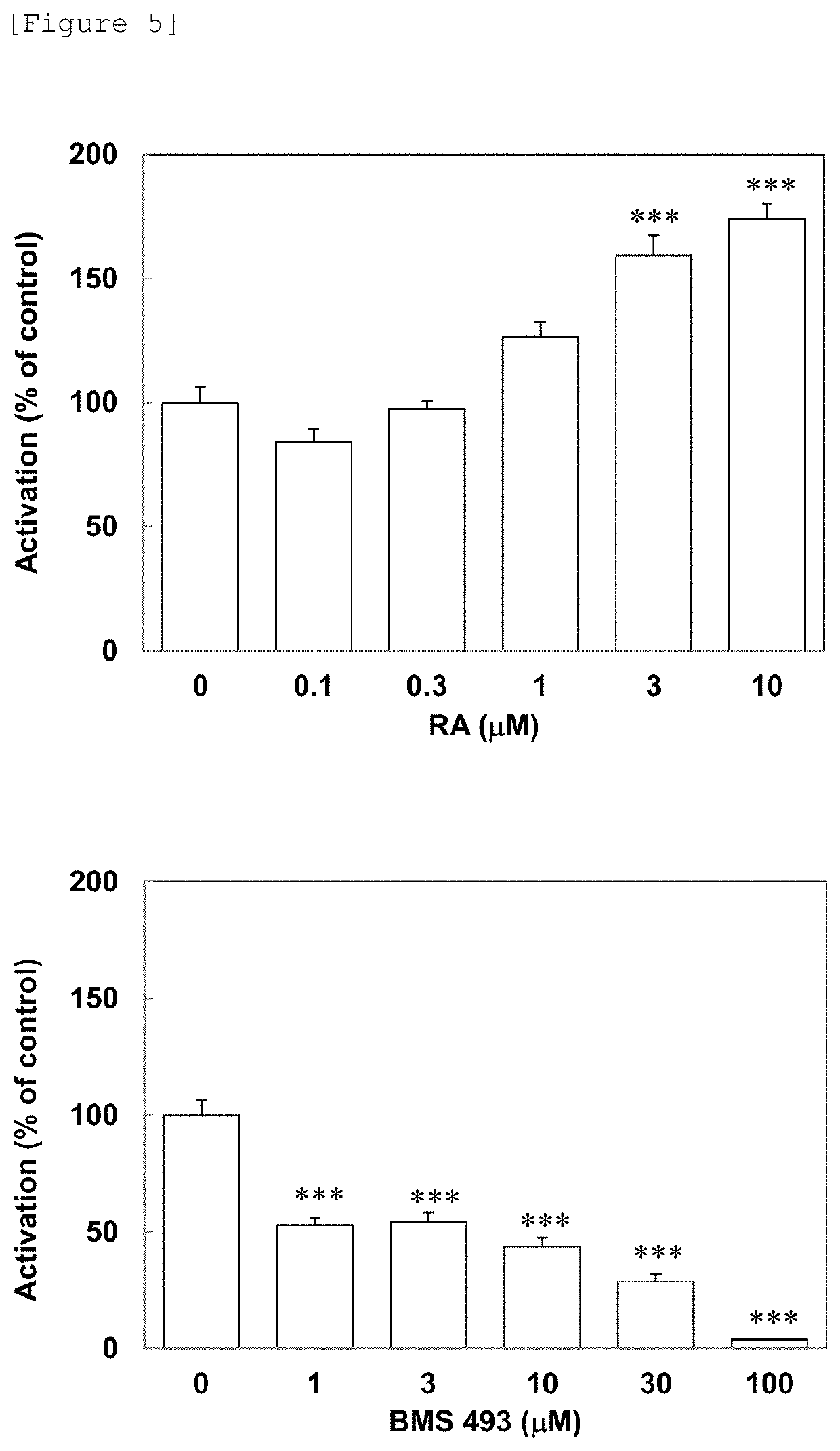
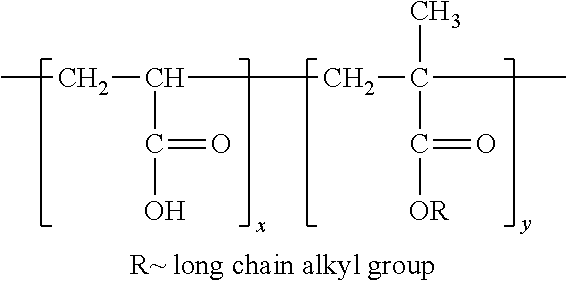
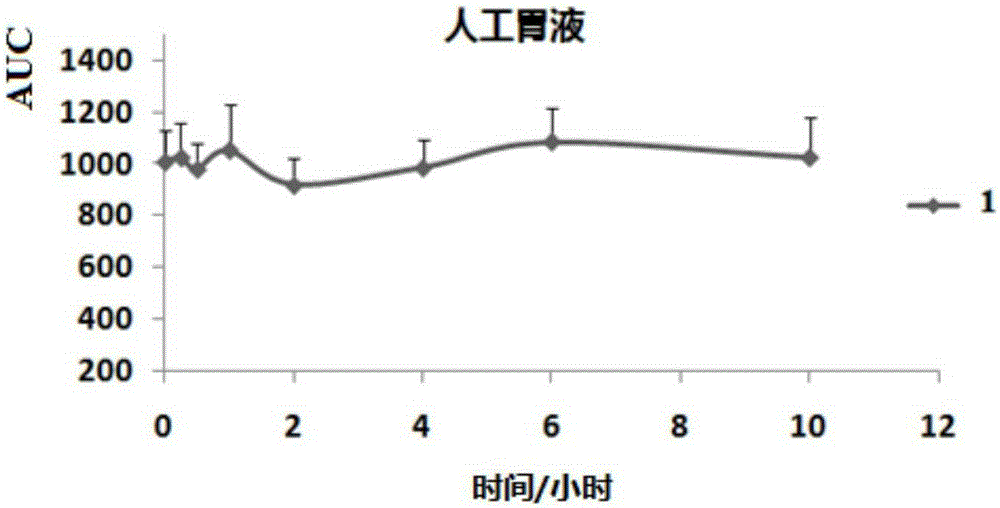
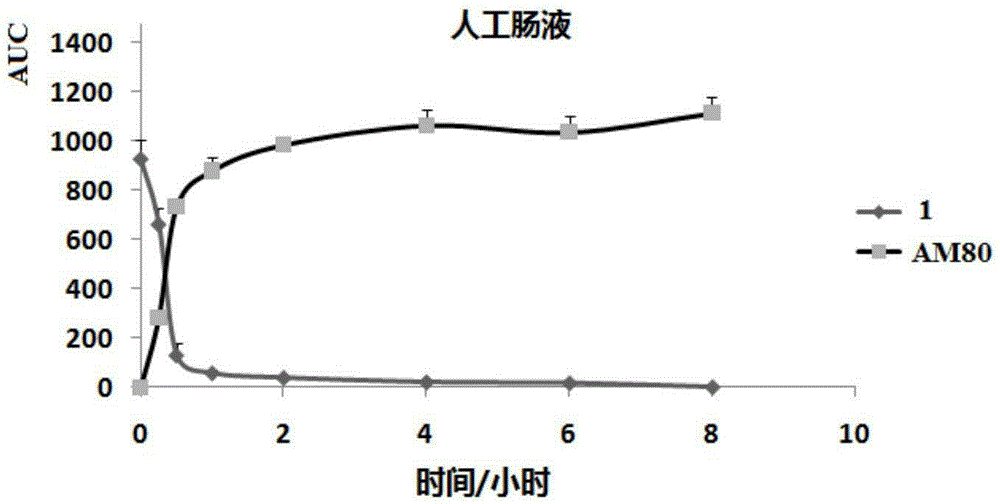
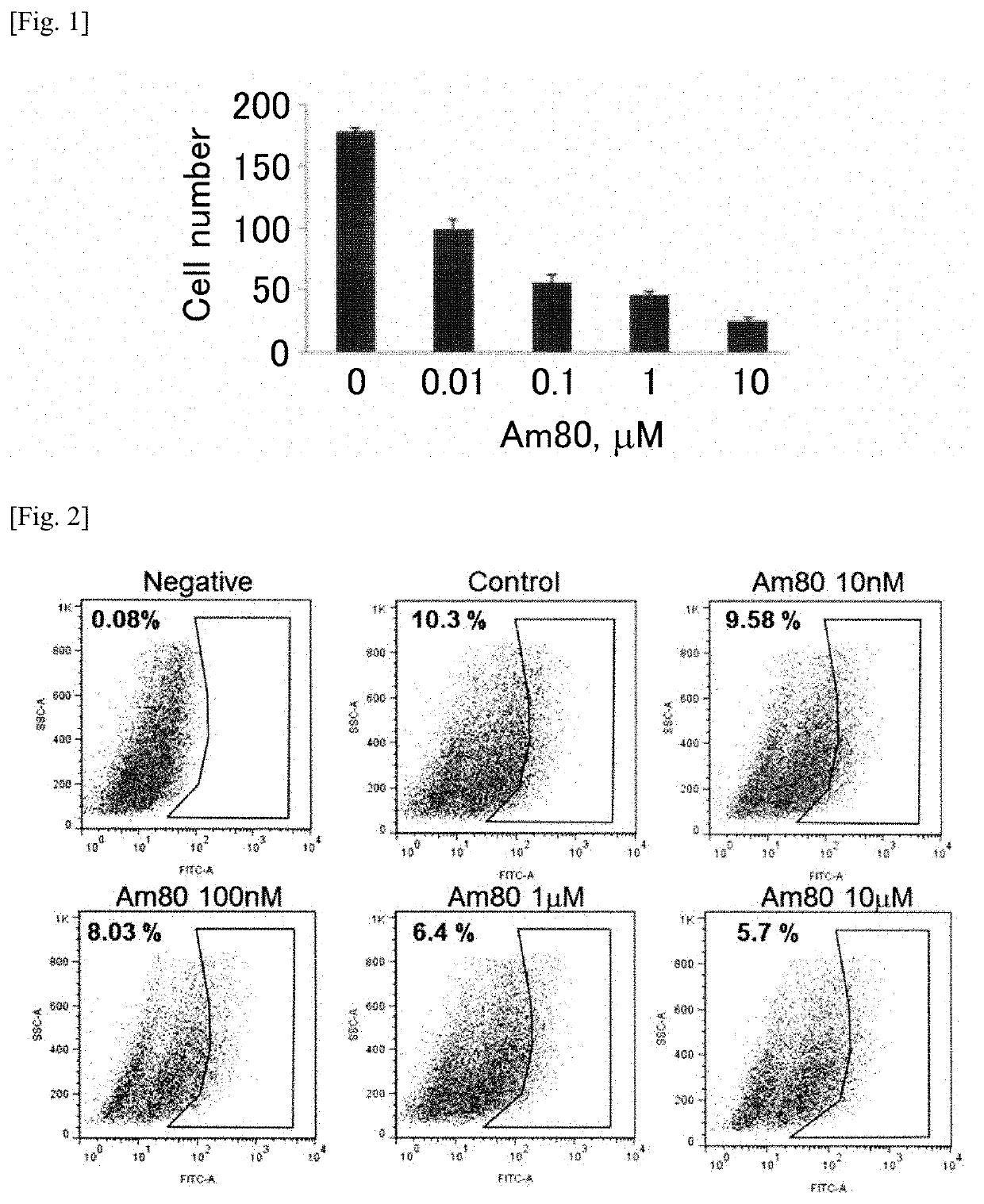
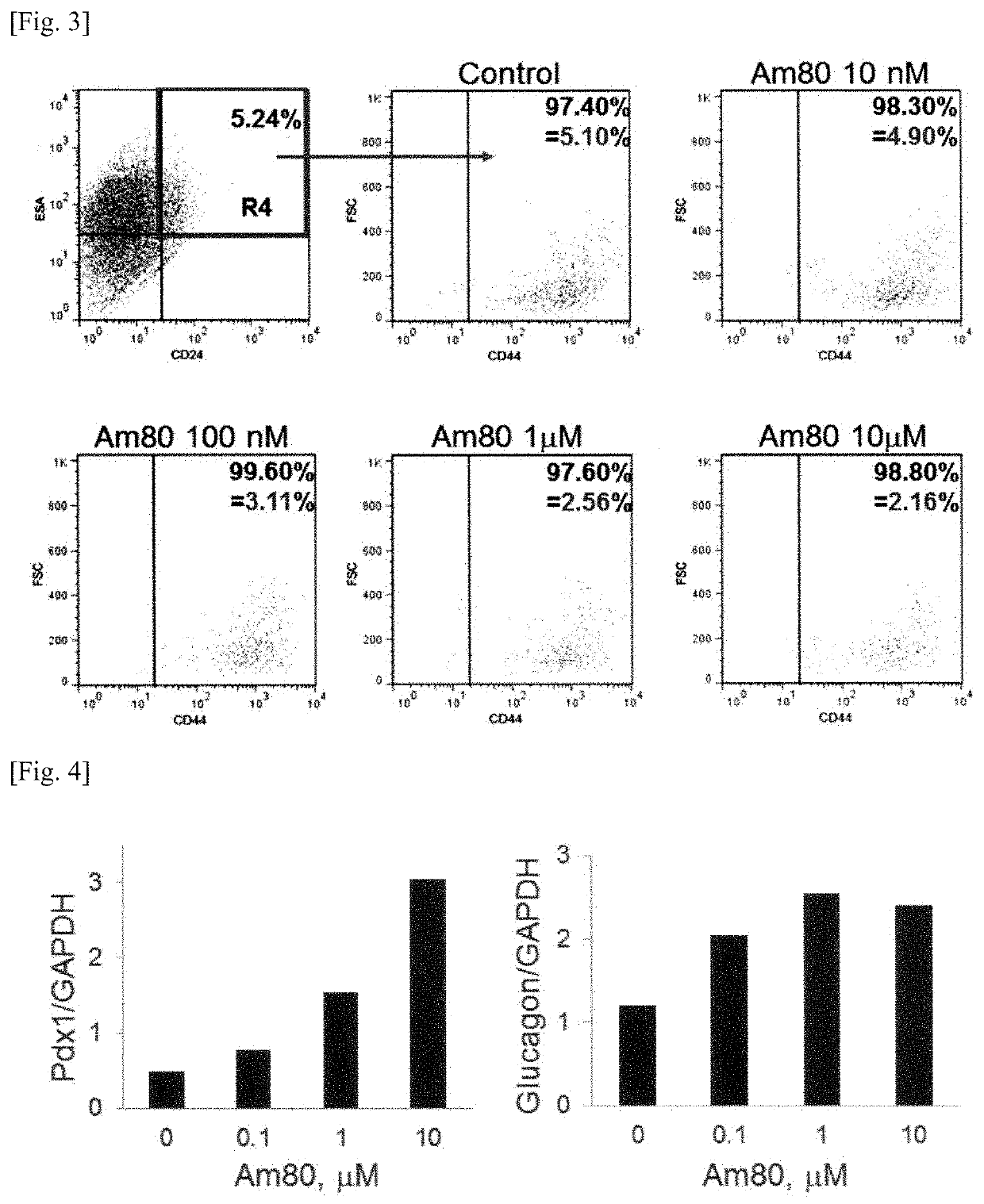
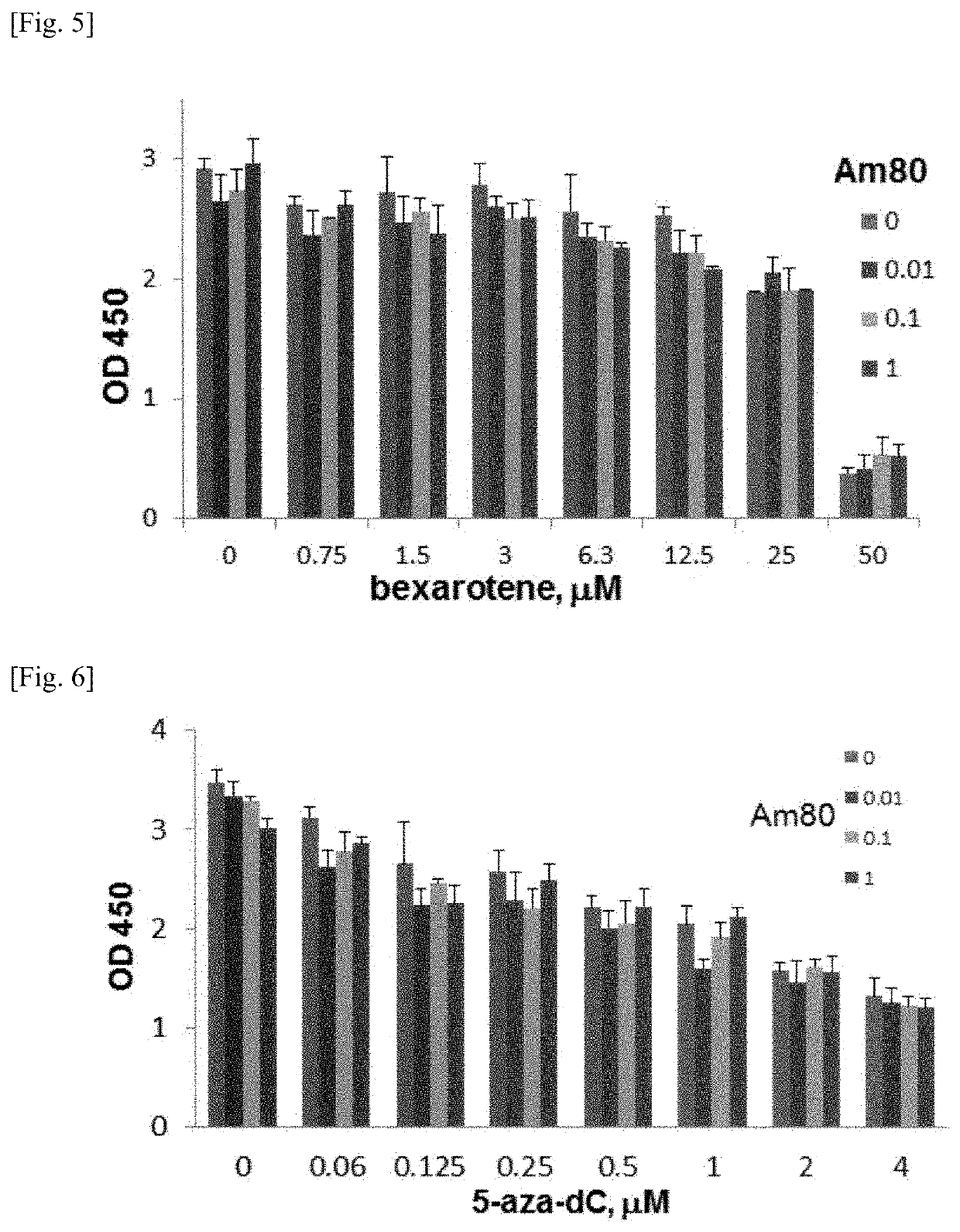
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
31 results about "Tamibarotene" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
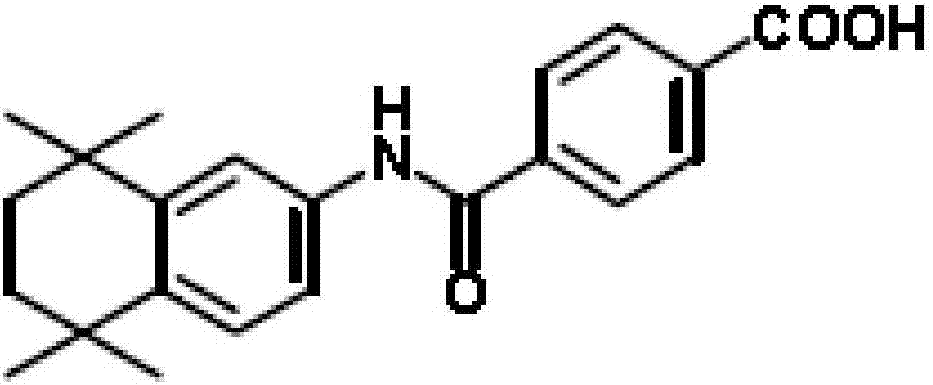
Tamibarotene (brand name: Amnolake), also called retinobenzoic acid, is orally active, synthetic retinoid, developed to overcome all-trans retinoic acid (ATRA) resistance, with potential antineoplastic activity against acute promyelocytic leukaemia (APL) . It is currently marketed only in Japan and early trials have demonstrated that it tends to be better tolerated than ATRA. It has also been investigated as a possible treatment for Alzheimer's disease, multiple myeloma and Crohn's disease.
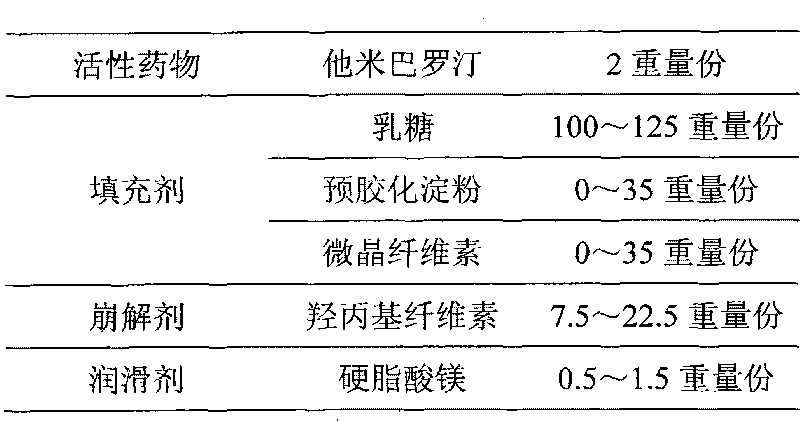
Tamibarotene solid preparation and preparation method thereof
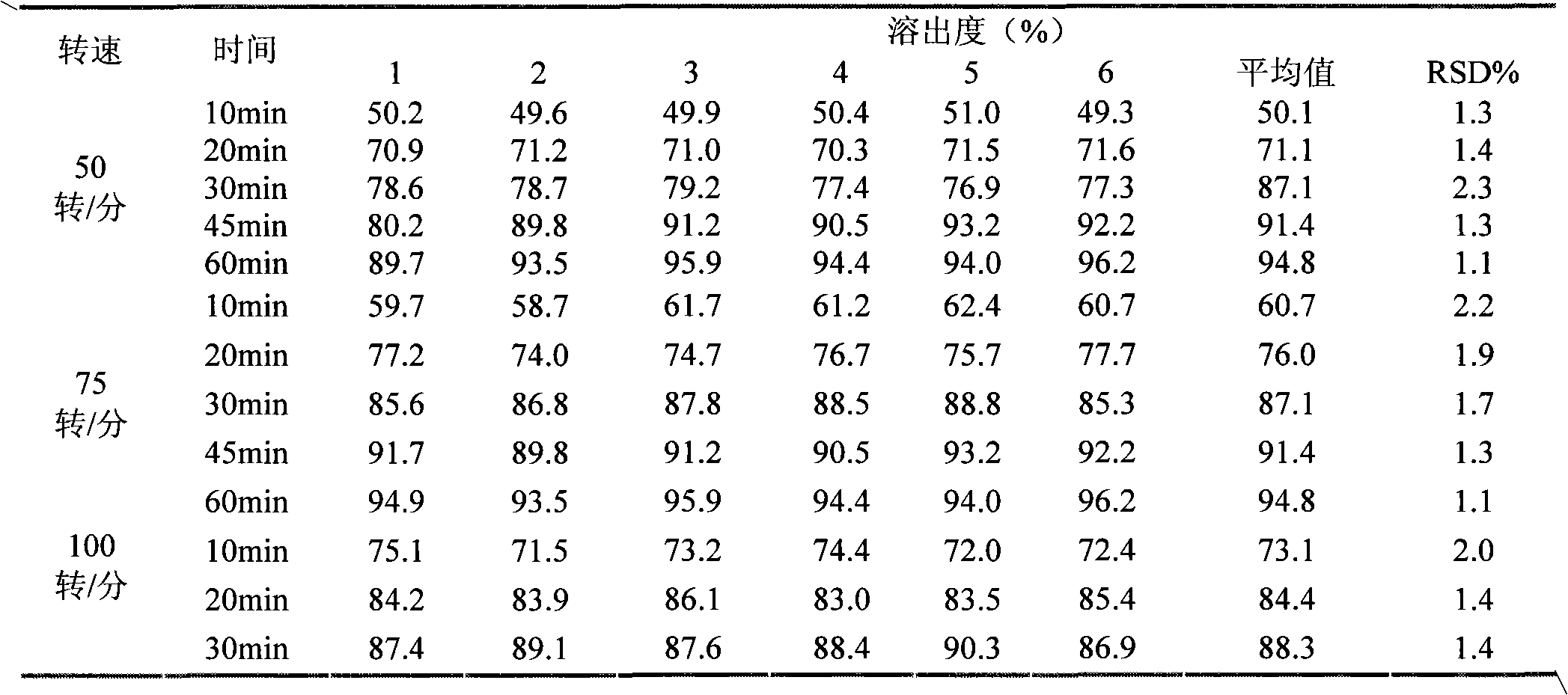
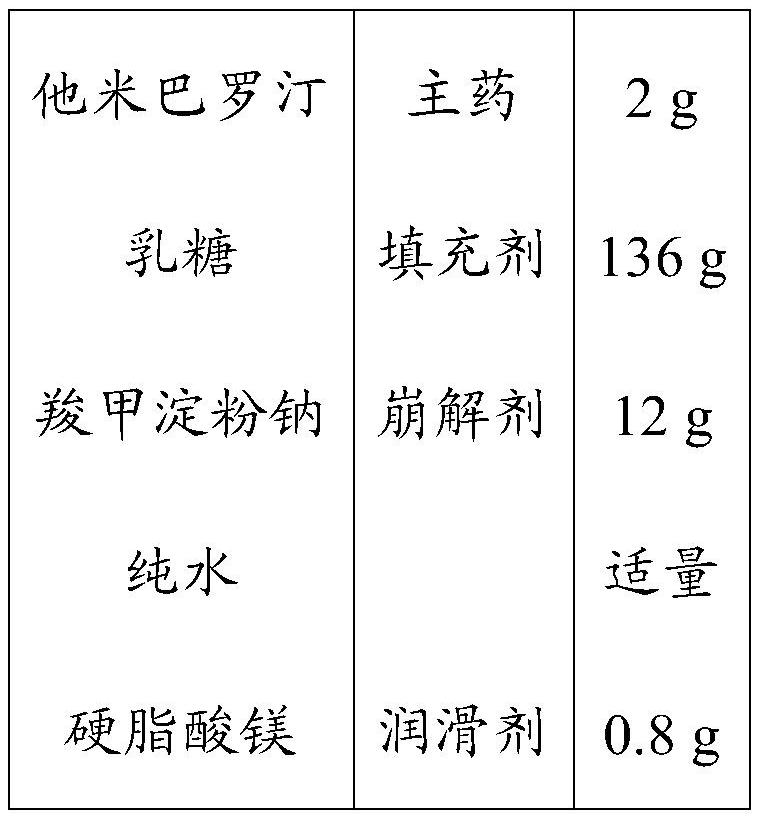
InactiveCN101829044AHigh dissolution rateQuality improvementOrganic active ingredientsPharmaceutical delivery mechanismTamibaroteneLubricant
The invention discloses a tamibarotene solid preparation, which comprises tamibarotene, disintegrant, filler and lubricant. The tamibarotene solid preparation is characterized in that over 70 percent of the tamibarotene solid preparation can be dissolved in 20 minutes. Formula study experiments prove that the tamibarotene solid preparation comprises 2 to 3 weight percent of tamibarotene, 5 to 7 weight percent of disintegrant, 90 to 92 weight percent of filler and 0.5 to 0.7 weight percent of lubricant. Preferably, the tamibarotene solid preparation comprises 2.3 weight percent of tamibarotene, 5.8 weight percent of disintegrant, 91.2 weight percent of filler and 0.6 weight percent of lubricant.
Owner:PHARMA RES INST OF BENCAO TIANYUAN OF BEIJING
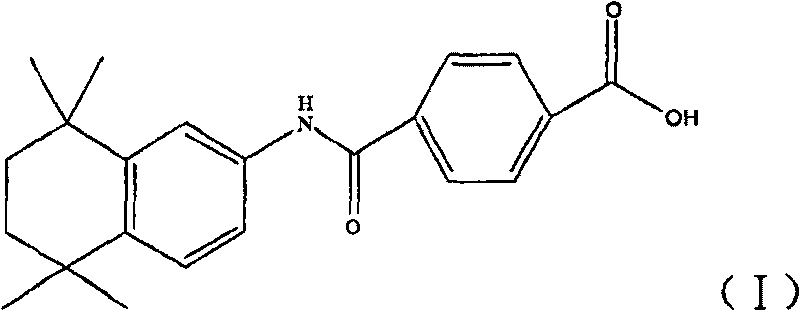
Water-soluble prodrug of tamibarotene, and preparation method and applications thereof
InactiveCN101665449AAppropriate drug crystal formGood water solubilityOrganic active ingredientsOrganic compound preparationSolubilityDrug crystals
The invention provides a water-soluble prodrug of tamibarotene, and a preparation method and applications thereof and belongs to the technical fields of organic compound synthesis and medical applications. The water-soluble prodrug of tamibarotene has favorable water solubility and suitable drug crystal form, and therefore, is suitable to be used as a raw material for medical preparations and especially suitable for preparing injections. Particularly, the invention provides medical acceptable salts of tamibarotene DMEA ester, which have the general formula (I), wherein HA is HCl, H2SO4, HNO3,H3PO4, HOAc, paratoluenesulfonic acid, maleic acid, succinic acid, citric acid or L(+)-tartaric acid.
Owner:SHANDONG UNIV
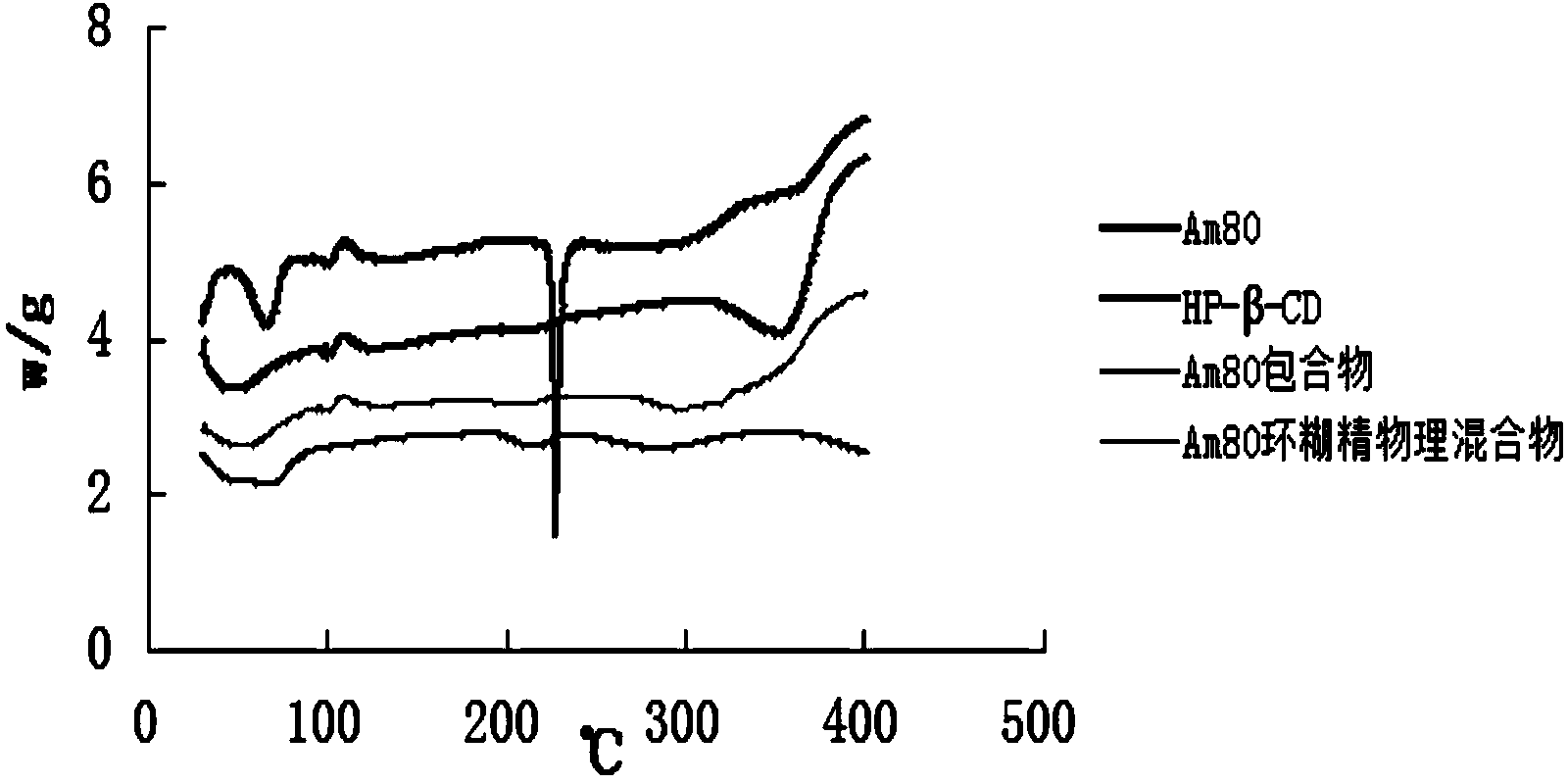
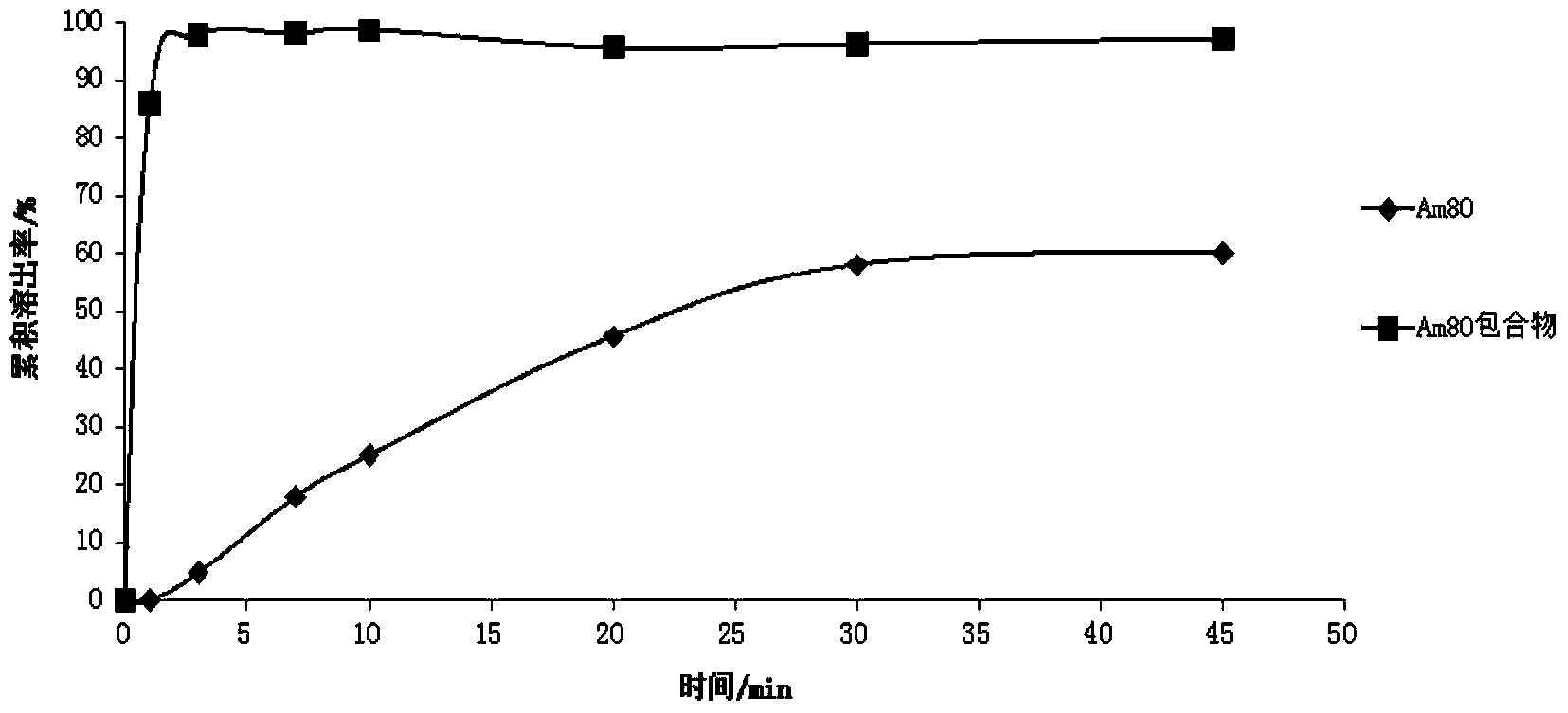
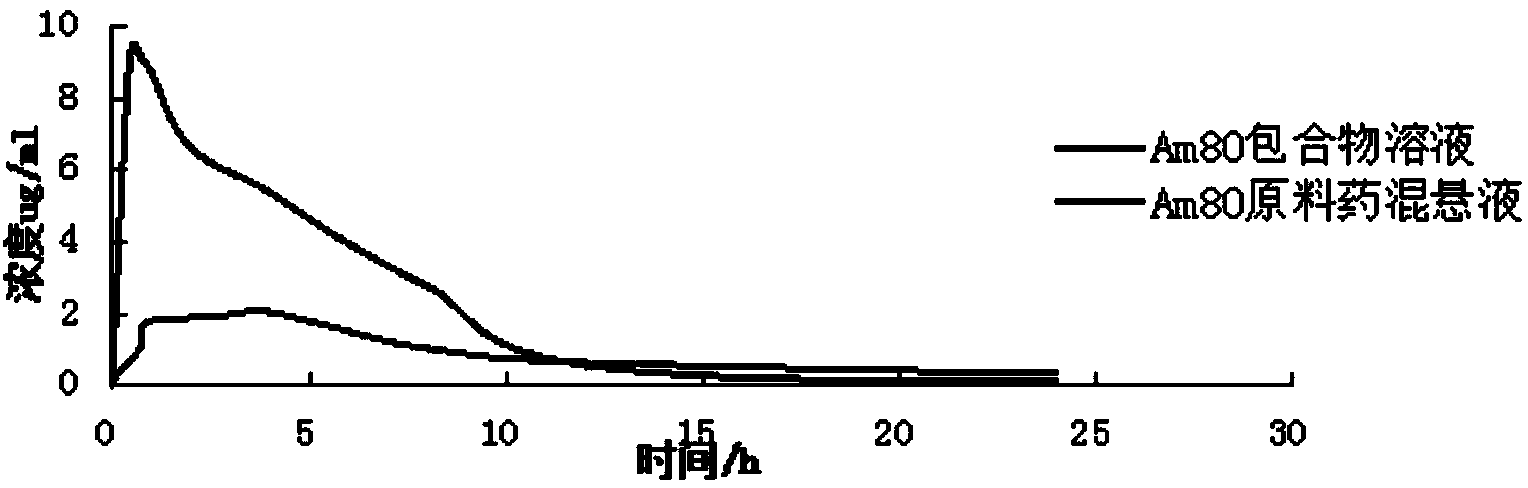
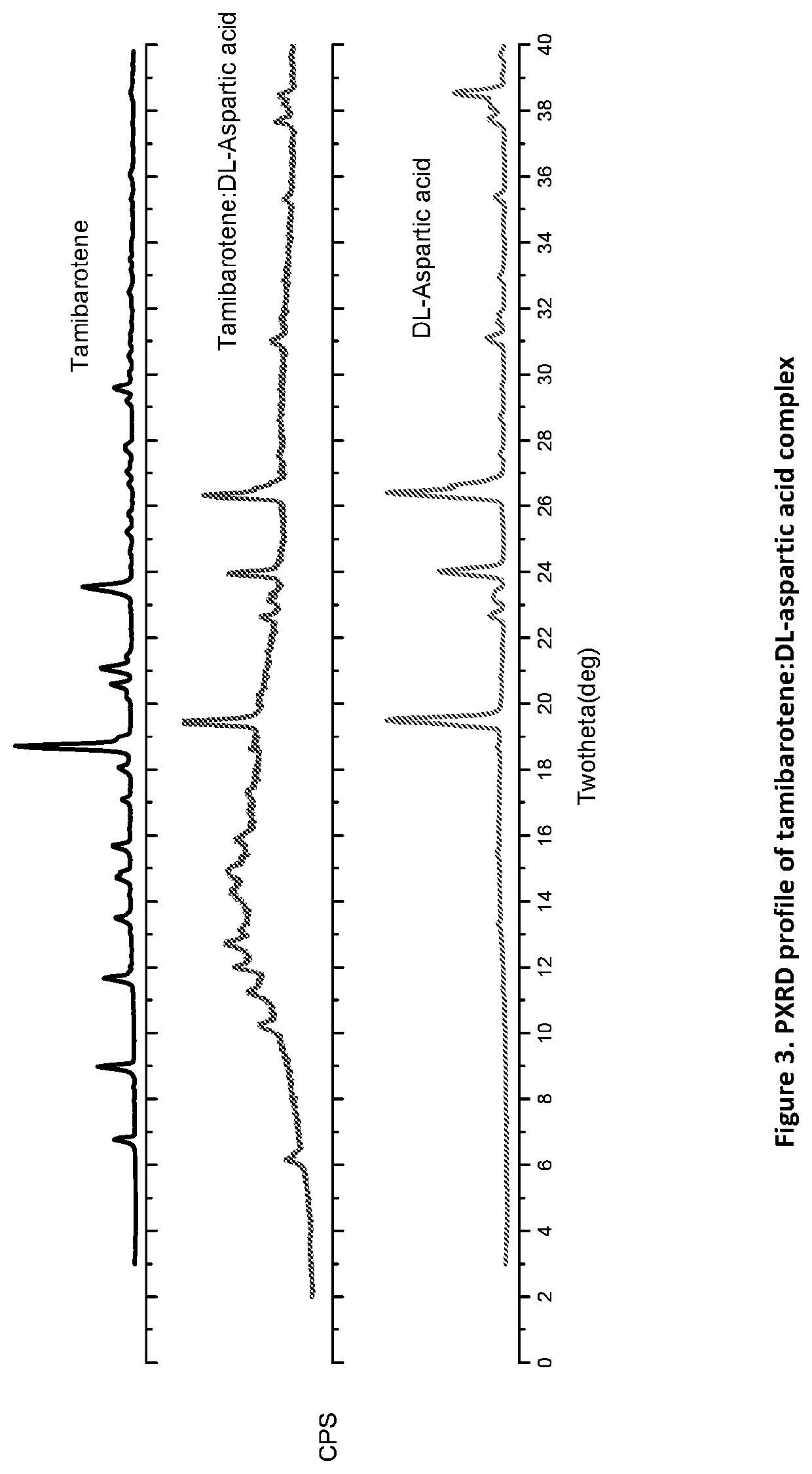
Tamibarotene cyclodextrin or cyclodextrin derivative clathrate and preparation method thereof
InactiveCN104162167AImprove solubilityIncrease dissolution rateOrganic active ingredientsPharmaceutical non-active ingredientsSolubilityFreeze-drying
The invention discloses a tamibarotene cyclodextrin or cyclodextrin derivative clathrate which is prepared from tamibarotene and cyclodextrin or a cyclodextrin derivative, the molar ratio of tamibarotene to cyclodextrin or cyclodextrin derivative is 1:1-1:100, and the preparation method is as follows: the cyclodextrin or cyclodextrin derivative is added to a solvent to produce a solution or suspension, and then the tamibarotene is added for stirring, grinding or ultrasonic mixing to obtain the tamibarotene cyclodextrin or cyclodextrin derivative clathrate; or the cyclodextrin or cyclodextrin derivative is put in a colloid mill or mortar, a solvent is added for stirring to make a paste, then the tamibarotene is added into the paste for grinding for 1-5 hours to obtain a homogeneous thick paste, and the tamibarotene cyclodextrin or cyclodextrin derivative clathrate is obtained by filtration, concentration or freeze drying. The invention also discloses a tamibarotene-containing composition. The tamibarotene cyclodextrin or cyclodextrin derivative clathrate improves the solubility and dissolution rate of the tamibarotene, has good water solubility, less vascular stimulation, quick disintegration, higher bioavailability and other characteristics.
Owner:SHANDONG UNIV
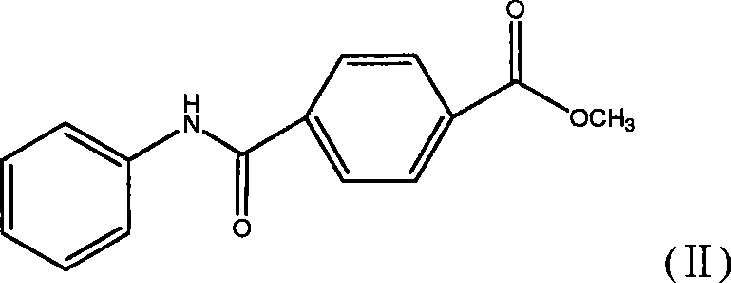
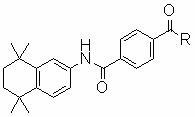
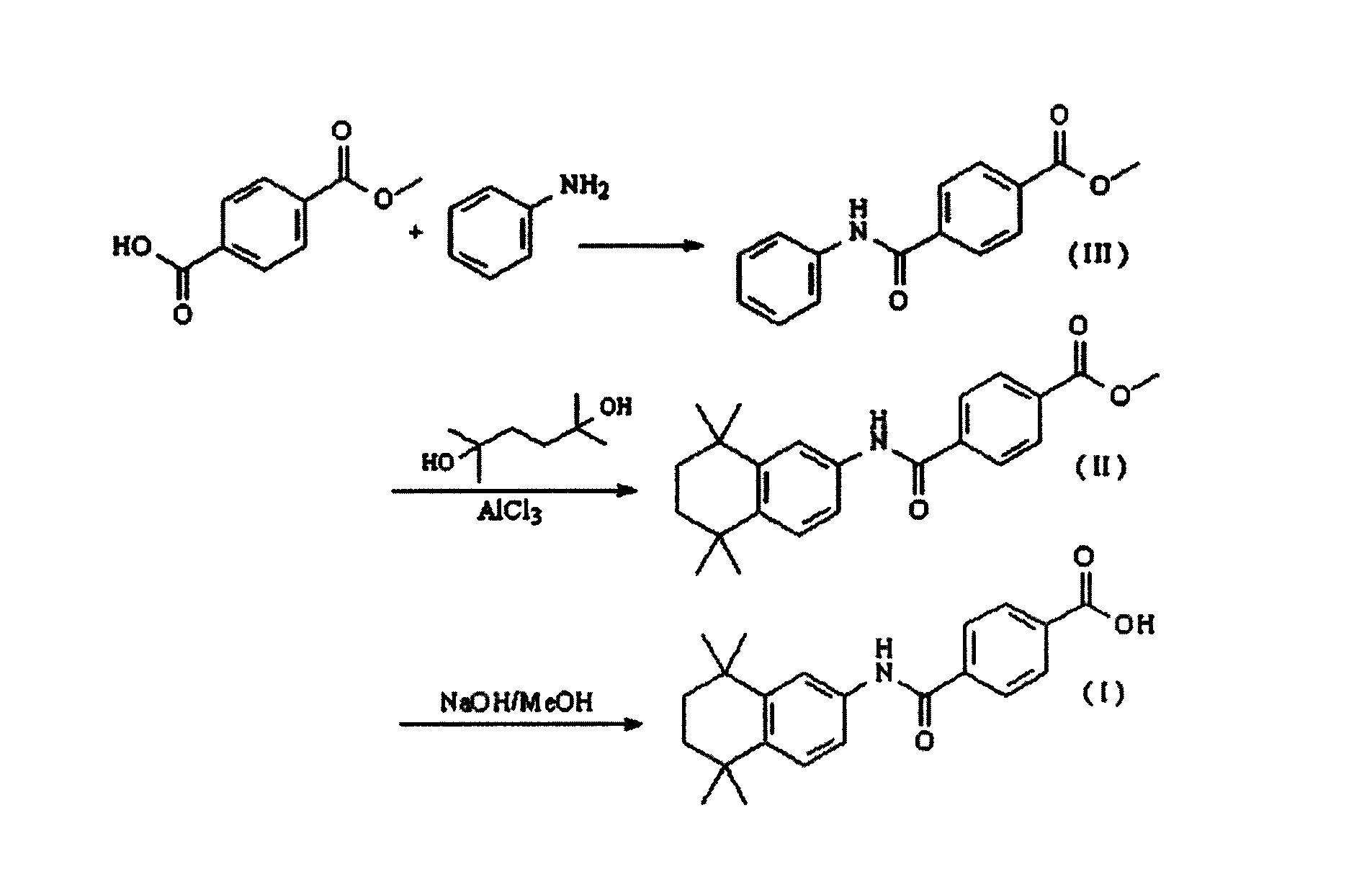
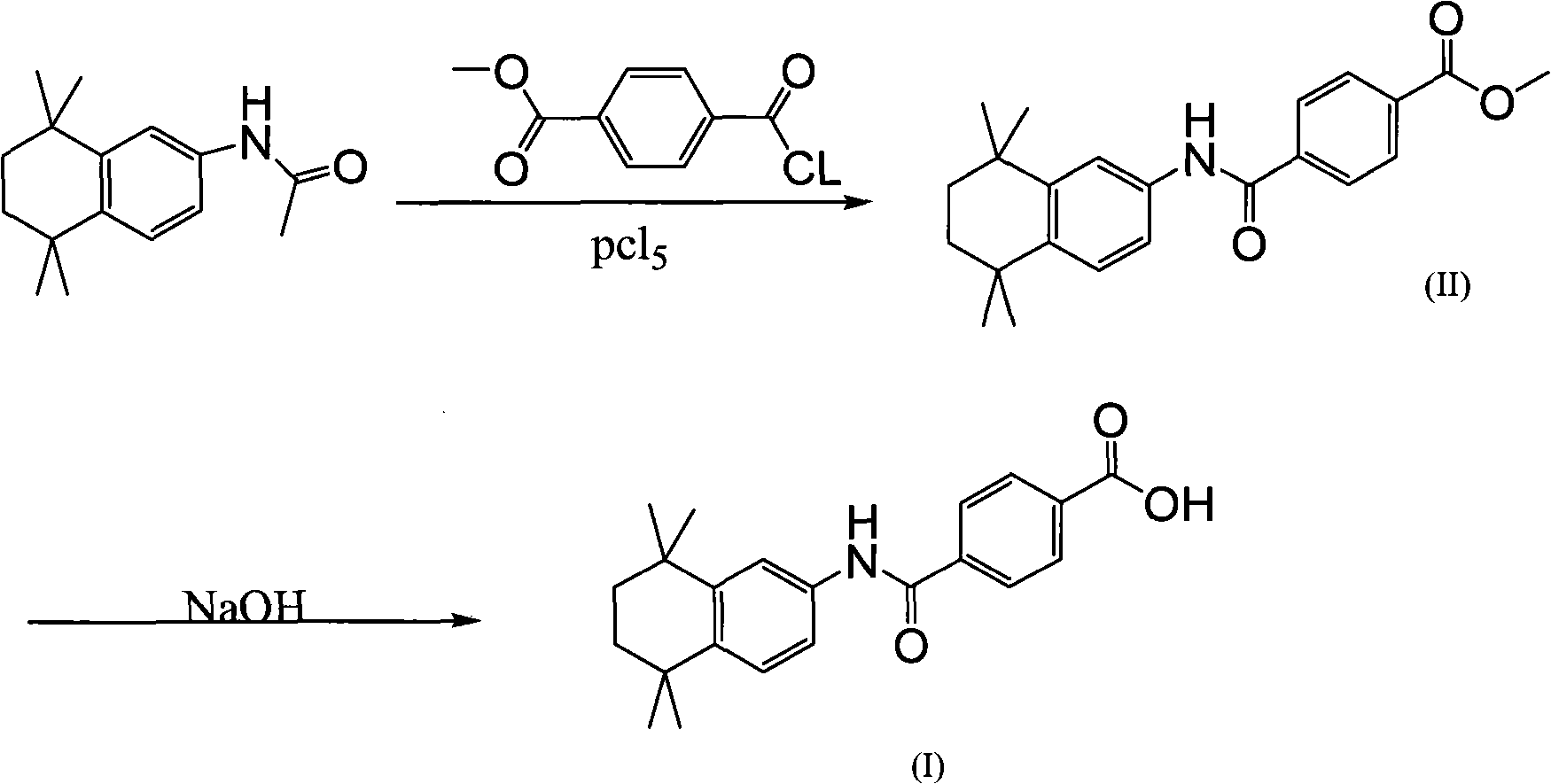
Synthetic technique for tamibarotene
InactiveCN101121675AFew stepsThe synthetic route is simpleOrganic compound preparationCarboxylic acid amides preparationBenzoic acidSynthesis methods
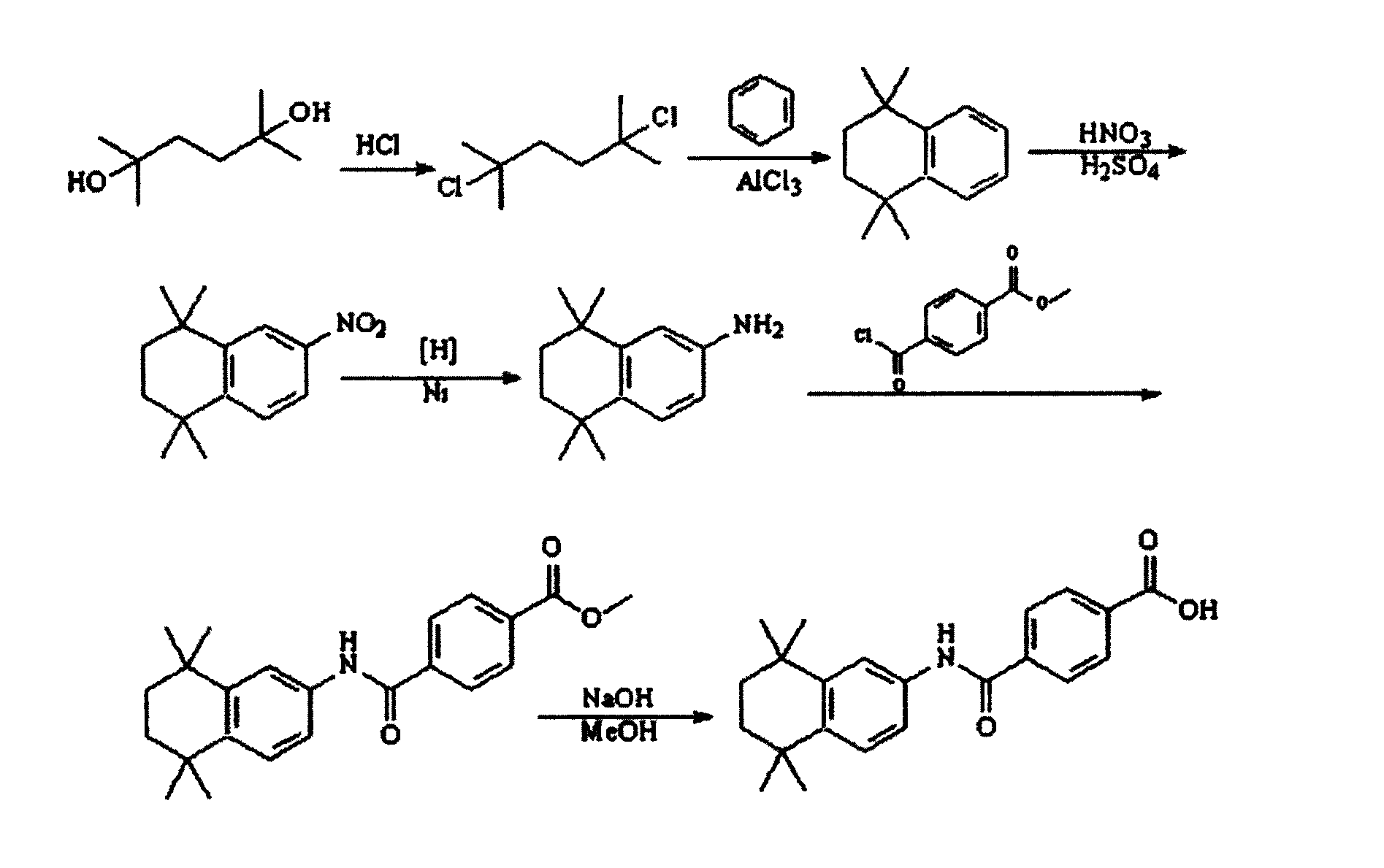
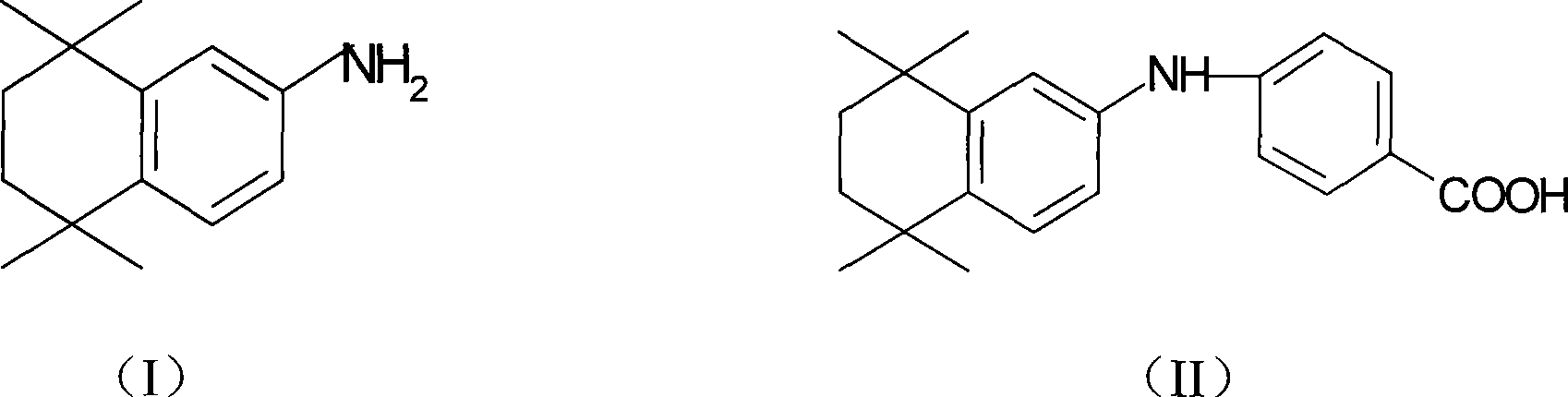
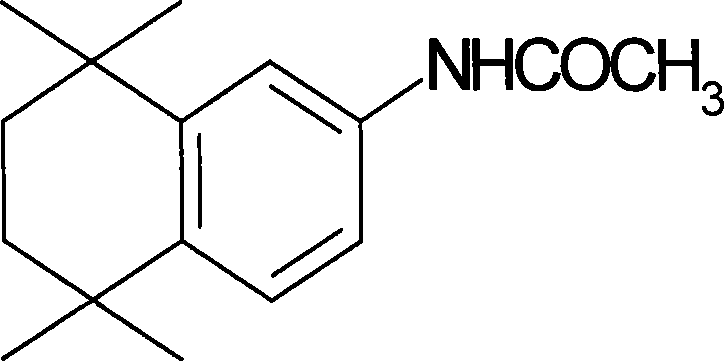
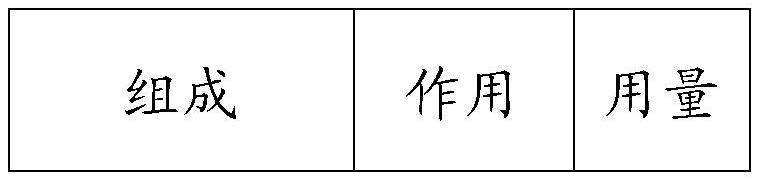
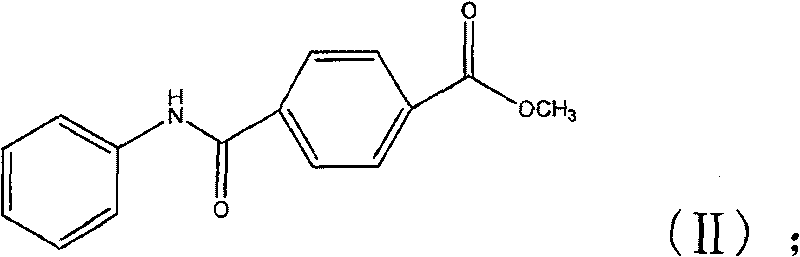
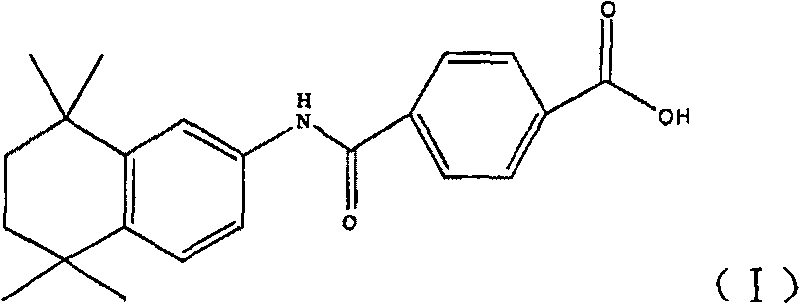
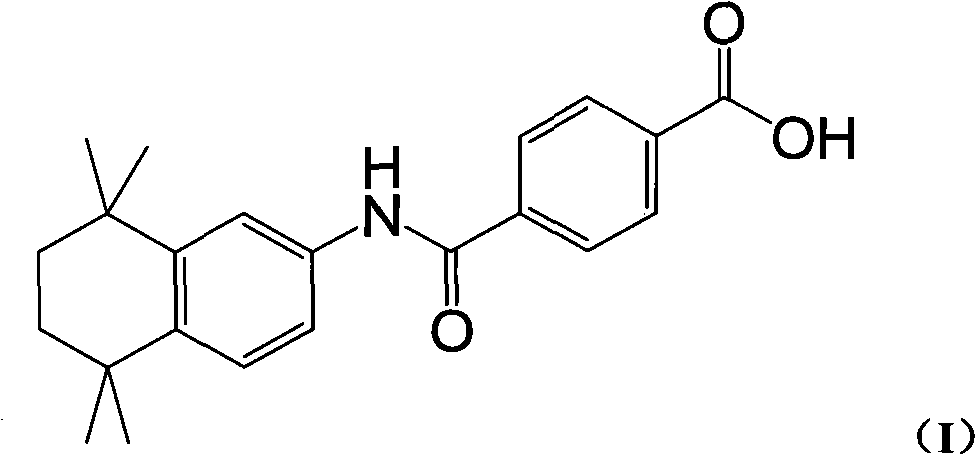
The invention discloses a synthesis method for the (I) Linarotene (I), a leukemia treatment drug. The invention mainly proposes a novel synthetic route with the steps as follows: the aniline, and p-chlorocarbonyl benzoic methyl ester are used as the raw materials; the intermediate is the p-chlorocarbonyl benzoic methyl ester and can do the cyclization with the 2,5 - dichloro-2 ,5 - dimethyl hexane under the anhydrous condition to get the intermediate 4-[(5,6,7,8-tetrahydrocannabinol-5,5,8,8- tetramethyl-2-naphthalene) carbamoyl] methyl benzoate. After the hydrolysis, the 4-[(5,6,7,8-tetrahydrocannabinol-5,5,8,8- tetramethyl-2-naphthalene) carbamoyl] benzoate (I) can be made, that is, the Linarotene. The synthetic route is simple; the operation is convenient and conducive to the environmental protection; the invention is suitable for the industrial production.
Owner:CHINA PHARM UNIV
Method for preparing dispersible tablets of tamibarotene
InactiveCN101708168AGood content uniformityHigh dissolution rateOrganic active ingredientsPill deliveryOrganic solventDissolution
The invention discloses a method for preparing dispersible tablets of tamibarotene. The process in the method adopts the solvent deposition method, and the dispersible tablets of tamibarotene, prepared by the method, are good in stability, high in content uniformity and also high in dissolution rate; the method is operated according to the requirements of the Chinese Pharmacopoeia on the dissolution rate of the dispersible tablets, and the dispersible tablets are completely disintegrated within 3 minutes and can pass the No 2 screen(24 meshes), so that the dispersible tablets can enhance the absorption in the human body and improve the biological utilization rate; the preparation method is simple in operation and causes little pollution; by dissolving the tamibarotene in the organic solvents and depositing the tamibarotene in the auxiliary materials, the dust problem in operation can be solved, the caused pollution can be lessened, the untoward reactions of operators are avoided; and the mixed powder of raw materials and auxiliary materials, prepared by the method, can be tabletted directly, so that the cost is saved, and the requirement for being suitable for industrialized production is met.
Owner:深圳万乐药业有限公司
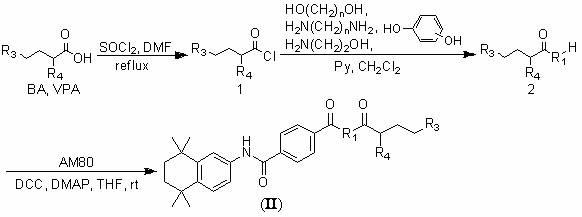
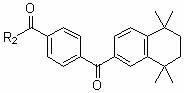
Multiple-target-point tamibarotene derivatives, preparation method and purposes thereof
The invention discloses multiple-target-point tamibarotene derivatives, a preparation method and purposes thereof. More specifically speaking, the invention provides a compound represented by a structural general formula (I), wherein the definition of R is referred to an instruction book; the derivatives are multiple-target-point compounds which are obtained by connecting tamibarotene as an RAR (retinoic acid receptors) excitant with various histone deacetylase inhibitors, various RXR (retinoid X receptors) excitants or other pharmacophoric groups directly through ester bonds or amido bonds or indirectly through connecting groups; and the derivatives are suitable to be used as antitumor drugs to be used for treating various malignant tumors, and are particularly suitable for treating various leukemia.
Owner:济南铂卅医药科技有限公司
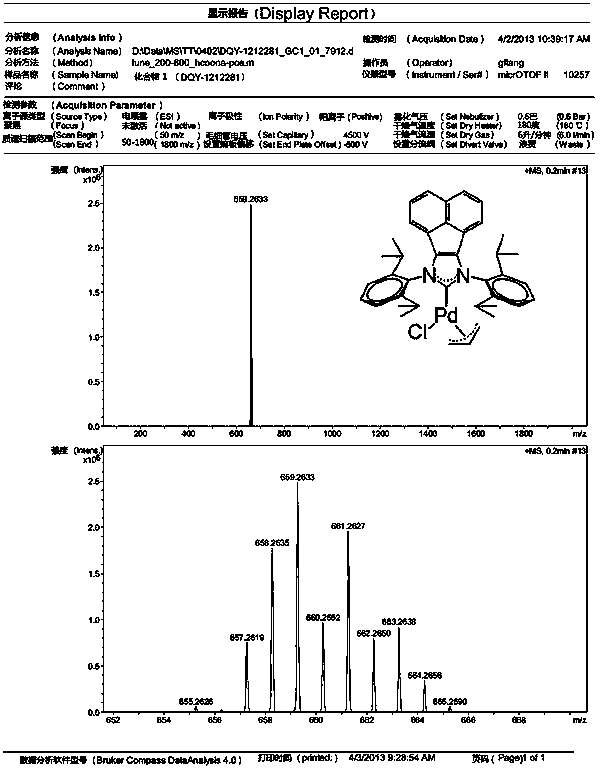
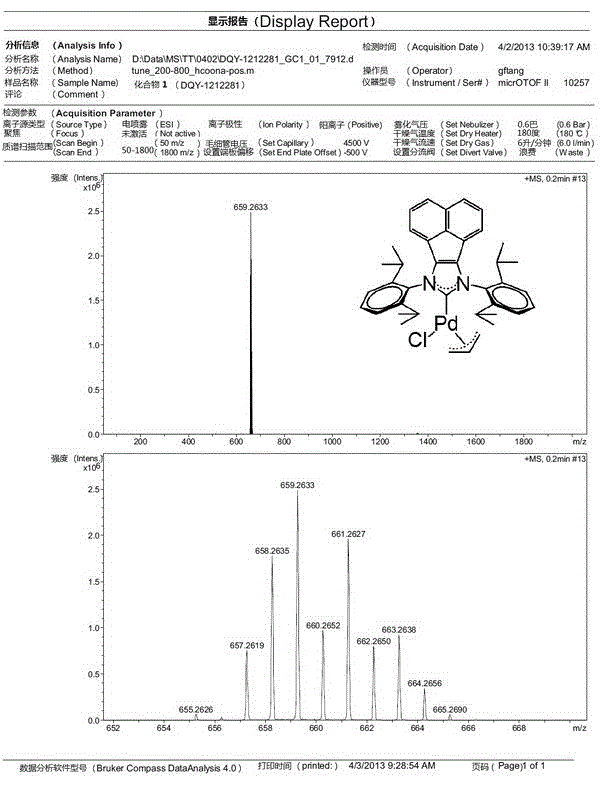
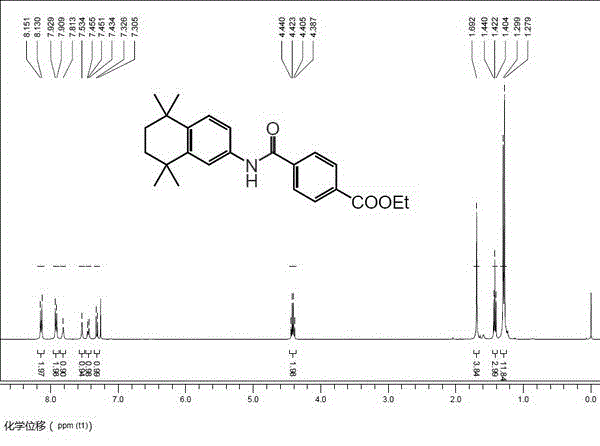
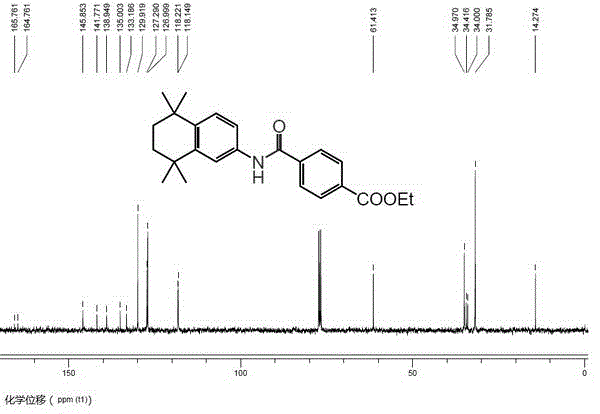
Method for catalytically synthesizing tamibarotene through acenaphthene imidazole n-heterocyclic carbine allyl palladium chloride compound
InactiveCN103408450AShort synthetic routeGood atom economyOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsPtru catalystPharmaceutical Substances
The invention belongs to the technical field of medicine pharmaceutical synthesis, and particularly relates to a method for catalytically synthesizing tamibarotene through an acenaphthene imidazole n-heterocyclic carbine allyl palladium chloride compound, aiming at providing a novel synthesis method of tamibarotene. The method comprises the following steps of: by taking a novel acenaphthene imidazole n-heterocyclic carbine allyl palladium chloride compound with high catalytic activity as a catalyst; performing amide carbonylation coupled reaction under carbon monoxide in order to directly synthesize a key precursor of tamibarotene by such one step; and then simply hydrolyzing to obtain the target compound, namely, tamibarotene. According to the method, the catalyst adopted has high catalytic performance, so that the catalytic coupling reaction brings high yield; and the catalyst is naturally easily synthesized, the raw materials adopted in the synthetic line are easily available, so that such synthetic line has high competitive advantages and high utility value in industrial production.
Owner:FUDAN UNIV
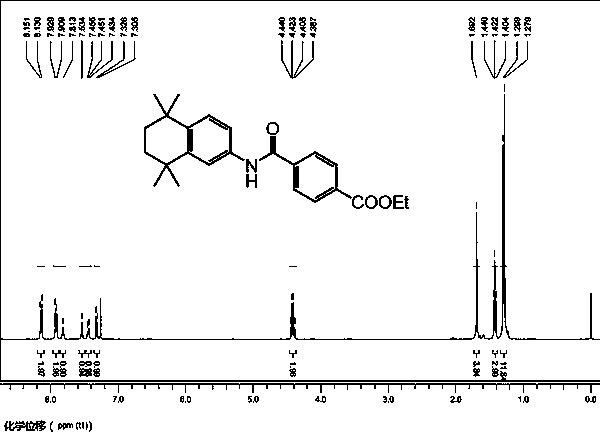
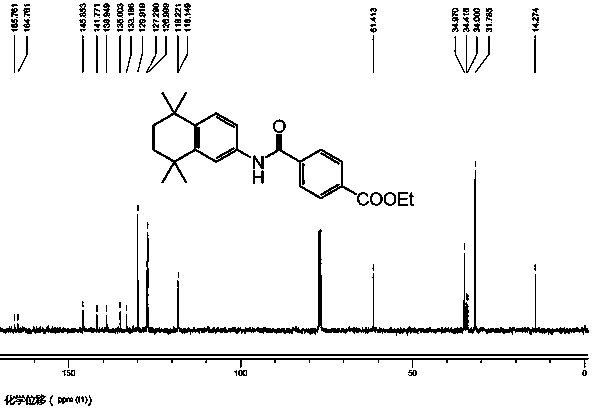
Synthesis method of tamibarotene
InactiveCN102633673AInhibit productionAvoid it happening againOrganic compound preparationCarboxylic acid amides preparationAlkaneSynthesis methods
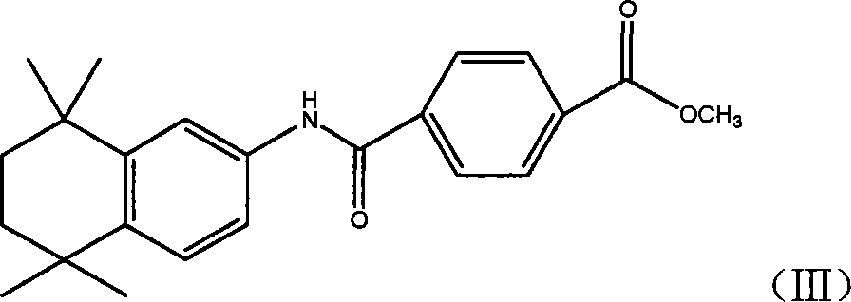
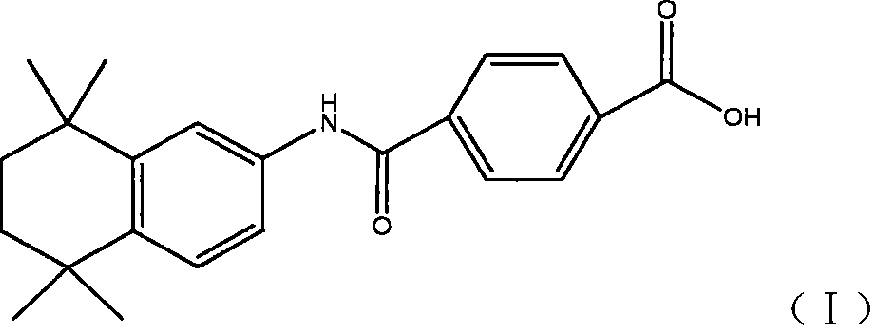
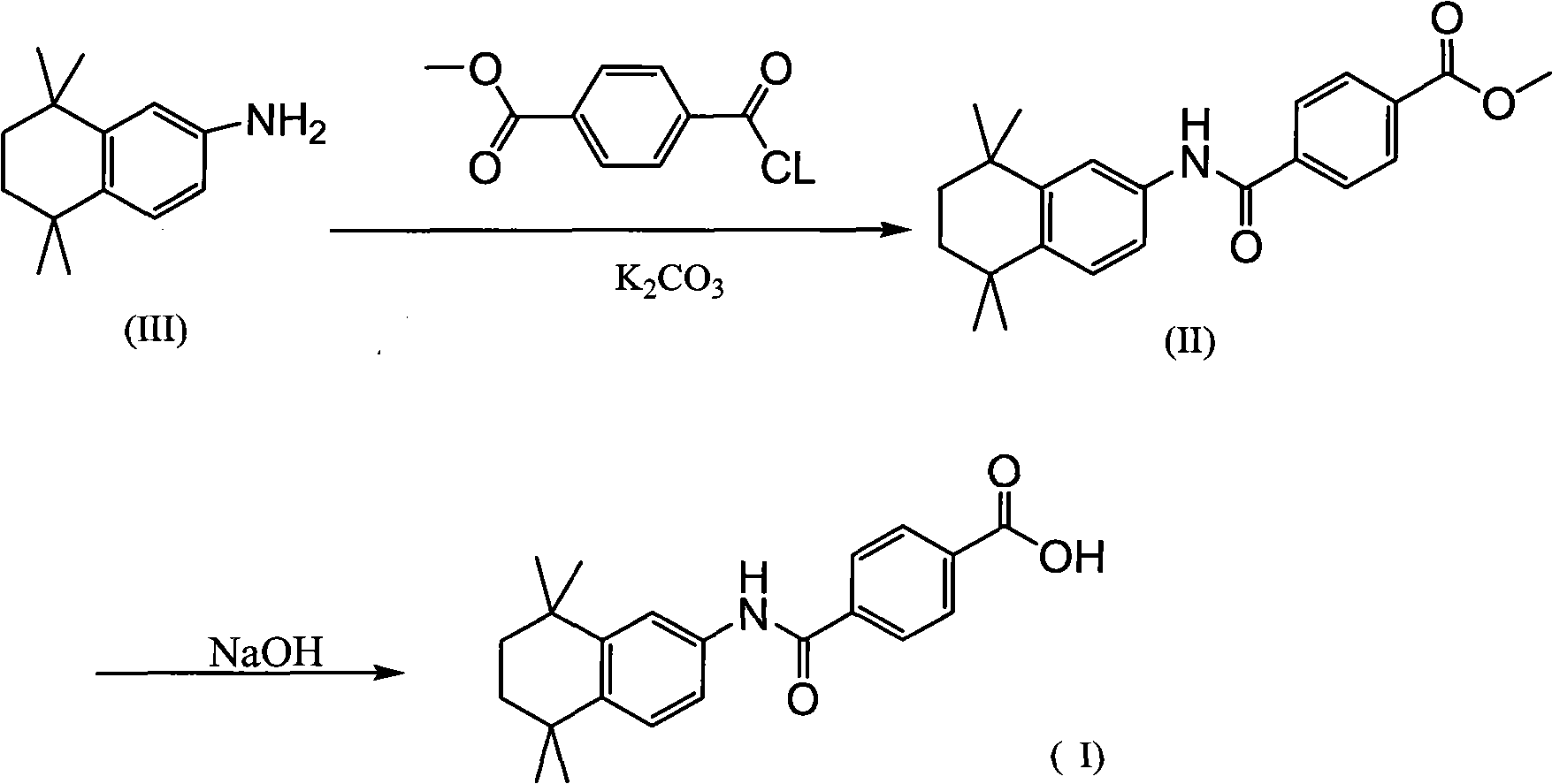
The invention relates to a synthesis method of tamibarotene, which comprises the steps of: 1)taking aniline and monomethyl ester terephthalate as raw materials, and synthesizing p-carbaniloyl methyl benzoate (III); 2) under the protection of nitrogen in an anhydrous condition, carrying out cyclization on the intermediate III and 2, 5-dimethyl-2, 5-hexanediol at a low temperature, to obtain an intermediate II; and 3) hydrolyzing the intermediate II to obtain the tamibarotene (I), wherein dicyclohexyl carbodiimide (DCC) / hydroxyl benzotriazole (HOBt), diisopropylcarbodiimide (DIC) / HOBt, HATU or HBTU taken as a condensing agent as well as triethylamine and diisopropylethylamine (DIEA) taken as acid-binding agents can be added into the step 1); halogen acid taken as a catalyst is added into the step 2); and the reaction solvent is halogenated alkanes. The synthesis method of the tamibarotene avoids the link with high pollution, thus reducing the environmental cost.
Owner:SHANGHAI MEDPEP
Tamibarotene capsule preparation
ActiveUS8252837B2Risk of contaminationImprove absorption rateBiocideOrganic active ingredientsPolyethylene glycolActive ingredient
The present invention provides a practical preparation form of Tamibarotene and dosage form thereof, which exhibit high absorptivity by the body with minimized toxicity, and which are safe and stable without any risk of contamination. It is provided a Tamibarotene capsule preparation which encapsulates a composition comprising an oil component as its base and Tamibarotene as an active ingredient dissolved in the base. It is preferred that the oil component be propylene glycol fatty acid esters or polyethylene glycols. It is also preferred that the Tamibarotene capsule preparation comprise 0.1-50 mg / mL of the Tamibarotene based on the oil component.
Owner:TMRC
Preparation of tamibarotene-cytarabine conjugate and nano pharmacosomes and anti-tumor application of tamibarotene-cytarabine conjugate and nano pharmacosomes
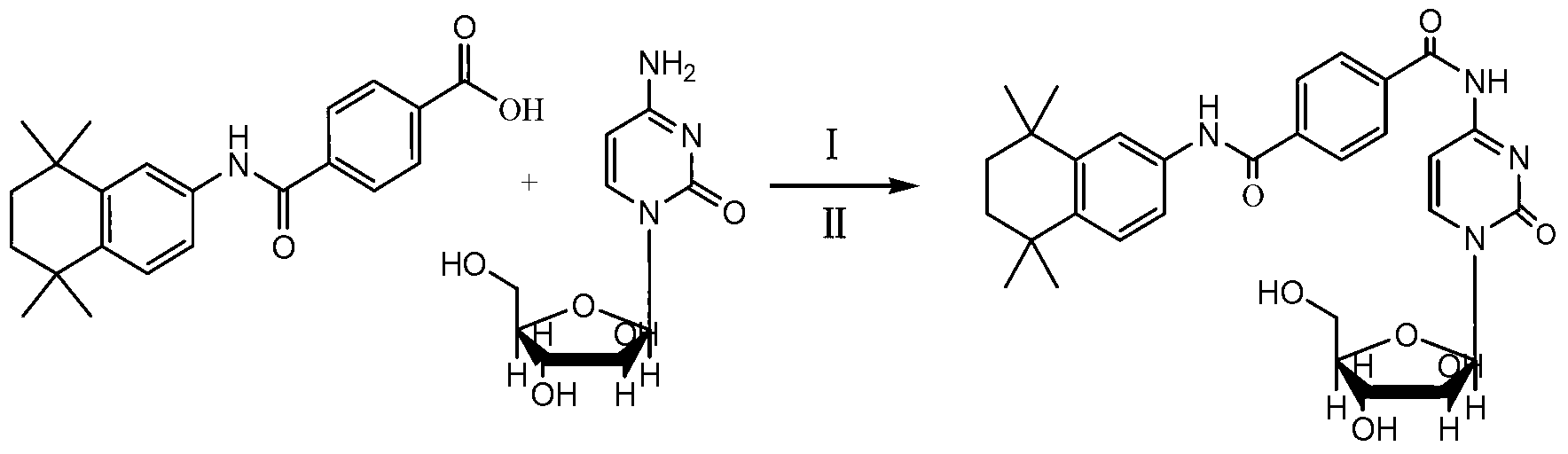
The invention discloses preparation of tamibarotene-cytarabine conjugate, nano pharmacosomes and an anti-tumor application of the tamibarotene-cytarabine conjugate and nano pharmacosomes. The tamibarotene-cytarabine conjugate is prepared by coupling the carboxyl of tamibarotene with four locations of amino groups of cytarabine. The tamibarotene-cytarabine conjugate has a proper amphipathy, can form a nanoscale dispersive self-assembly transfer system in water and has good anti-tumor activity in vitro and vivo.
Owner:CAPITAL UNIVERSITY OF MEDICAL SCIENCES
Drug for retinal degenerative disease associated with photoreceptor degeneration
PendingUS20200330415A1Increase the number ofSymptoms improvedCompounds screening/testingSenses disorderAcitretinPharmacology
An object of the present invention is to provide a medicine that can simply treat and / or prevent a retinal degenerative disease associated with photoreceptor degeneration, including retinitis pigmentosa. The solution is to provide an agent for treating and / or preventing a retinal degenerative disease associated with photoreceptor degeneration, containing a compound having a retinoic acid receptor agonistic activity (for example, tamibarotene, tamibarotene methyl ester, tamibarotene ethyl ester, tazarotene, tazarotenic acid, adapalene, palovarotene, retinol, isotretinoin, alitretinoin, etretinate, acitretin or bexarotene) or a salt thereof.
Owner:DAIICHI SANKYO CO LTD
Topical compositions for reducing the effects of aging
The present invention comprises ternary compositions of tamibarotene and / or ammonium lactate in Pemulen for treatment of effects of aging.
Owner:SAMSON PHARMA LLC
Multiple target point type Tamibarotene derivative as well as preparation method and application thereof
ActiveCN105175285AAchieve synergyEnhanced inhibitory effectUrea derivatives preparationOrganic active ingredientsLeukemiaHydroxycarbamide
The invention provides a multiple target point type Tamibarotene derivative as well as a preparation method and an application thereof. Particularly, RAR (retinoic acid receptor) agonist Tamibarotene is connected with antineoplastic drug hydroxycarbamide, fluorouracil and lenalidomide on sales through ester bonds or amido bonds respectively to obtain three multiple target point mutual prodrugs. The invention provides a method for preparing the compound and the application of the compound in preparing antineoplastic drugs, particularly drugs for curing various leukemias. The invention further relates to a drug combination of the compound.
Owner:SHANDONG UNIV
Cancer stem cell proliferation inhibitor
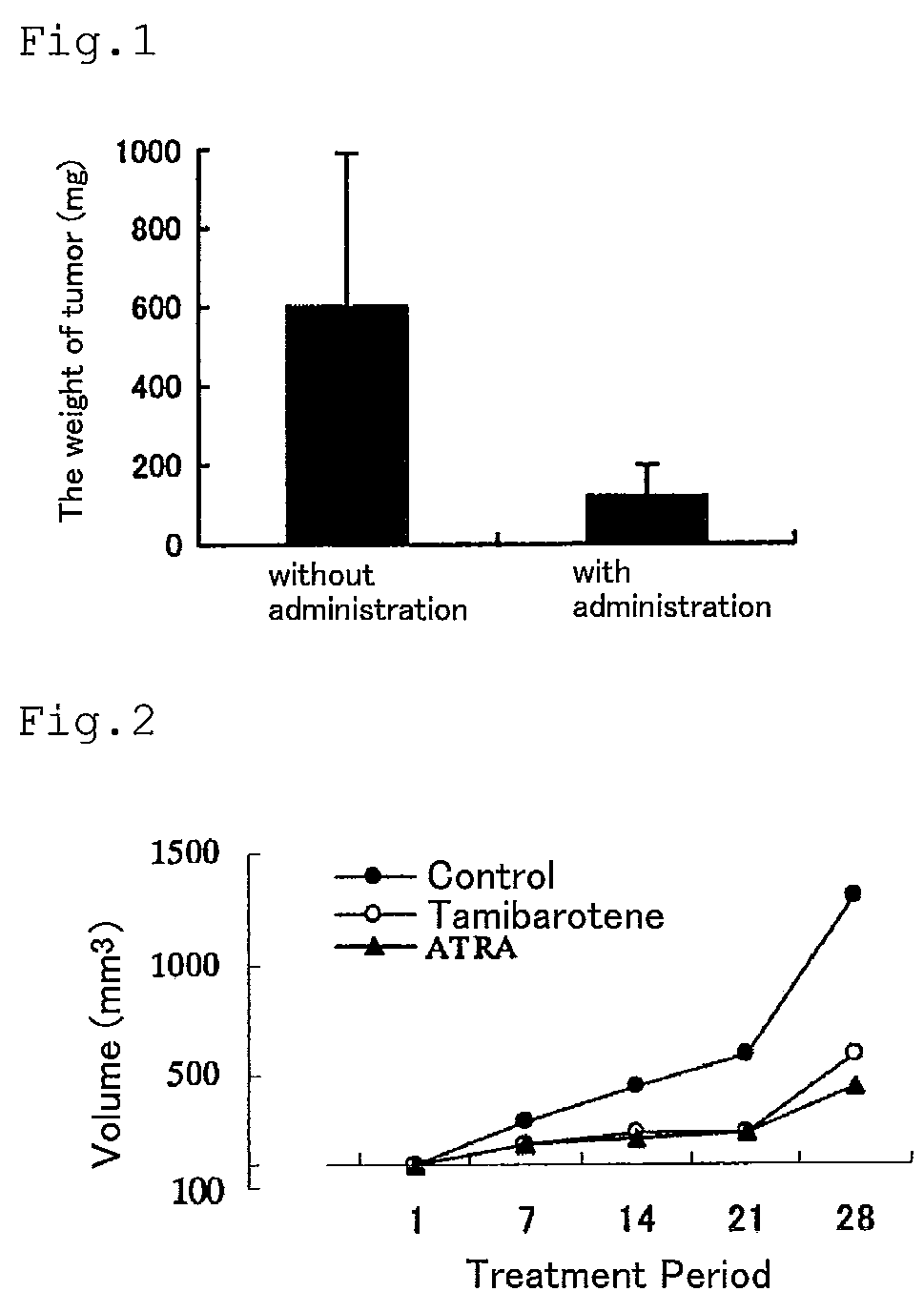
PendingUS20200061007A1Cancer stem cells can be reducedSize of tumor is reduced and decreasedHydroxy compound active ingredientsAntineoplastic agentsPharmaceutical drugAgonist
The present invention aims to provide a growth inhibitor for cancer stem cells resistant to existing anticancer drug therapies, which growth inhibitor acts on the cells through growth inhibition and apoptosis. The growth inhibitor for cancer stem cells contains a retinoid agonist, preferably tamibarotene, alone or in combination with a rexinoid agonist, preferably bexarotene, as an effective component(s). The growth inhibitor for cancer stem cells enhances the effects of various anticancer drugs when the growth inhibitor is used in combination with the anticancer drugs.
Owner:NAT INST OF ADVANCED IND SCI & TECH +1
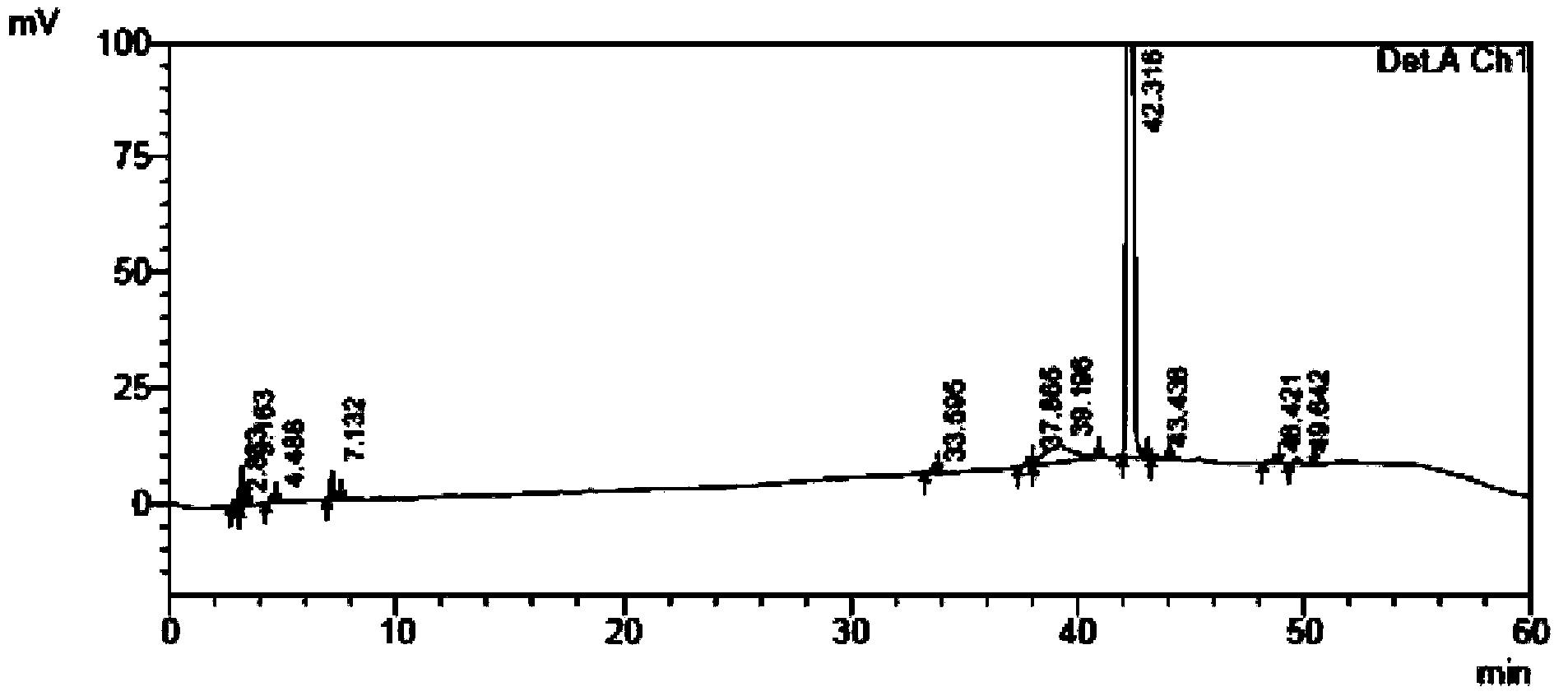
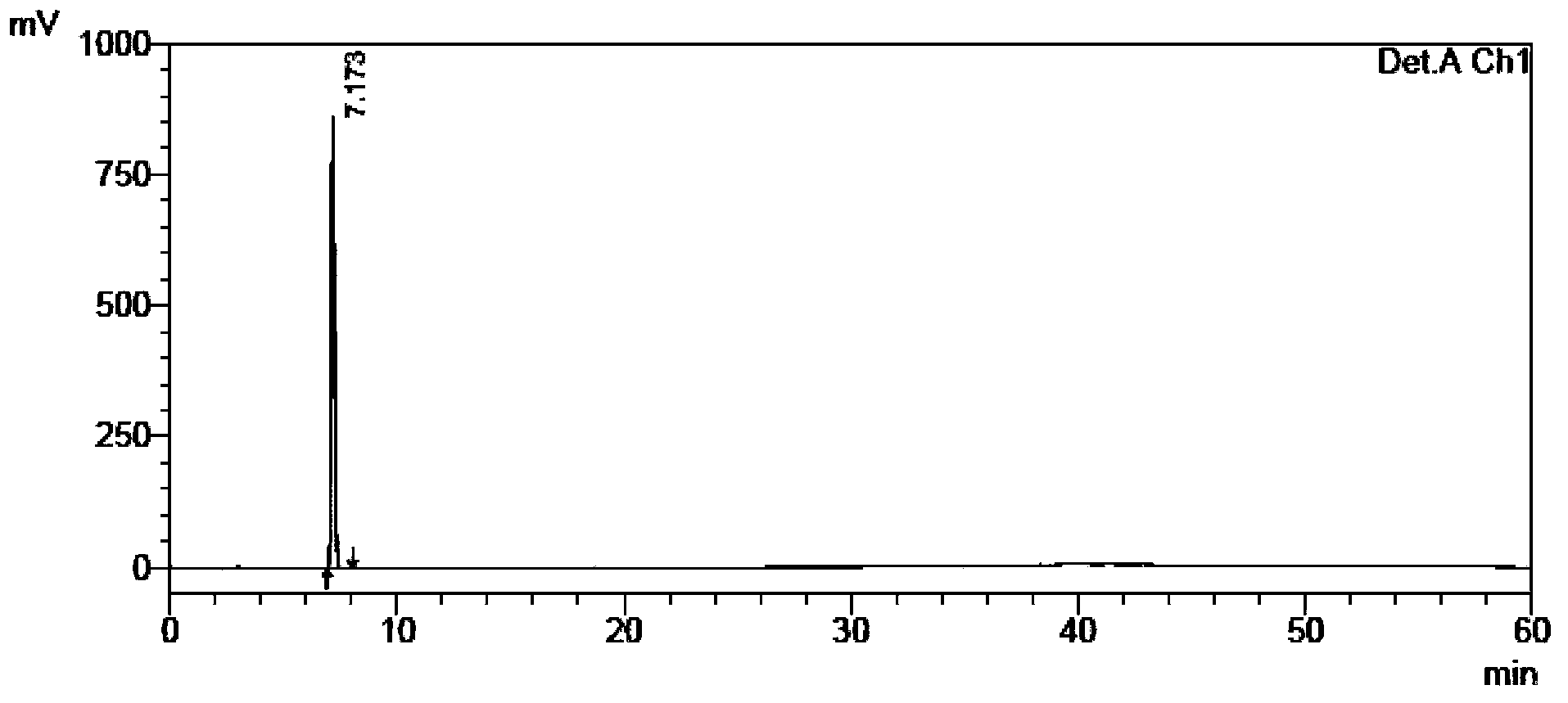
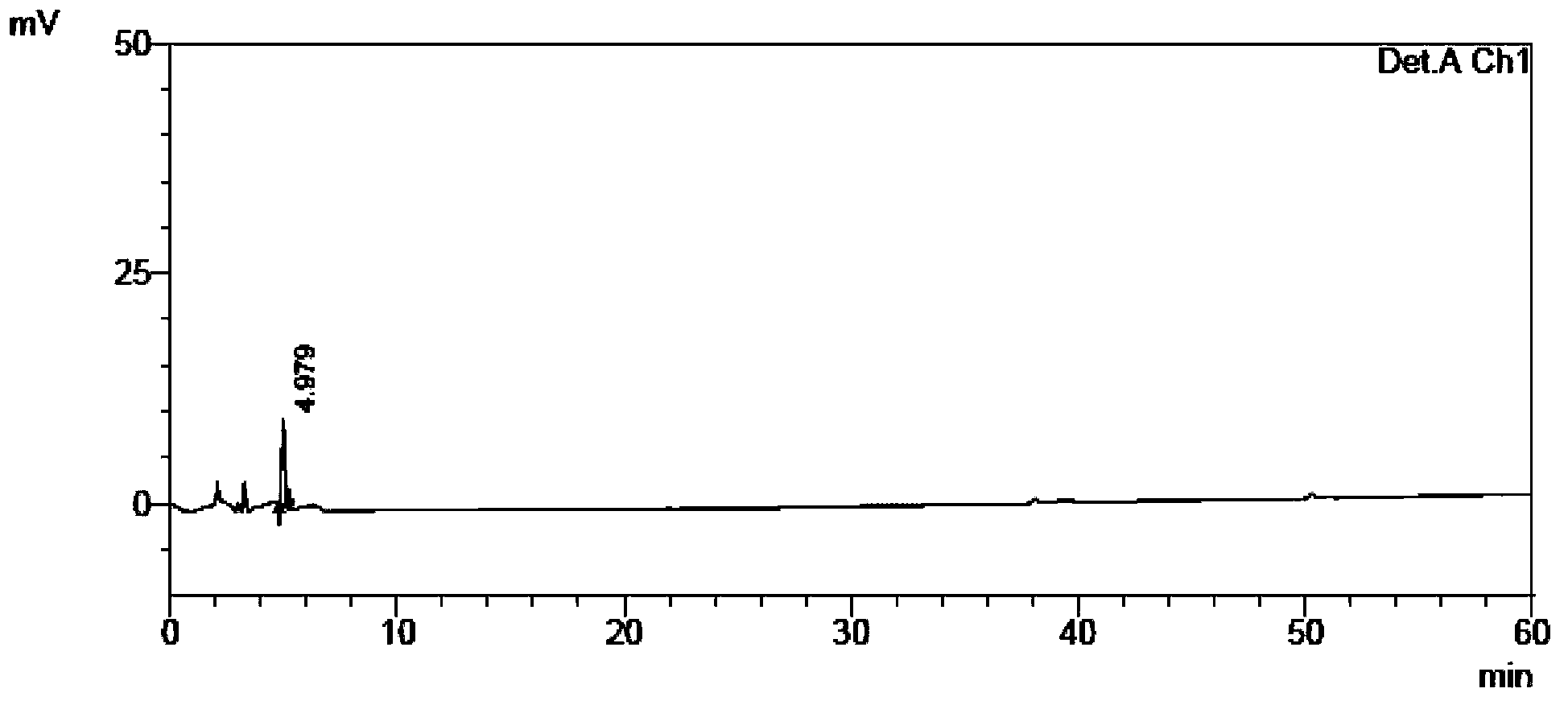
Content and impurity measuring method for tamibarotene and preparation thereof
The invention relates to a medicine analyzing method and particularly relates to a method for measuring impurities in tamibarotene and a preparation thereof by using high performance liquid chromatography. Octadecyl silane bonding silica gel is used as a filling agent, a phosphoric acid buffer solution with a volume ratio of water to phosphoric acid being 2000:1-2000:5 is used as a mobile phase A, acetonitrile is used as a mobile phase B, and a gradient elution process is adopted to establish a sensitive, exclusive and comprehensive tamibarotene impurity detecting and analyzing method. The method can be adopted to effectively detect changes of impurities in production and storage processes of the tamibarotene, and has important practical significance to medicine quality control.
Owner:PHARMA RES INST OF BENCAO TIANYUAN OF BEIJING
Method for preparing dispersible tablets of tamibarotene
InactiveCN101708168BGood content uniformityHigh dissolution rateOrganic active ingredientsPill deliveryOrganic solventDissolution
The invention discloses a method for preparing dispersible tablets of tamibarotene. The process in the method adopts the solvent deposition method, and the dispersible tablets of tamibarotene, prepared by the method, are good in stability, high in content uniformity and also high in dissolution rate; the method is operated according to the requirements of the Chinese Pharmacopoeia on the dissolution rate of the dispersible tablets, and the dispersible tablets are completely disintegrated within 3 minutes and can pass the No 2 screen(24 meshes), so that the dispersible tablets can enhance the absorption in the human body and improve the biological utilization rate; the preparation method is simple in operation and causes little pollution; by dissolving the tamibarotene in the organic solvents and depositing the tamibarotene in the auxiliary materials, the dust problem in operation can be solved, the caused pollution can be lessened, the untoward reactions of operators are avoided; and the mixed powder of raw materials and auxiliary materials, prepared by the method, can be tabletted directly, so that the cost is saved, and the requirement for being suitable for industrialized production is met.
Owner:深圳万乐药业有限公司
Novel crystalline forms of tamibarotene for treatment of cancer
Synthesis and characterization of novel tamibarotene forms suitable for pharmaceutical compositions in drug delivery systems to treat human or warm-blooded mammal diseases.
Owner:TRANSGENEX NANOBIOTECH
A method for content determination and impurity determination of tamibarotene and preparation thereof
The invention relates to a medicine analyzing method and particularly relates to a method for measuring impurities in tamibarotene and a preparation thereof by using high performance liquid chromatography. Octadecyl silane bonding silica gel is used as a filling agent, a phosphoric acid buffer solution with a volume ratio of water to phosphoric acid being 2000:1-2000:5 is used as a mobile phase A, acetonitrile is used as a mobile phase B, and a gradient elution process is adopted to establish a sensitive, exclusive and comprehensive tamibarotene impurity detecting and analyzing method. The method can be adopted to effectively detect changes of impurities in production and storage processes of the tamibarotene, and has important practical significance to medicine quality control.
Owner:PHARMA RES INST OF BENCAO TIANYUAN OF BEIJING
Method for synthesizing 5,6,7,8-tetrahydro-5,5,8,8-tetramethyl-2-naphthylamine
ActiveCN101486656AMethod environmentally friendlyReduce pollutionOrganic compound preparationAmino compound preparationHydrogenBoiling point
The invention discloses a synthesis method of 5, 6, 7, 8-tetrahydro-5, 5, 8, 8-tetramethyl-2-naphthylamine. The synthesis method comprises the following steps: the 5, 6, 7, 8-tetrahydro-5, 5, 8, 8-tetramethyl-2-naphthoamide reacts with sodium hydroxide in a high boiling point solvent; a protective group of the amino group is removed at temperature between 100 DEG C and 200 DEG C; a reaction solution is deposited with water and filtered; a solid product obtained is recrystallized to obtain the 5, 6, 7, 8-tetrahydro-5, 5, 8, 8-tetramethyl-2-naphthylamine. The method is environmentally friendly,has little pollution, low cost, simple operation, high safety, high reaction yield and high transformation rate reaching more than 80 percent and requires no adoption of expensive Pd-C catalysts and no catalytic hydrogenation. If necessary, the obtained 5, 6, 7, 8-tetrahydro-5, 5, 8, 8-tetramethyl-2-naphthylamine can be condensed and hydrolyzed to obtain tamibarotene.
Owner:重庆莱美隆宇药业有限公司
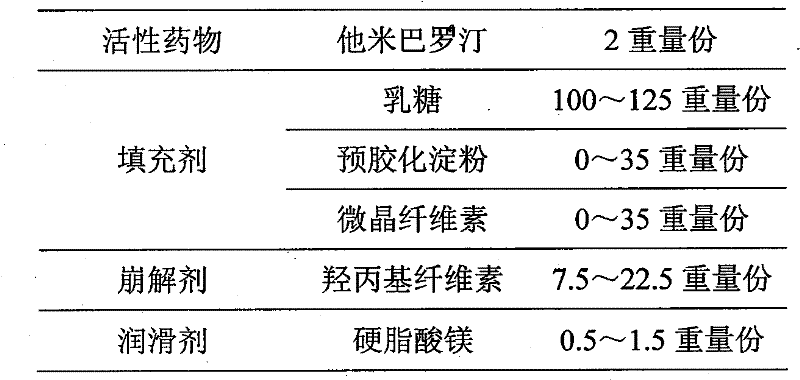
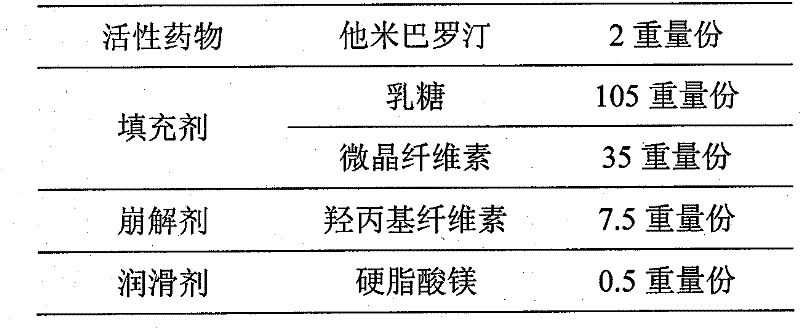
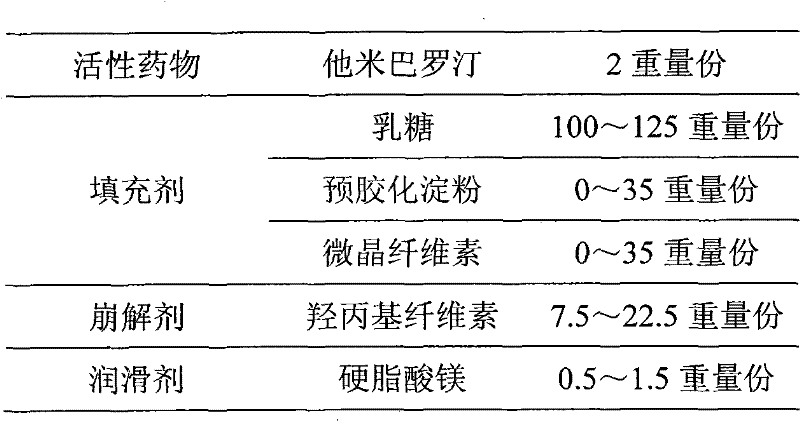
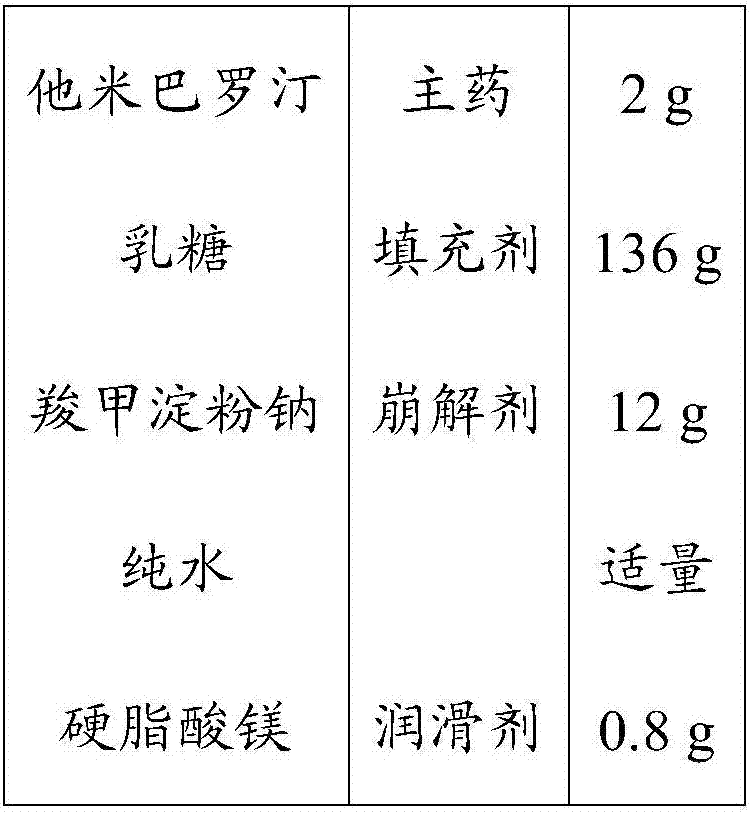
A kind of oral solid preparation of Tamibarotene, its preparation method and application
ActiveCN107213119BImprove solubilityPromote absorptionOrganic active ingredientsPill deliveryBioavailabilityOrganic chemistry
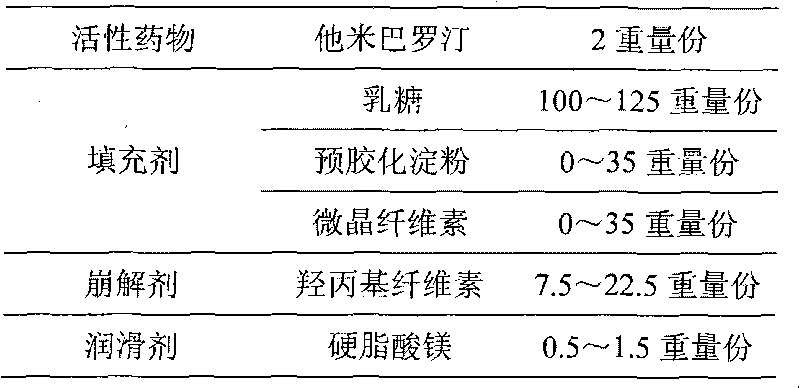
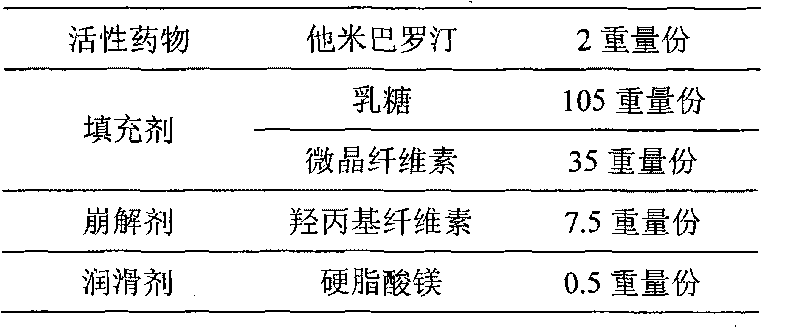
The invention provides an orally taken tamibarotene solid preparation. The solid preparation comprises tamibarotene, disintegrating agent, filling agent and lubricating agent, wherein the weight of the tamibarotene is 1%-2% of the weight of the solid preparation, the weight of the filling agent is 70%-90% of the weight of the solid preparation, the weight of the disintegrating agent is 8%-25% of the weight of the solid preparation and the weight of the lubricating agent is 0.5%-0.6% of the weight of the solid preparation. The orally taken tamibarotene solid preparation prepared according to the invention has high dissolution rate, content uniformity and stability, is beneficial to the promotion of the bioavailability of human body and is more suitable for clinical application. The invention also provides a preparation method and an application of the solid preparation.
Owner:HAIKOU PHARMA FACTORY
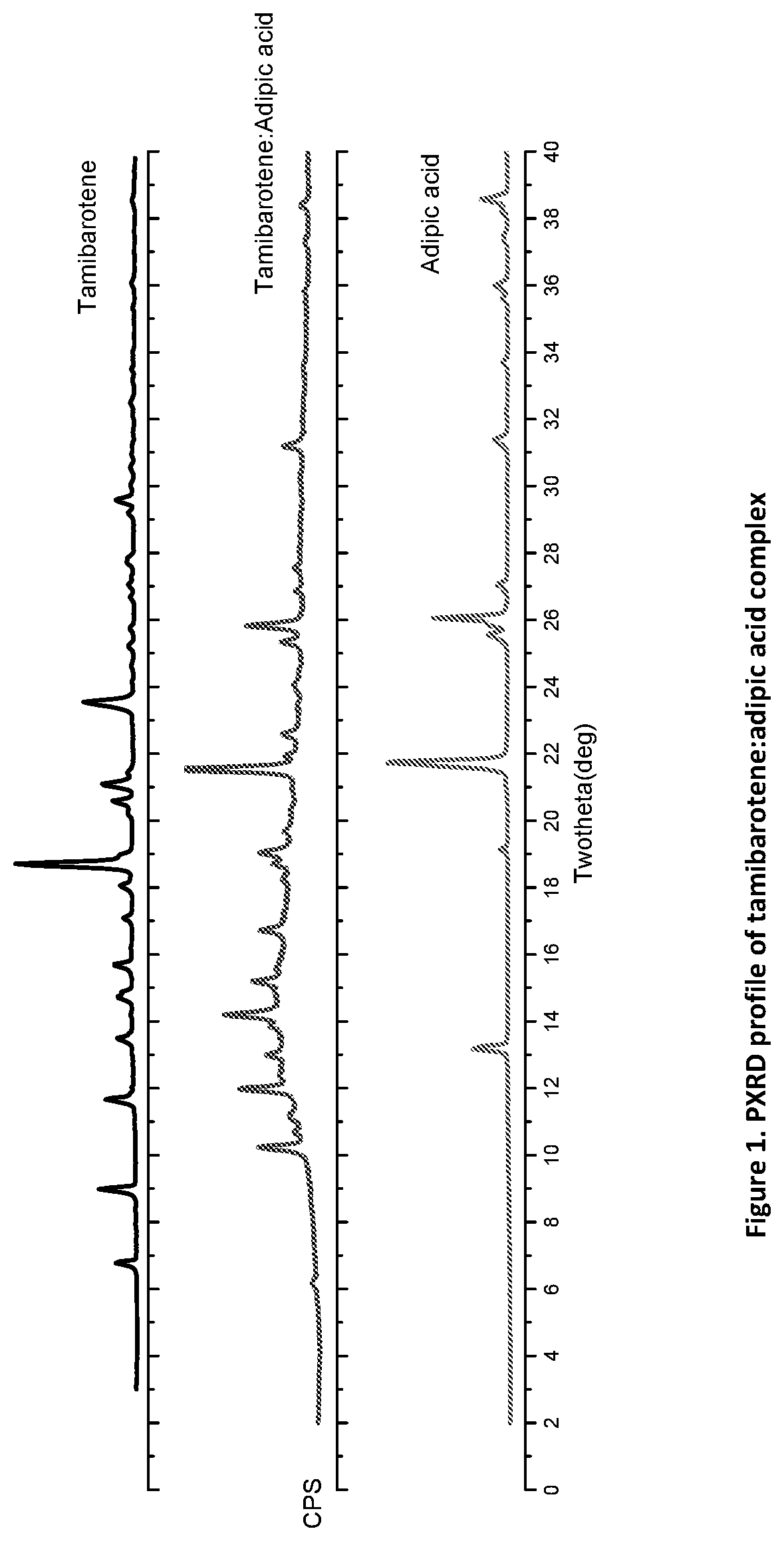
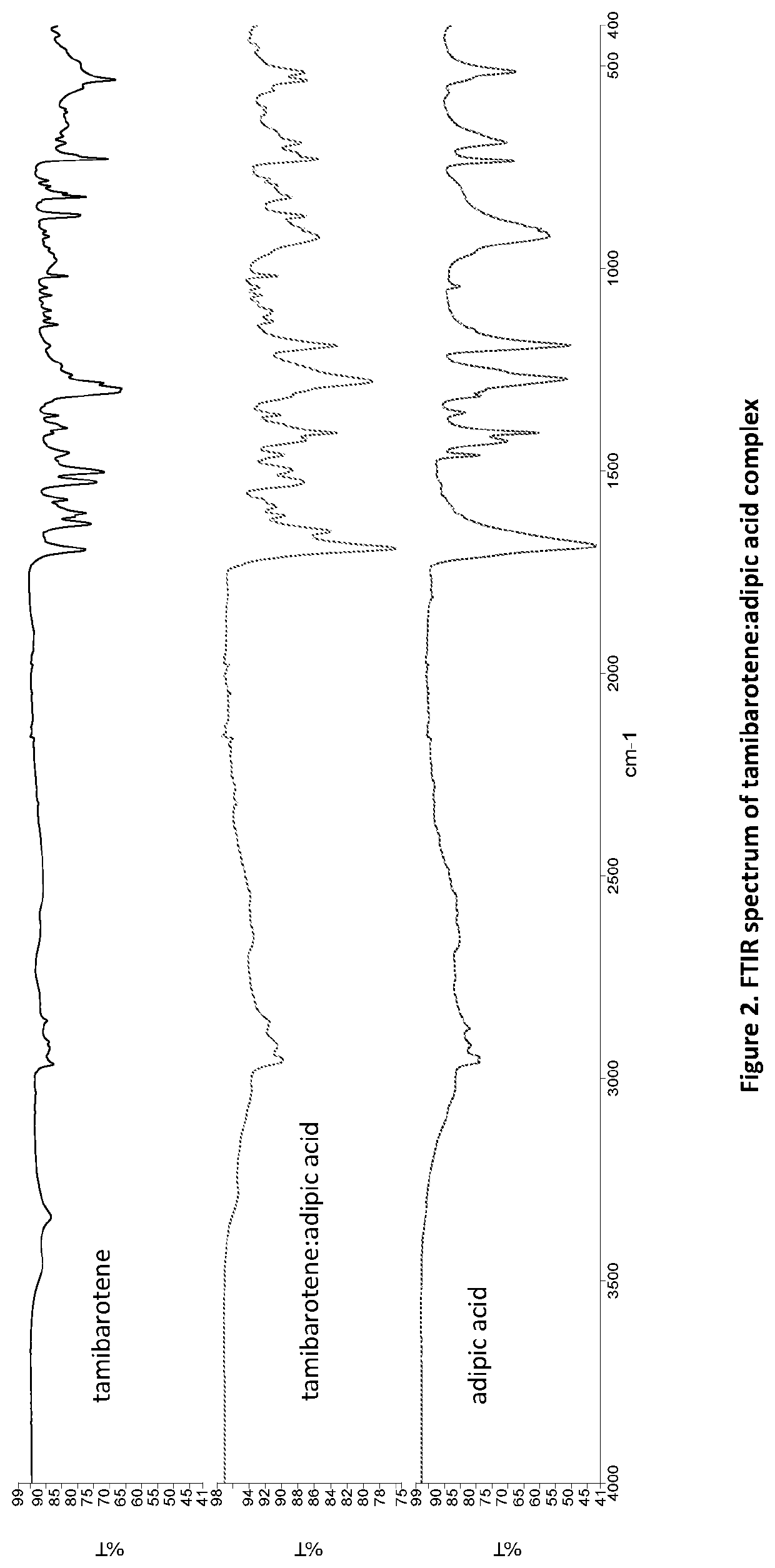
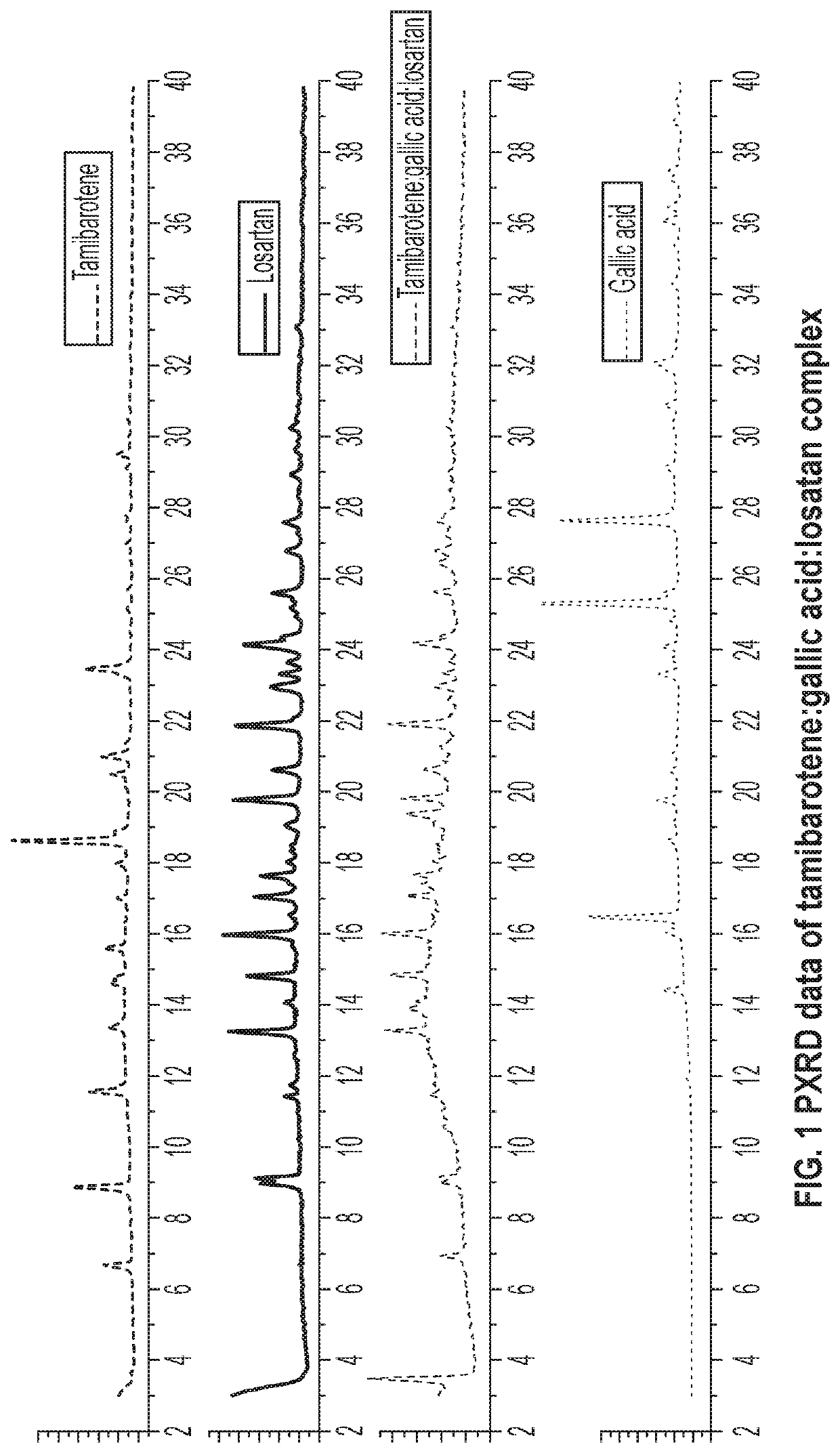
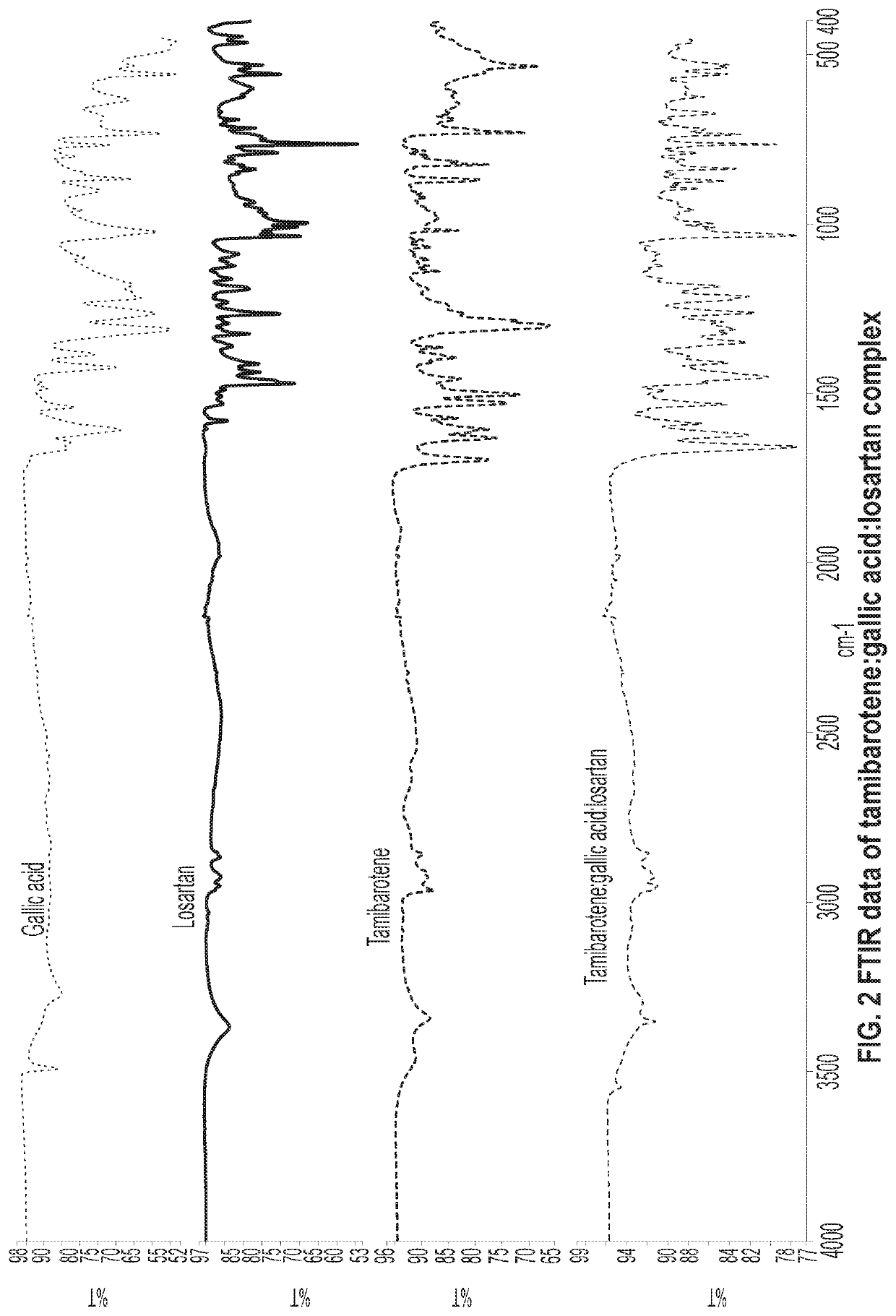
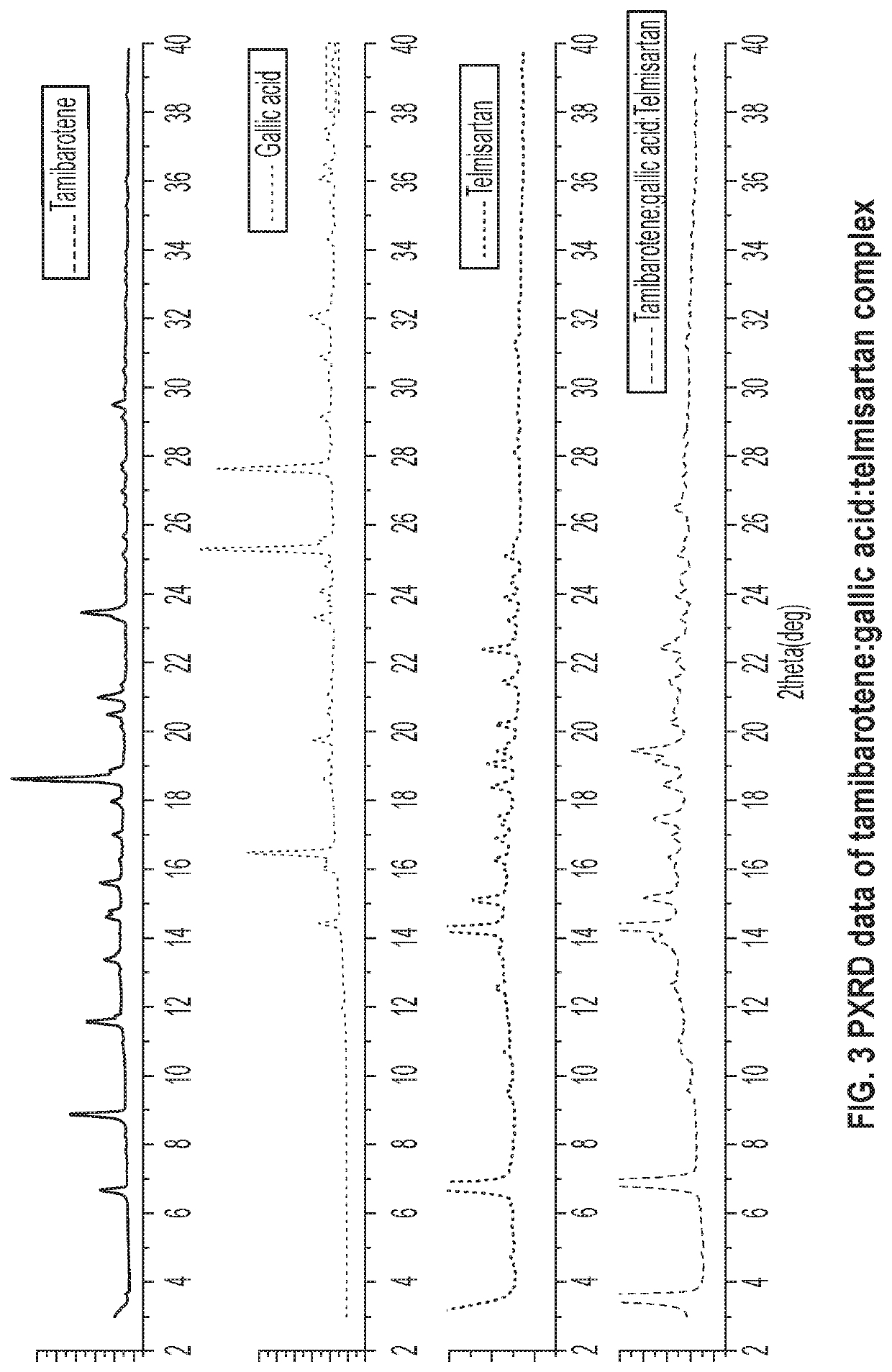
Novel ternary molecular complex of tamibarotene for cancer stem cells treatment
Preparation and characterization of novel tamibarotene forms suitable for pharmaceutical compositions in drug delivery systems for the treatment of cancer stem cells. The tamibarotene compositions can be used for the safe and effective treatment of human diseases including a variety of cancers, cancer stem cells, drug resistant cancers, and used as a radio sensitizer.
Owner:TRANSGENEX NANOBIOTECH
Method for catalytically synthesizing tamibarotene through acenaphthene imidazole n-heterocyclic carbine allyl palladium chloride compound
InactiveCN103408450BShort synthetic routeGood atom economyOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsPtru catalystPharmaceutical Substances
Owner:FUDAN UNIV
Synthetic technique for tamibarotene
InactiveCN101121675BEase of industrial productionOrganic compound preparationCarboxylic acid amides preparationBenzoic acidSynthesis methods
The invention discloses a synthesis method for the (I) Linarotene (I), a leukemia treatment drug. The invention mainly proposes a novel synthetic route with the steps as follows: the aniline, and p-chlorocarbonyl benzoic methyl ester are used as the raw materials; the intermediate is the p-chlorocarbonyl benzoic methyl ester and can do the cyclization with the 2,5 - dichloro-2 ,5 - dimethyl hexaneunder the anhydrous condition to get the intermediate 4-[(5,6,7,8-tetrahydrocannabinol-5,5,8,8- tetramethyl-2-naphthalene) carbamoyl] methyl benzoate. After the hydrolysis, the 4-[(5,6,7,8-tetrahydrocannabinol-5,5,8,8- tetramethyl-2-naphthalene) carbamoyl] benzoate (I) can be made, that is, the Linarotene. The synthetic route is simple; the operation is convenient and conducive to the environmental protection; the invention is suitable for the industrial production.
Owner:CHINA PHARM UNIV
New process for synthesizing tamibarotene
InactiveCN103664680AOrganic compound preparationCarboxylic acid amides preparationBenzoic acidHydrogen
The invention discloses a synthetic method of tamibarotene, namely a medicament for treating leukemia and provides a new synthetic route. The synthetic method mainly comprises the following steps: carrying out condensation reaction on raw materials 5,5,8,8-tetramethyl-5,6,7,8-tetrahydronaphthalene-2-amine and mono-methyl terephthalate to obtain 4-[(4,5,6,7-tetrahydro-5,5,8,8-tetramethyl-2-naphthyl)carbamoyl]methyl benzoate; further hydrolyzing the intermediate in potassium carbonate to obtain 4-[(5,5,8,8-tetramethyl-6,7-dihydronaphthalene-2-yl)carbamoyl]benzoic acid, namely tamibarotene. The synthetic route is simple and environment-friendly and is beneficial to industrial production.
Owner:SHANDONG LUBEI PHARMA
Multi-target tamibarotene derivative and its preparation method and application
ActiveCN105175285BAchieve synergyEnhanced inhibitory effectUrea derivatives preparationOrganic active ingredientsLeukemiaHydroxycarbamide
The invention provides a multiple target point type Tamibarotene derivative as well as a preparation method and an application thereof. Particularly, RAR (retinoic acid receptor) agonist Tamibarotene is connected with antineoplastic drug hydroxycarbamide, fluorouracil and lenalidomide on sales through ester bonds or amido bonds respectively to obtain three multiple target point mutual prodrugs. The invention provides a method for preparing the compound and the application of the compound in preparing antineoplastic drugs, particularly drugs for curing various leukemias. The invention further relates to a drug combination of the compound.
Owner:SHANDONG UNIV
Topical compositions for reducing the effects of aging
Owner:SAMSON PHARMA LLC
Method for synthesizing 5,6,7,8-tetrahydro-5,5,8,8-tetramethyl-2-naphthylamine
ActiveCN101486656BMethod environmentally friendlyReduce pollutionOrganic compound preparationAmino compound preparationHydrogenBoiling point
The invention discloses a synthesis method of 5, 6, 7, 8-tetrahydro-5, 5, 8, 8-tetramethyl-2-naphthylamine. The synthesis method comprises the following steps: the 5, 6, 7, 8-tetrahydro-5, 5, 8, 8-tetramethyl-2-naphthoamide reacts with sodium hydroxide in a high boiling point solvent; a protective group of the amino group is removed at temperature between 100 DEG C and 200 DEG C; a reaction solution is deposited with water and filtered; a solid product obtained is recrystallized to obtain the 5, 6, 7, 8-tetrahydro-5, 5, 8, 8-tetramethyl-2-naphthylamine. The method is environmentally friendly,has little pollution, low cost, simple operation, high safety, high reaction yield and high transformation rate reaching more than 80 percent and requires no adoption of expensive Pd-C catalysts and no catalytic hydrogenation. If necessary, the obtained 5, 6, 7, 8-tetrahydro-5, 5, 8, 8-tetramethyl-2-naphthylamine can be condensed and hydrolyzed to obtain tamibarotene.
Owner:重庆莱美隆宇药业有限公司
Nitric oxide donor tamibarotene derivatives and their preparation method and use
InactiveCN102295618BOrganic active ingredientsOrganic chemistryPharmaceutical drugCombinatorial chemistry
The invention relates to the field of medicinal chemistry, and discloses a nitric oxide donor type tamibarotene derivative, a preparation method and application thereof. More specifically, the present invention provides a compound shown in general structural formula (I), wherein the definition of R is shown in the description of this patent. Rotin is a kind of multi-target compound obtained by hybridizing various linking groups with ester bonds or amide bonds, and is suitable as an antitumor drug for treating various leukemias.
Owner:济南铂卅医药科技有限公司
Water-soluble prodrug of tamibarotene, and preparation method and applications thereof
InactiveCN101665449BEasy to administerImprove bioavailabilityOrganic active ingredientsOrganic compound preparationSolubilityWater soluble prodrug
The invention provides a water-soluble prodrug of tamibarotene, and a preparation method and applications thereof and belongs to the technical fields of organic compound synthesis and medical applications. The water-soluble prodrug of tamibarotene has favorable water solubility and suitable drug crystal form, and therefore, is suitable to be used as a raw material for medical preparations and especially suitable for preparing injections. Particularly, the invention provides medical acceptable salts of tamibarotene DMEA ester, which have the general formula (I), wherein HA is HCl, H2SO4, HNO3,H3PO4, HOAc, paratoluenesulfonic acid, maleic acid, succinic acid, citric acid or L(+)-tartaric acid.
Owner:SHANDONG UNIV
Orally taken tamibarotene solid preparation, preparation method and application thereof
ActiveCN107213119AImprove solubilityPromote absorptionOrganic active ingredientsPill deliveryMedicineDissolution
The invention provides an orally taken tamibarotene solid preparation. The solid preparation comprises tamibarotene, disintegrating agent, filling agent and lubricating agent, wherein the weight of the tamibarotene is 1%-2% of the weight of the solid preparation, the weight of the filling agent is 70%-90% of the weight of the solid preparation, the weight of the disintegrating agent is 8%-25% of the weight of the solid preparation and the weight of the lubricating agent is 0.5%-0.6% of the weight of the solid preparation. The orally taken tamibarotene solid preparation prepared according to the invention has high dissolution rate, content uniformity and stability, is beneficial to the promotion of the bioavailability of human body and is more suitable for clinical application. The invention also provides a preparation method and an application of the solid preparation.
Owner:HAIKOU PHARMA FACTORY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com