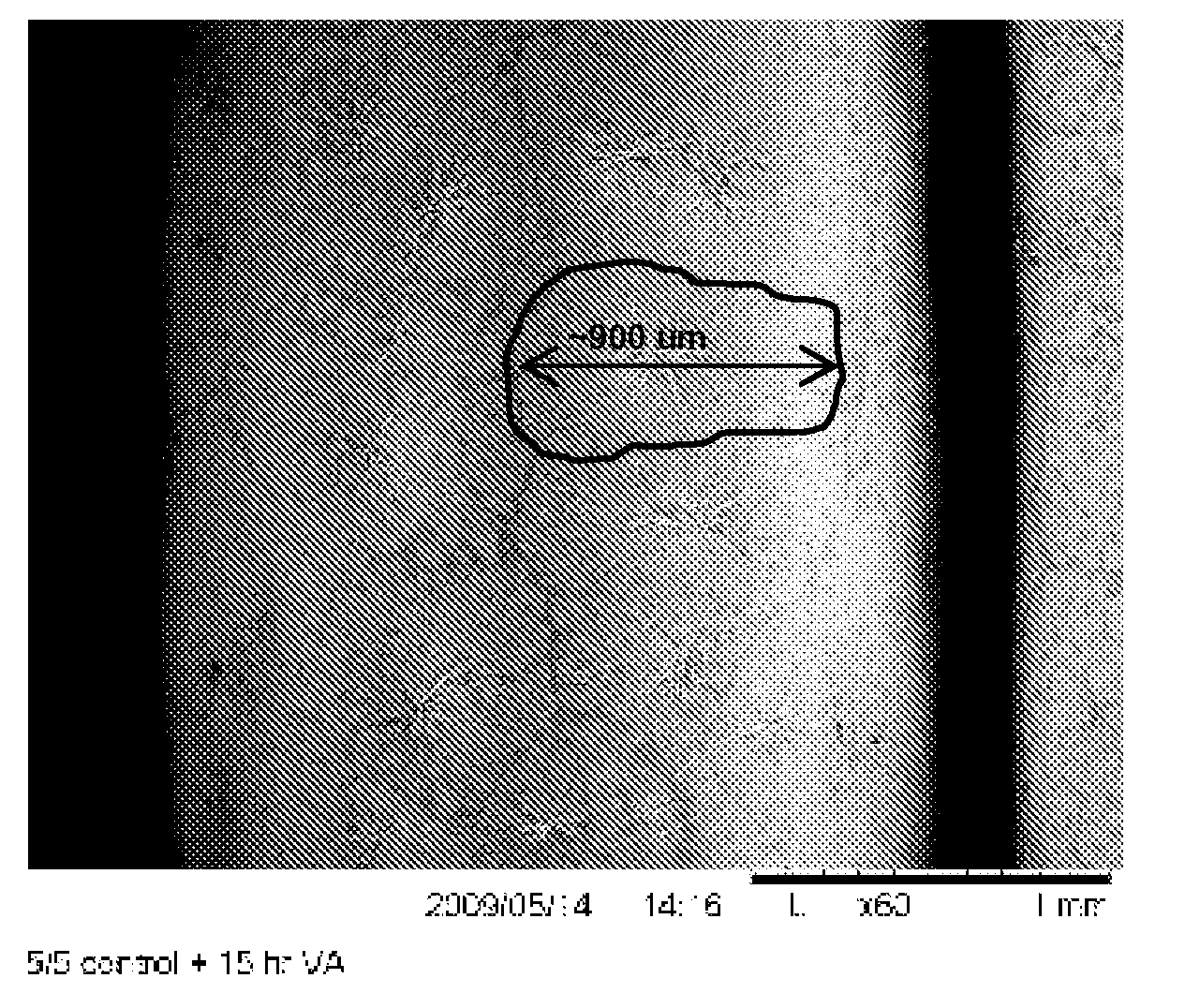
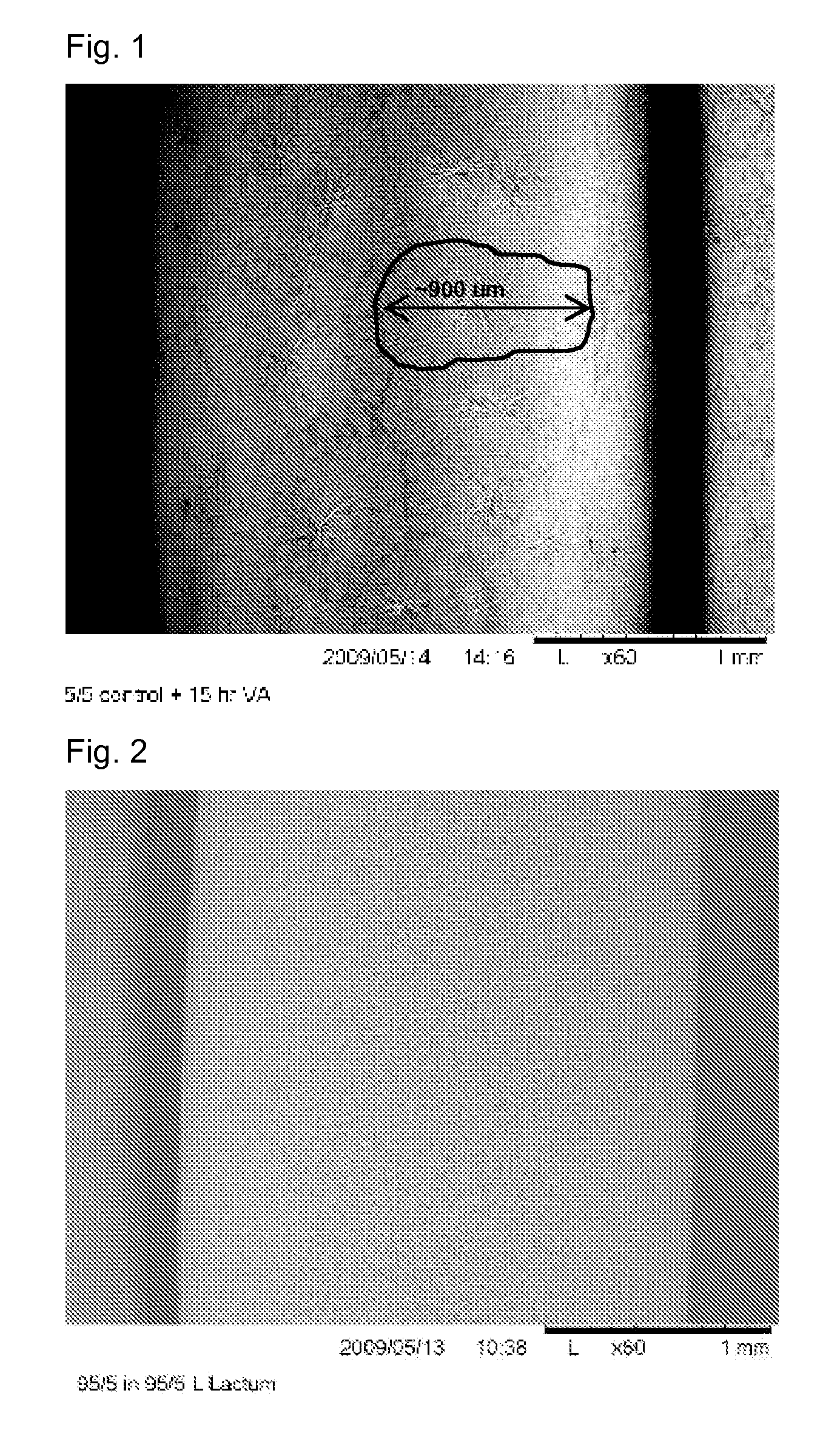
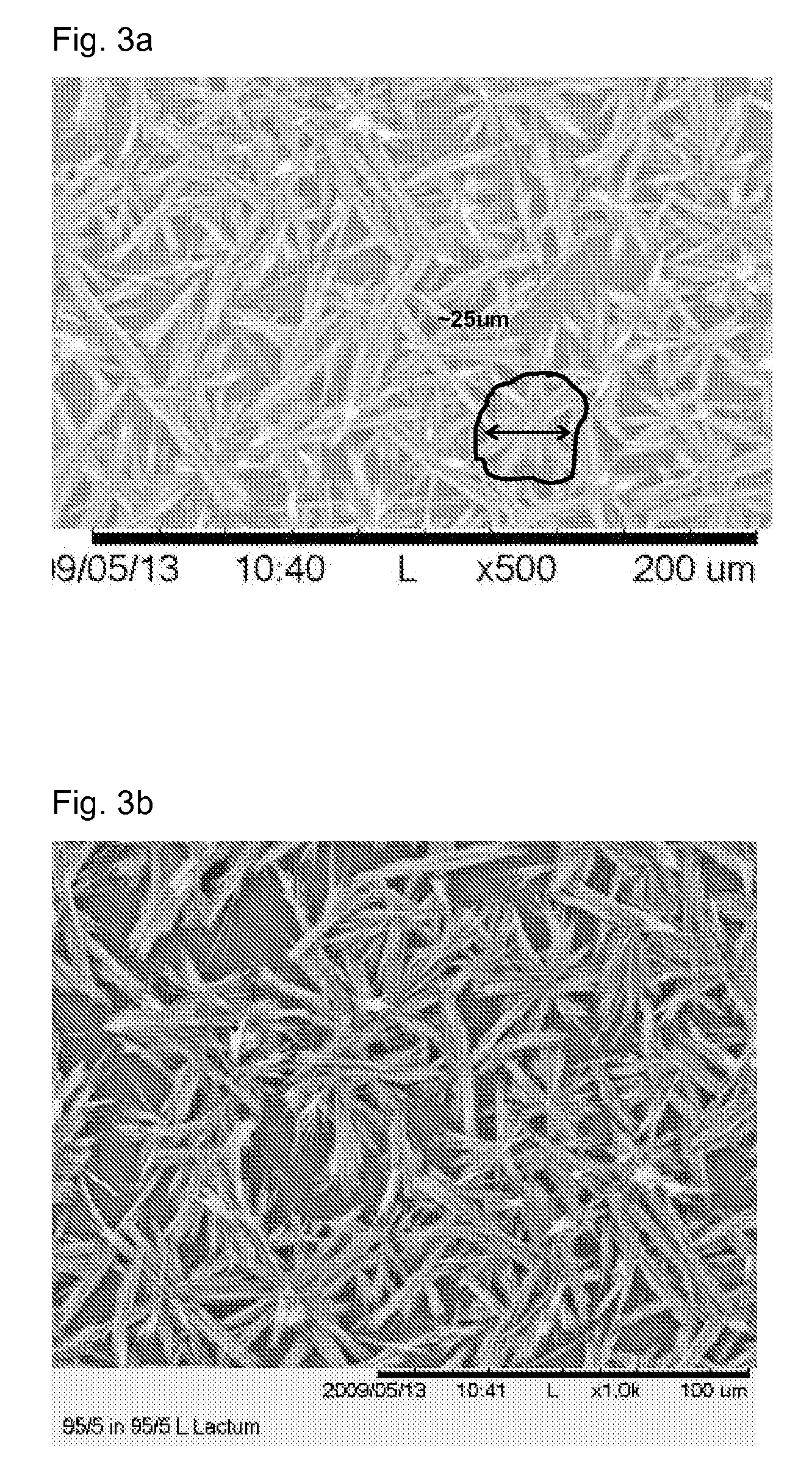
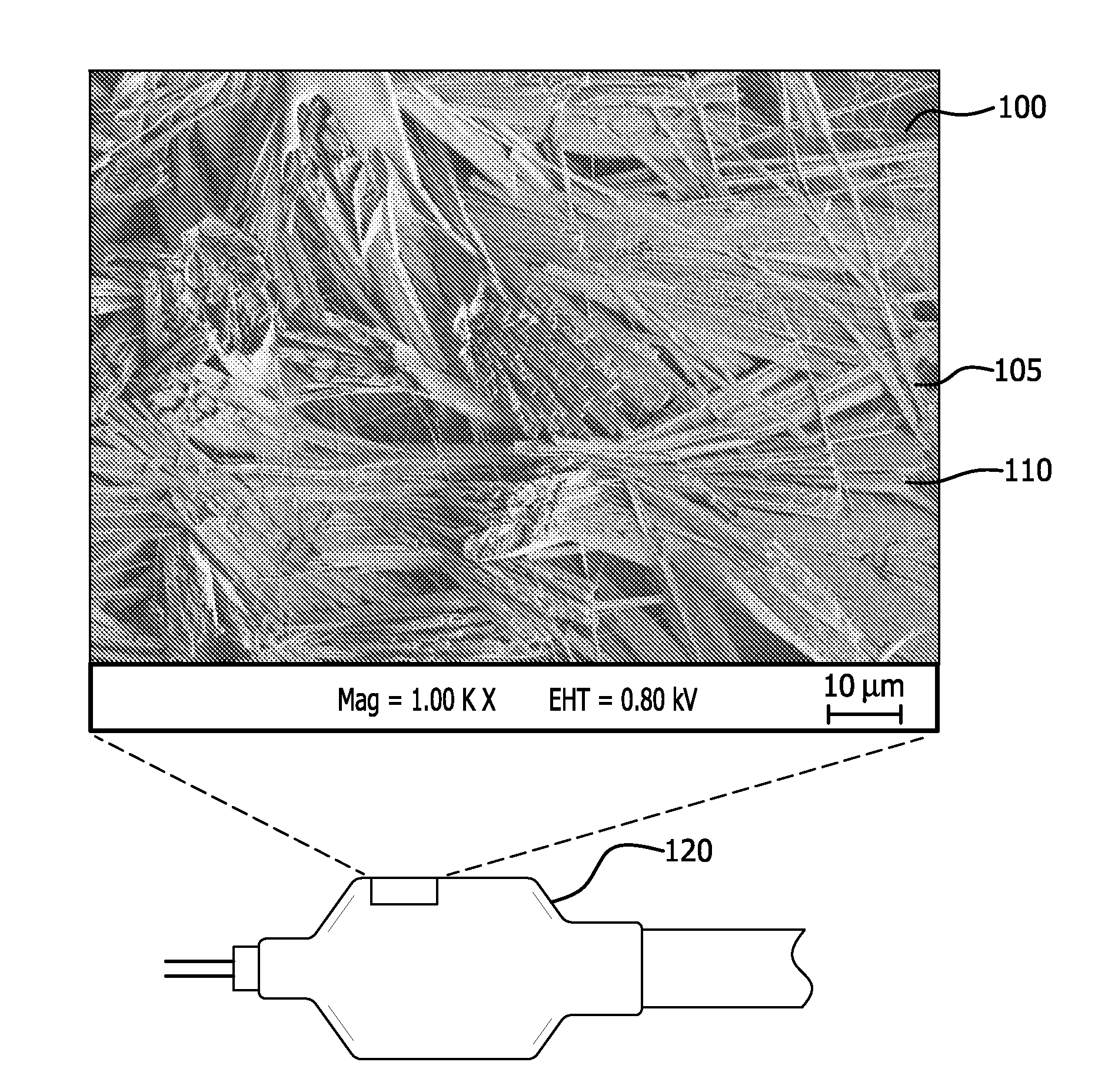
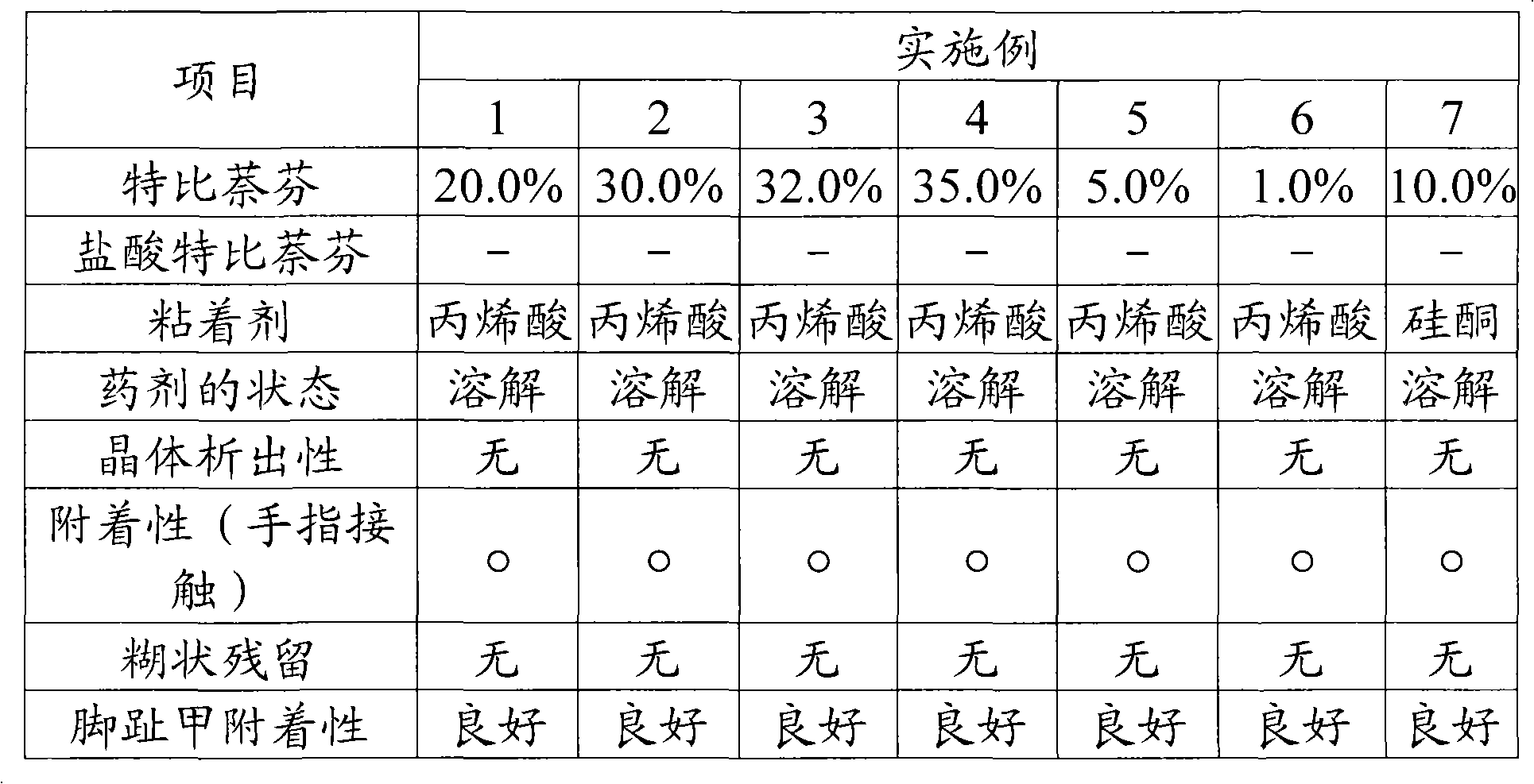
Patents
Literature
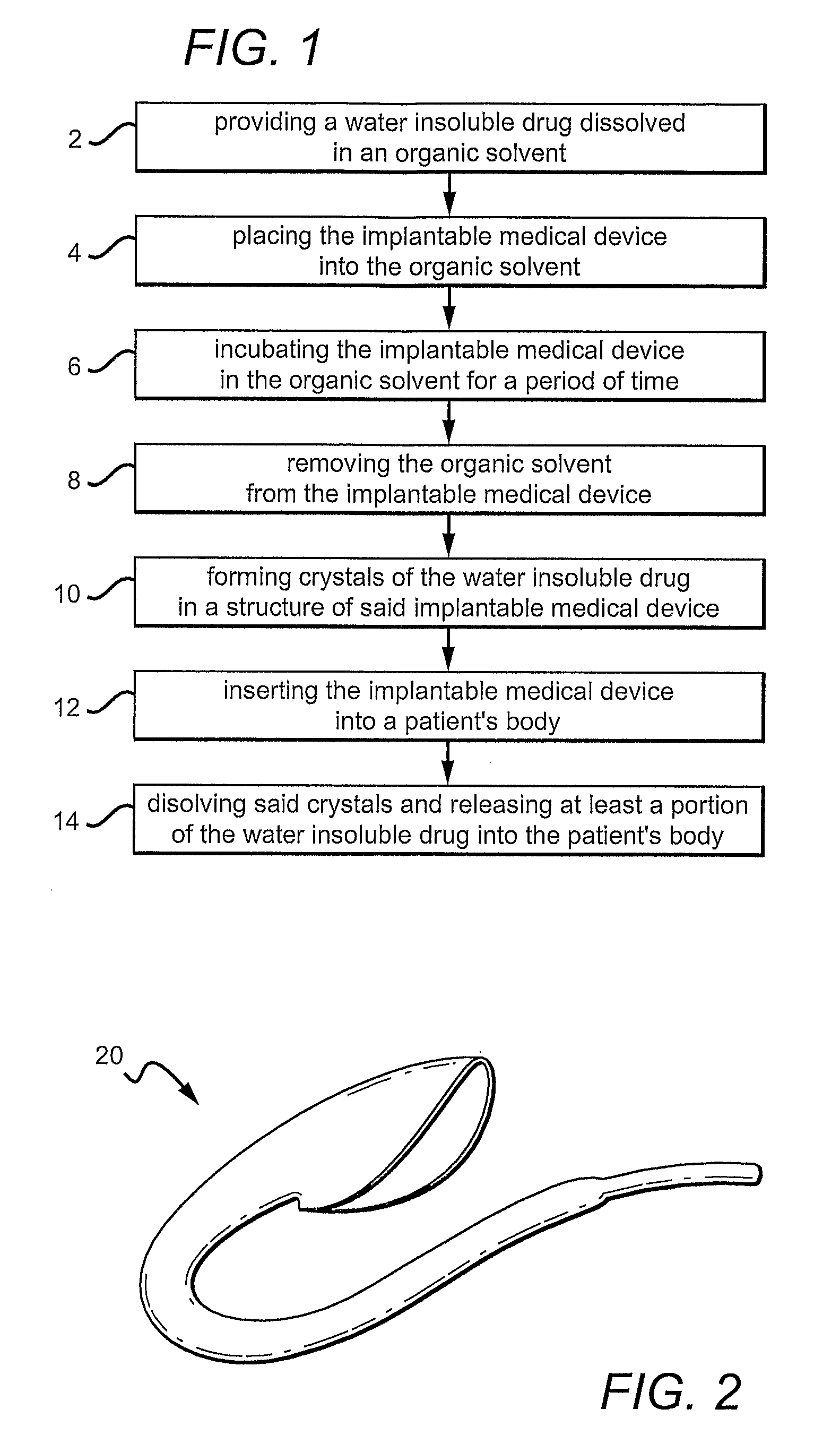
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
86 results about "Drug crystals" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Known informally as meth, ice or blue ice, or glass, it resembles shiny blue-white "rocks" or fragments of glass of varying sizes. It is known more formally as crystal methamphetamine. The drug is an odorless, blue, or colorless form of d-methamphetamine, a synthetic psychostimulant.
Nucleation of Drug Delivery Balloons to Provide Improved Crystal Size and Density
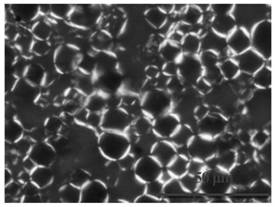
Drug delivery balloons have densely packed crystals of small particle size of the drug thereon. An amorphous drug coating is applied to a balloon surface and annealed to provide the crystals. The balloon surface is nucleated to induce formation of drug crystals in the annealing step to provide the crystals in high density with small size.
Owner:BOSTON SCI SCIMED INC
Porous composites with high-aspect ratio crystals
ActiveUS20140271775A1Easy to attachHigh aspect ratioOrganic active ingredientsBiocideVascular diseaseMedicine
The present disclosure is directed toward composite materials comprising high aspect ratio habits of drug crystals which can be partially or fully extending into a substrate, and additionally, can be projecting from a substrate at an angle of about 20° to about 90°. The present disclosure is directed toward medical devices, such as medical balloons, comprising said composite and methods of using and making the same. The described composite can be used for the local treatment of vascular disease. The present disclosure is also directed toward paclitaxel crystals with a hollow acicular habit.
Owner:WL GORE & ASSOC INC
Drug-releasing graft
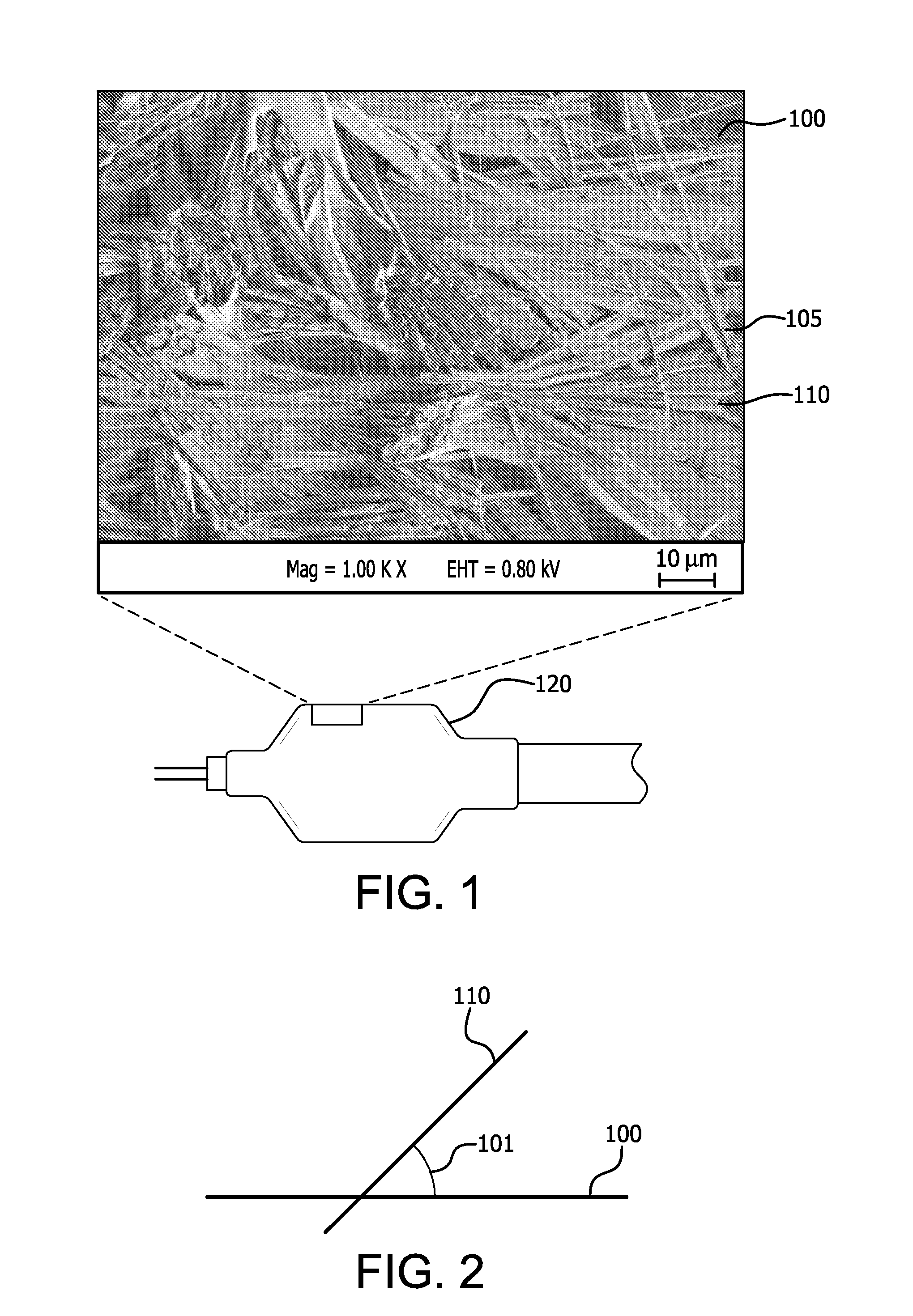
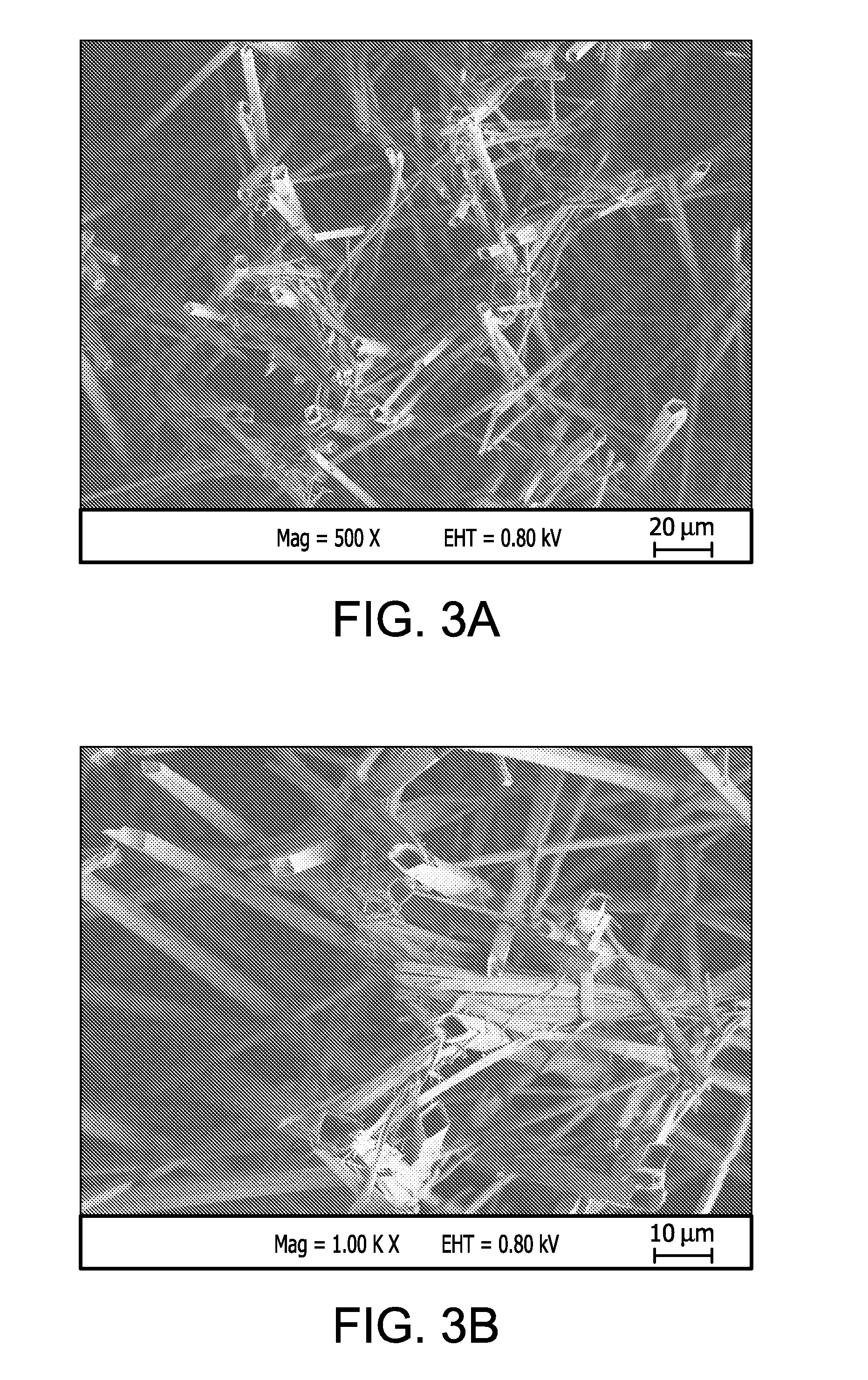
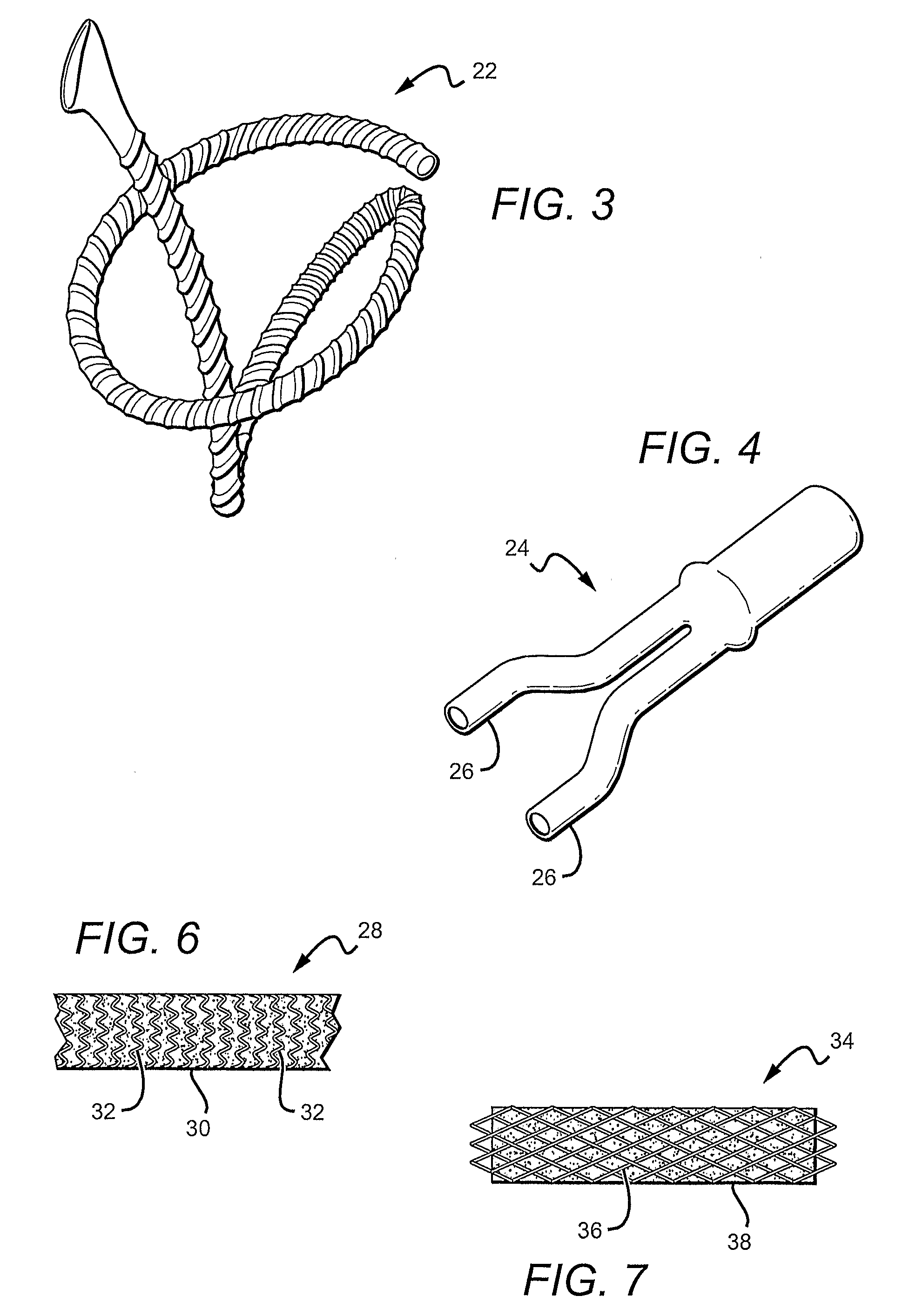
InactiveUS20100268321A1Minimize partial impairmentAvoid occlusionBiocideStentsMedicineWater insoluble
A method of incorporating drugs into an implantable medical device. In one variation, water insoluble drugs are used to form crystals within the porous structure of the device. Upon implantation, the drug crystals dissolve slowly and release the drug into the surrounding tissue. In one example, a water insoluble drug is crystallized within the pores of an ePTFE vascular graft.
Owner:CR BARD INC
Process employing controlled crystallization in forming crystals of a pharmaceutical
InactiveUS20050256314A1Improve filtration efficiencyImprove washing efficiencyOrganic chemistryCrystallization separationState of artCrystallization kinetics
A process is provided which employs reactive controlled crystallization to produce drug substance having desirable crystal properties which process involves providing reactants A and B in liquid or solution form and adding reactant B to reactant A using a cubic or incremental addition technique to control extent of reaction and thus crystallization kinetics, including supersaturation and nucleation, to produce crystals of drug substance which are generally larger, better quality and with few fines and narrow particle size distribution than normally obtainable employing prior art crystallization techniques. In addition, crystals of drug substance produced by the above process is also provided.
Owner:BRISTOL MYERS SQUIBB CO
Drug-coating balloon catheter and production method and application thereof
ActiveCN107362439ANot easy to wash offHigh drug loading efficiencyBalloon catheterMedical devicesDrugs solutionDrug crystals
The invention provides a drug-coating balloon catheter and a production method and application thereof. The method includes: preparing active drug seed crystals, and screening the seed crystals 1-3 micrometers in length to prepare active drug seed crystal suspension; adding the active drug seed crystal suspension, an active drug solution and an additive solution into different channels of a coating machine, mixing and atomizing the active drug seed crystal suspension, the active drug solution and the additive solution at the nozzle tip end of the ultrasonic sprayer of the coating machine, spraying to the surface of a balloon dilatation catheter to obtain drug coating with an appropriate crystal size, and performing homogenizing post-processing to obtain the drug-coating balloon catheter with homogeneous crystals. The drug-coating balloon catheter and the production method thereof have the advantages that the drug coating is firmly combined with a balloon, and drug crystals are even and complete; small drug loss during balloon preparation and in-vivo conveying can be guaranteed, concentration of effective drugs entering the blood vessel wall of a lesion part is high, high drug loading efficiency is achieved, intravascular in-situ stenosis or restenosis can be treated effectively, the risks of late thrombosis and restenosis are reduced, and the positive remodeling of blood vessels can be formed at the same time.
Owner:LEPU MEDICAL TECH (BEIJING) CO LTD
Crystallization method for preparing high-purity I-type clopidogrel hydrogen sulfate
The invention discloses a method for preparing high-purity I-type clopidogrel hydrogen sulfate, belonging to the technical field of finding and preparing of drug crystal forms. The method comprises the following four steps of: (A) transforming a clopidogrel salt into free alkali thereof at a lower temperature; (B) dropwise adding sulfuric acid into aqueous alkali at 20 to 25 DEG C, reacting and crystallizing; (C) growing crystals for 1 to 2 hours at the same temperature; and (D) washing an obtained solid by using ethyl acetate, and performing vacuum drying. An I-type clopidogrel hydrogen sulfate crystal prepared by using the method is determined to be the high crystal form purity I-type clopidogrel hydrogen sulfate after being subjected to X-ray powder diffraction, infrared spectrum and thermal analysis. An appropriate amount of seed crystal is added during crystallization, the crystallization speed is obviously increased, and the crystallization can be completed within about 5 hours.
Owner:JIANGNAN UNIV
Method and application for constructing polarized force fields and method and system for predicting drug crystal forms
ActiveCN106372400APrecise Design DirectionHigh chemical precisionChemical property predictionComputational theoretical chemistryAb initio quantum chemistry methodsQuantum chemical
The invention discloses a method and application for constructing polarized force fields and a method and system for predicting drug crystal forms. The method for constructing chemical molecule polarized force fields is suitable for being executed in one or more computation apparatuses, and comprises the following steps of: carrying out optimization computation on a quantum chemical structure of a chemical molecule on the basis of an ab initio calculation method so as to obtain a locally optimized molecular structure; calculating the difference between first energy of the locally optimized molecular structure when the charge of the molecular structure is neutral and second energy of the locally optimized molecular structure when the molecular structure has a predetermined positive-valence charge, and taking the difference as a vertical ion potential corresponding to the molecular structure; calculating polarized force field parameters of the molecular structure on the basis of the vertical ion potential and the locally optimized molecular structure, wherein the polarized force field parameters comprise a multi-pole vector of atom distribution, a multi-pole polarization rate of the atom distribution and a frequency-related polarization rate; and constructing a corresponding polarized force field model on the basis of the locally optimized molecular structure and the calculated polarized force field parameters.
Owner:SHENZHEN JINGTAI TECH CO LTD
Water-soluble prodrug of tamibarotene, and preparation method and applications thereof
InactiveCN101665449AAppropriate drug crystal formGood water solubilityOrganic active ingredientsOrganic compound preparationSolubilityDrug crystals
The invention provides a water-soluble prodrug of tamibarotene, and a preparation method and applications thereof and belongs to the technical fields of organic compound synthesis and medical applications. The water-soluble prodrug of tamibarotene has favorable water solubility and suitable drug crystal form, and therefore, is suitable to be used as a raw material for medical preparations and especially suitable for preparing injections. Particularly, the invention provides medical acceptable salts of tamibarotene DMEA ester, which have the general formula (I), wherein HA is HCl, H2SO4, HNO3,H3PO4, HOAc, paratoluenesulfonic acid, maleic acid, succinic acid, citric acid or L(+)-tartaric acid.
Owner:SHANDONG UNIV
Micro- and nano-particulate drugs and methods of making thereof
InactiveUS20080145438A1Promote absorptionImprove solubilityBiocideDrug compositionsActive agentDecomposition
A micro and nano-particulate drug comprising a drug substance and a surfactant in which the drug and surfactant form a eutectic mixture. The matrix formed between the drug substance and the surfactant has a melting point less than the decomposition temperature of the drug substance and thus provides the advantages of reduced irritation due to the melting process without the prior art problem of decomposition of the drug substance. In one embodiment, crystals are formed while the mixture is cooled at room temperature under high shear conditions. In a second embodiment, a flowable material may be formed which also contains the drug and that may be incorporated into a pharmaceutical delivery system is also disclosed. Methods of preparing the micro and nano-particulate drug crystals and non-crystalline substance are also contemplated in the inventive subject matter.
Owner:BOGUE BEUFORD ARLIE
Antimycotic Patch
InactiveUS20090203797A1Easy to controlAvoid bleedingBiocideOrganic active ingredientsHigh concentrationDrug crystals
A nail and / or skin patch for preventing or treating mycoses is provided that is able to maintain a drug concentration in the nails and / or skin horny layer at a high concentration for a long period of time without adding a dissolving agent that prevents precipitation of drug crystals or permeation enhancer that promotes penetration of drug into the nails and / or skin, retains superior adhesion even when adhered for a long period of time and has causes little skin irritation.A nail and / or skin patch for preventing or treating mycoses, which contains an antimycotic, having an octanol / water partition coefficient in the form of a logKo / w value of 4 or more, in a dissolved state in an acrylic-based pressure-sensitive adhesive layer or silicone-based pressure-sensitive adhesive layer.
Owner:NICHIBAN CO LTD
Mesotrione oil suspension agent composition
ActiveCN103503862ASmall molecular weightReduce oxidationBiocideAnimal repellantsJatrophaMethyl carbonate
The invention relates to a mesotrione oil suspension agent composition. The composition is characterized in that, the composition comprises, by mass: 8-15% of mesotrione, 10-20% of an emulsifying dispersant, 1-3% of a suspension stabilizer, 1-5% of water, 0.1-0.2% of an organosilicon defoaming agent, and balance of a green and environment-friendly solvent. The green and environment-friendly solvent mainly comprises a mixture of dimethyl carbonate and at least one of jatropha pine nut oil and castor oil. According to the mesotrione oil suspension agent provided by the invention, non-edible plant oil with wide source and the non-toxic green solvent are adopted as suspension carriers, such that the composition is green and environment-friendly, and has a characteristic of high safety to plants. Also, a crystal transition inhibiting agent is added, such that mesotrione original drug crystal transition and decomposition can be effectively inhibited.
Owner:JIANGSU CHANGQING AGROCHEMICAL CO LTD
Angiotensin receptor antagonist as well as NEP inhibitor drug crystal form and preparation thereof
The invention relates to an amorphous crystal form of a latest-generation cardiotonic agent valsartan ((2R, 4S)-5-biphenyl-4-group-5-(3-carboxyl-propionyl-amino)-2-methyl-ethyl valerate) trisodium and a preparation method of the cardiotonic agent.
Owner:JIANGSU CAREFREE PHARM CO LTD
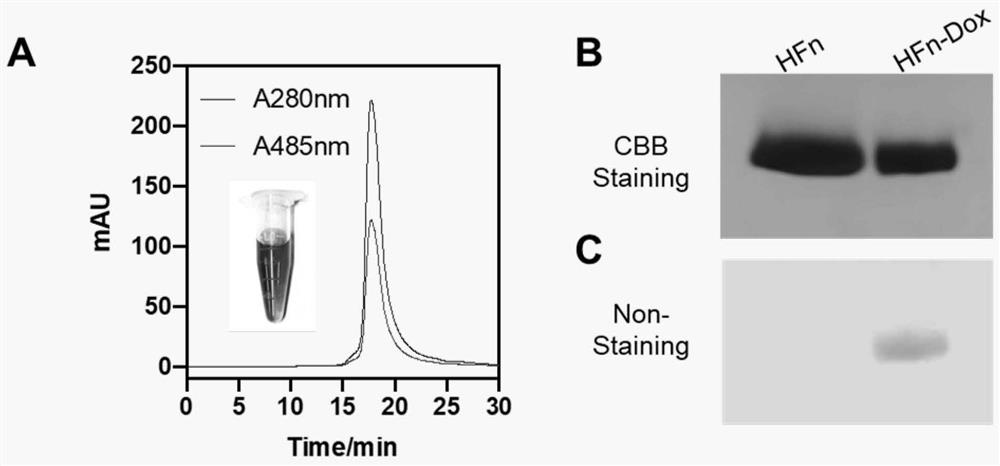
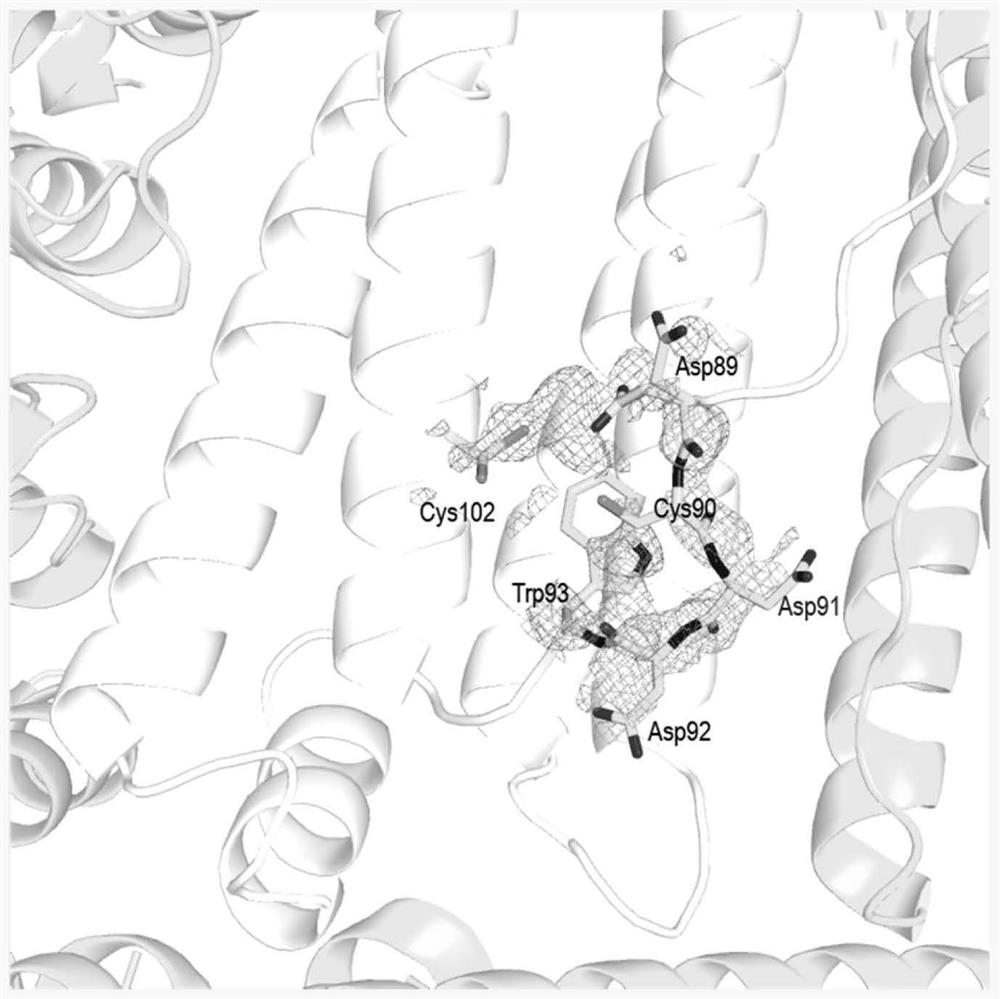
Method for loading drug on non-denatured human H ferritin
PendingCN112409446AEfficient use ofTake advantage of shortcutsOrganic active ingredientsInorganic active ingredientsDrug crystalsPharmaceutical Substances
The invention belongs to the technical field of ferritin packaging drugs, and particularly discloses a method for loading drugs on non-denatured human H ferritin. Through HFn-small molecule drug crystal structure analysis and identification, the findings show that 12 drug channels exist on a protein shell of HFn, the key amino acid determining the channels is Asp89-Cys90-Asp91-Asp92, and meanwhile, amino acids of the small molecule drug channels has the property of temperature factor regulation and control. Based on the discovery, under the conditions that the ferritin structure is not changedand the ferritin is not denatured, by exploring different incubation temperatures and / or different incubation conditions, on the premise that the ferritin structure is kept complete, determination ofthe optimal condition of the HFn entrapping the drugs is achieved. By utilizing the obtained entrapment condition under the non-denaturation condition, the HFn can be conveniently, quickly, efficiently and sufficiently used for entrapment of different small molecular drugs.
Owner:NANJING NAMOMEI TECH CO LTD
Drug eluting balloon with preferred drug orientation to improve drug transfer efficiency
A catheter comprises a medical balloon having a drug coating. The drug coating comprises drug crystals on a surface of the balloon. The majority of the drug crystals on the surface of the balloon are oriented drug crystals which extend within 5 degrees of a predetermined, non-zero common angle relative to the surface of the balloon from which the crystals extend.
Owner:BOSTON SCI SCIMED INC
Self-assembled luminous current-conducting nano medicine crystal and ultra-thin film and preparation method thereof
The invention provides a self-assembling light-emitting conductive nano medicinal crystal and ultra-thin film, and the preparing process, wherein non-resilient electronic tunneling interaction is employed for self-assembling oxidation resistant enzyme oxygen free radical antagonist, beta-receptor agonist, P2 receptor agonist, benzene alkylamines calcium antagonist monomer, and bibasic, ternary and quaternary compound.
Owner:ZHONGSHAN HOSPITAL FUDAN UNIV
Drug coating layer and method for forming same
PendingCN111032101ADoes not interfere with vascular metastasesAvoid breakingOrganic active ingredientsBalloon catheterBiological bodyDrug crystals
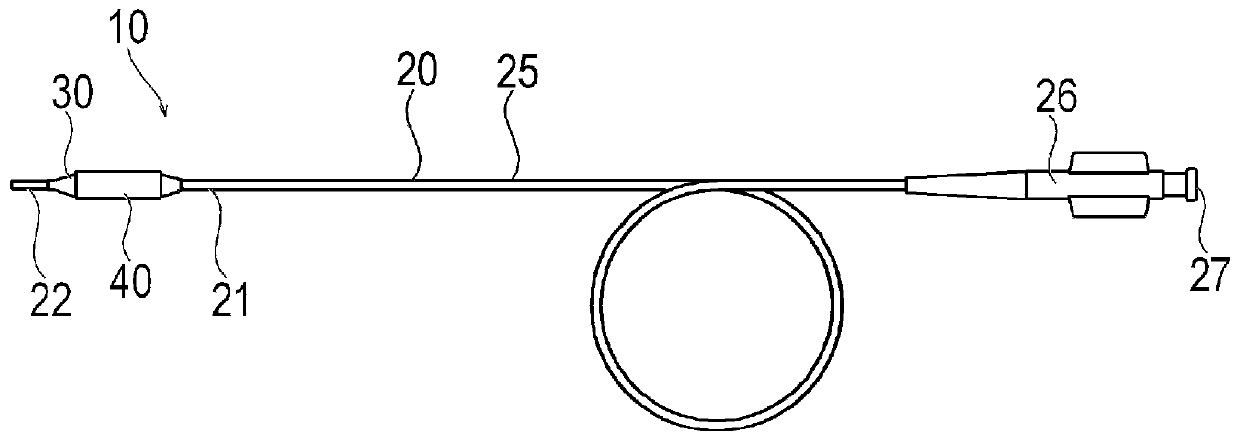
Provided are a drug coating layer and a method for forming the same, the layer being capable of suppressing damage to drug crystals having a long balloon surface, and of maintaining drug crystals in an appropriate shape for having an effect on a living body. A drug coating layer (40) formed on the surface of a balloon (30), the layer comprising: a plurality of long bodies (42), which are crystalsof a water insoluble drug, having a longitudinal axis extending from the surface of the balloon (30) at various lengths and angles; and a water soluble additive layer (41) provided in the space between the surface of the balloon (30) and an outer surface (51) so as to fill the space between the long bodies (42), the outer surface (51) being provided with protrusions and recesses connecting a plurality of tip ends (46) and side surfaces (43) of the long bodies (42), and positioned at an outside of an aggregate (50) of a plurality of the long bodies (42). The side surfaces (43) and / or tip end surfaces (47) of the long bodies (42) are exposed at the surface of the additive layer (41) without the tip ends of the long bodies (42) almost protruding from the additive layer (41).
Owner:TERUMO KK
Flurbiprofen cataplasm and preparation method thereof
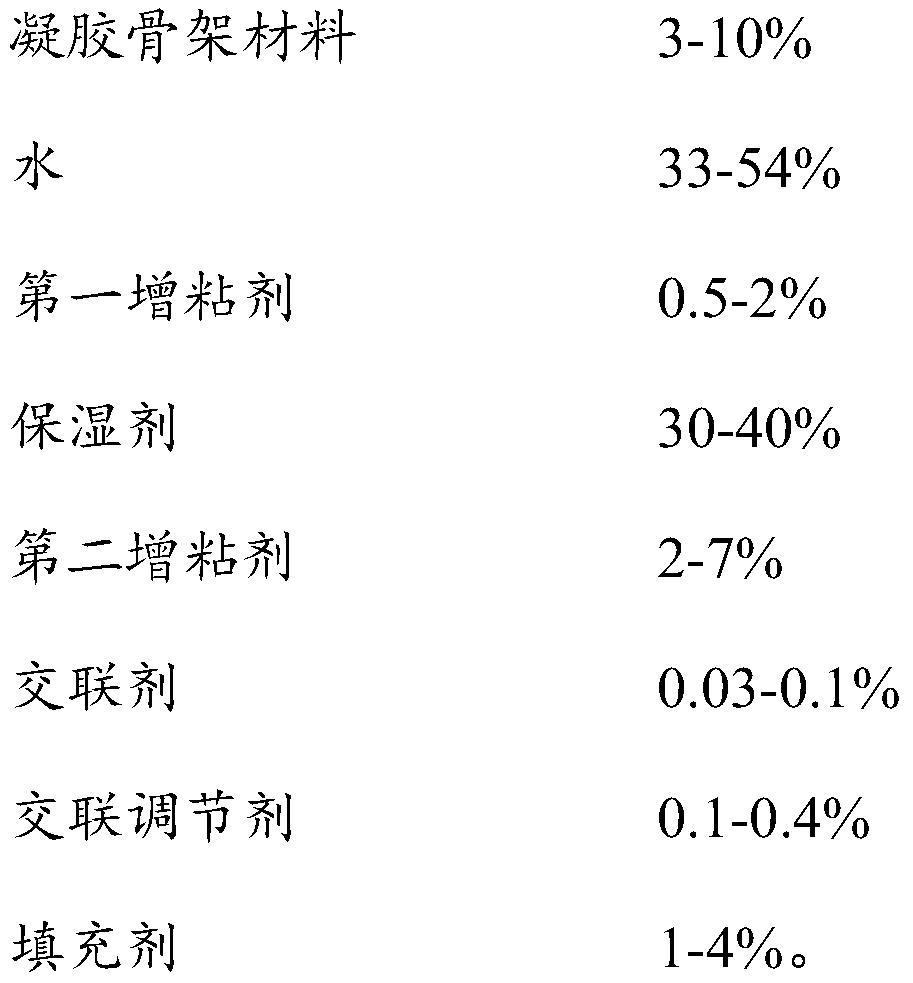
ActiveCN113041236AImprove solubilityPromote transdermal absorptionOrganic active ingredientsAntipyreticActive agentPyrrolidinones
The invention provides a flurbiprofen cataplasm and a preparation method thereof. The cataplasm consists of a backing, a paste body and an anti-adhesion membrane, wherein the paste body is prepared from the following components: emulsion, a first tackifier, a humectant, a gel skeleton material, a cross-linking agent, a cross-linking regulator and a filler; the emulsion is obtained by mixing a component A and a component B; the component A is obtained by mixing flurbiprofen, a surfactant and a solvent; the component B is prepared from a pH regulator, a second tackifier and water; the surfactant is prepared from benzyl ether and span, or is prepared from Kolliphor HS 15 and span; and the gel skeleton material is selected from at least one of partially neutralized sodium polyacrylate, carbomer, polyacrylic acid, polyvinylpyrrolidone and polybutylene. The flurbiprofen cataplasm is good in percutaneous absorption and good in storage stability, and drug crystals cannot be separated out after long-term storage.
Owner:GUANGZHOU NEWORLD PHARMA CO LTD
Sodium fusidate novel crystal form and preparation method and application thereof
InactiveCN112979739AImprove liquidityEasy to packAntibacterial agentsOrganic active ingredientsDrug crystalsPharmaceutical Substances
The invention relates to the technical field of medicine crystals, in particular to a sodium fusidate novel crystal form and a preparation method and application thereof. In an X-ray powder diffraction spectrogram of the novel crystal form, the sodium fusidate novel crystal form has characteristic peaks at the positions of 9.646 degrees, 10.882 degrees, 11.034 degrees, 13.840 degrees, 14.046 degrees, 16.577 degrees, 17.318 degrees, 17.687 degrees, 18.072 degrees, 19.868 degrees, 23.036 degrees, 25.489 degrees and 27.400 degrees as shown in 2 theta+ / -0.2 degrees. The novel sodium fusidate crystal form prepared by the invention has the advantages of good fluidity, convenience in subpackage and preservation and the like, can be used for preparing a sodium fusidate injection, and is favorable for improving the medicine quality and the like.
Owner:BEIJING ZHENDONG GUANGMING PHARMA RES INST
Drug-loaded emulsion of liquid crystal coated crystal drug and preparation method thereof
ActiveCN112972381AImprove the disadvantage of poor water solubilityEnhanced barrier functionOrganic active ingredientsAntipyreticHuman skinDrug crystals
The invention belongs to the technical field of pharmaceutical preparations, and particularly relates to a drug-loaded emulsion of a liquid crystal coated crystal drug and a preparation method thereof. The emulsion comprises a crystal drug and a liquid crystal emulsifier. A lamellar liquid crystal structure similar to a human skin cuticle structure exists in the drug-loaded emulsion of the liquid crystal coated crystal drug, and meanwhile, a crystal drug crystal structure also exists in the emulsion. The drug-loaded emulsion provided by the invention overcomes the defects of poor water solubility, low biofilm permeability of active components of traditional drugs, and has the advantages of high skin safety, multiple slow release characteristics, drug entrapment rate of nearly 100% and the like; and provides a new design idea for the development of an efficient drug-loaded emulsion preparation with higher retention rate of the active pharmaceutical ingredients, better preparation stability and more effective entrapment of the active pharmaceutical ingredients.
Owner:EAST CHINA UNIV OF SCI & TECH +1
Method and device for forming drug coating layer
ActiveCN111032103AEfficient removalInhibition of the impact of the situationBalloon catheterMedical devicesOrganic solventDrug crystals
Provided are a method and device for forming a drug coating layer with which it is possible to suppress damage to elongated drug crystals on the surface of a balloon and to maintain a suitable shape for causing the drug crystals to act on a living body. A method for forming a drug coating layer (40) in which a plurality of elongated bodies (42), which are crystals of a water-insoluble drug havinga length axis, are formed on the surface of a balloon (30), wherein the method has: a step in which a coating solution that includes a water-insoluble drug, a water-soluble additive, an organic solvent, and water is supplied to the surface of the balloon (30), the organic solvent and the water are caused to evaporate, and each of an additive layer (41) which includes the water-soluble additive andprojecting crystals (48) the tips of which project from the additive layer (41) are formed; a step in which a surplus portion (49) of the projecting crystals (48) that projects from the additive layer (41) is cut out from a site surrounded by the additive layer (41), and the site surrounded by the additive layer (41) is formed into an elongated body (42); and a step in which the cut-out surplus portion (49) is removed from the drug coating layer (40).
Owner:TERUMO KK
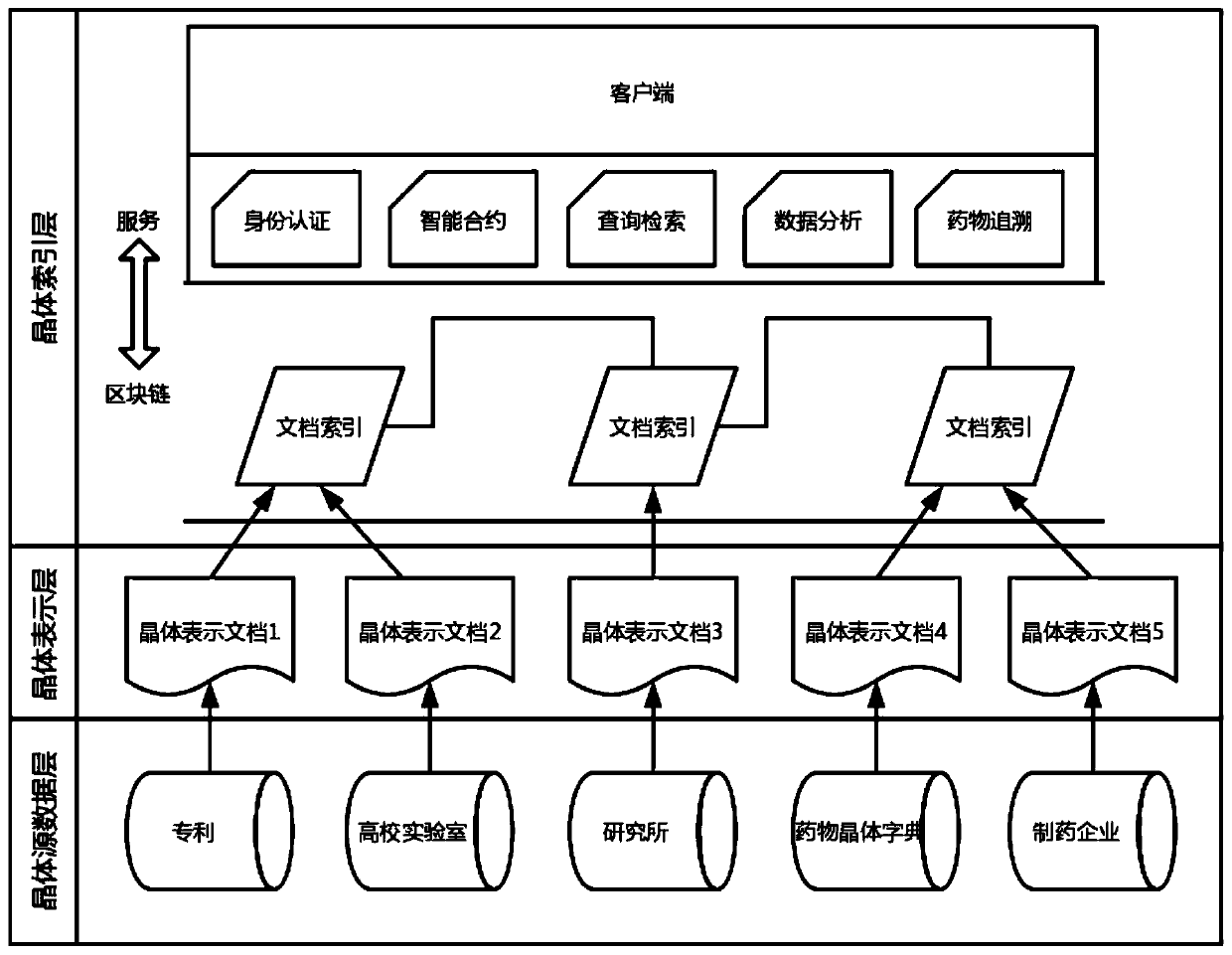
Drug crystal library based on block chain and construction method thereof
PendingCN110750492ASharing is fast and transparentProtection of attribution rightsDigital data protectionFile system administrationOriginal dataTheoretical computer science
The invention provides a drug crystal library based on a block chain and a construction method thereof, and the drug crystal library comprises that a first layer is a crystal source data layer and isused for acquiring crystal information and storing drug crystal original data, and the security and privacy are guaranteed by a data holder; the second layer is a crystal representation layer, is usedfor crystal information chaining, stores crystal information documents generated according to corresponding standards, and ensures unique identification of one crystal form; and the third layer is acrystal index layer, is used for querying crystal related information, is realized through blockchain nodes, and stores shared document index information and query information. The method is suitablefor construction of a drug molecular crystal library, drug molecular crystal form information is managed by using a block chain, functions of adding, searching, comparing and the like of drug molecular crystal forms are realized in combination with software, and the purposes of protecting, authorizing and the like of the molecular crystal forms are achieved.
Owner:SHENZHEN JINGTAI TECH CO LTD
Superfine powder of Fibrates lipid-lowering drug and preparation method therefor
InactiveCN105218370AQuality improvementImprove uniformityPowder deliveryOrganic compound preparationLipid formationSolubility
The invention relates to superfine powder of a Fibrates lipid-lowering drug and a preparation method therefor. The Fibrates lipid-lowering drug is a main therapeutic drug for resisting hyperlipidemia, and due to influence resulting from factors such as relatively poor water solubility and the like, the bioavailability of the Fibrates lipid-lowering drug is not high. The method for preparing the superfine powder of the Fibrates lipid-lowering drug, provided by the invention, comprises the steps of applying ultrasonic waves, of which the frequency is 10kHz to 500kHz, the power is 1mW to 5,000W and the sound intensity is 0.1mW / cm<2> to 500W / cm<2>, to a Fibrates lipid-lowering drug containing homogeneous solution so as to rapidly obtain Fibrates lipid-lowering drug crystals, and then, carrying out normal operations such as solid collecting, washing, drying and the like, thereby directly obtaining the superfine powder of the Fibrates lipid-lowering drug. According to the superfine powder prepared by the method, the bioavailability of the drug is improved, the dosage of the drug is reduced, the solubility of the drug is improved, and the drug absorption can be enhanced, so that the application is wider.
Owner:无锡康福特药物科技有限公司 +1
Method for preparing acicular acetaminophen crystal by time domain shaping femtosecond laser
InactiveCN109867610ALow costComponents are pureCarboxylic acid amide separation/purificationEnergy based chemical/physical/physico-chemical processesTime domainAcetaminophen crystals
The invention relates to a method for preparing acicular acetaminophen crystal by a time domain shaped femtosecond laser, and belongs to the field of preparation of pharmaceutical crystal materials. The method comprises the following steps: (1) preparing a supersaturated solution of acetaminophen by using a heating device; (2) using a femtosecond laser irradiation method to enable the acetaminophen supersaturated solution to be ionized to generate cavitation bubbles and generate acicular acetaminophen crystal as the bubbles rupture; and (3) drying filtered acicular acetaminophen crystal by using a heating device. The method for preparing the acicular paracetamol crystal provided by the invention is carried out under normal temperature and normal pressure without any addition in the production, the manufacturing time is short, the ratio of the acetaminophen crystal is high, and the size is controllable.
Owner:BEIJING INSTITUTE OF TECHNOLOGYGY
Preparation method of tedizolid phosphate composition tablets
ActiveCN107625737AImprove stabilityExcellent curative effectAntibacterial agentsOrganic active ingredientsSucrosePhosphate
The invention belongs to the field of medical preparation, and more specifically discloses a preparation method of tedizolid phosphate composition tablets. The preparation method comprise following steps: tedizolid phosphate, pregelatinized starch, sucrose, tyrosine, polacrilin potassium, and sodium lauryl sulfate are added into a 5% polyvinylpyrrolidone K30 solution, and wet granulation sodium lauryl sulfate is adopted so as to obtain the tedizolid phosphate composition tablets. The stability of the tedizolid phosphate composition tablets is improved obviously via screening on the accessory ingredients and controlling on the amount; and drug crystal transformation technical problem in wet granulation is solved via screening on the kind and the amount of a binding agent. It is found via experiments that, compared with the prior art, the preparation method possesses following advantages: the curative effect is better, the adverse reaction is reduced, and antimicrobial activity on MRSA is increased obviously.
Owner:SHANDONG YUXIN PHARMA CO LTD
A coating process for drug coating on implanted or interventional medical devices
ActiveCN104353132BChange release curveAvoid reunionSurgeryPharmaceutical containersPharmaceutical drugDrug crystals
The invention relates to the technical field of medical device preparation, in particular to a coating process of a drug coating on an implanted or interventional medical device. In this process, ultrasonic spraying is used to form a coating on the medical device. During the ultrasonic spraying process, the carrier gas is modified so that one or more auxiliary solvent gases that cannot dissolve the drug are introduced into the carrier gas. After the drug droplets meet the carrier gas, the drug is precipitated in the form of particles due to the change of solubility, so as to achieve the purpose of reducing the particle size of the final drug coating. The drug coating formed by this process is uniform, has good affinity with the surface of the stent or the balloon, the coating falls off little when it is folded and pressed, and does not form large drug crystal particles after being immersed in blood.
Owner:ZHEJIANG BELONGS TO A MEDICAL INSTR
Preparation method of drug-coated balloon catheter, prepared drug-coated balloon catheter and application thereof
ActiveCN107362439BNot easy to wash offHigh drug loading efficiencyBalloon catheterMedical devicesDrugs solutionThrombus
The invention provides a drug-coating balloon catheter and a production method and application thereof. The method includes: preparing active drug seed crystals, and screening the seed crystals 1-3 micrometers in length to prepare active drug seed crystal suspension; adding the active drug seed crystal suspension, an active drug solution and an additive solution into different channels of a coating machine, mixing and atomizing the active drug seed crystal suspension, the active drug solution and the additive solution at the nozzle tip end of the ultrasonic sprayer of the coating machine, spraying to the surface of a balloon dilatation catheter to obtain drug coating with an appropriate crystal size, and performing homogenizing post-processing to obtain the drug-coating balloon catheter with homogeneous crystals. The drug-coating balloon catheter and the production method thereof have the advantages that the drug coating is firmly combined with a balloon, and drug crystals are even and complete; small drug loss during balloon preparation and in-vivo conveying can be guaranteed, concentration of effective drugs entering the blood vessel wall of a lesion part is high, high drug loading efficiency is achieved, intravascular in-situ stenosis or restenosis can be treated effectively, the risks of late thrombosis and restenosis are reduced, and the positive remodeling of blood vessels can be formed at the same time.
Owner:LEPU MEDICAL TECH (BEIJING) CO LTD
Antimycotic patch
InactiveCN101541315AEasy to control adhesionPrevent seepageOrganic active ingredientsAntimycoticsHigh concentrationIrritation
A nail and / or skin patch for preventing or treating mycoses is provided that is able to maintain a drug concentration in the nails and / or skin horny layer at a high concentration for a long period of time without adding a dissolving agent that prevents precipitation of drug crystals or permeation enhancer that promotes penetration of drug into the nails and / or skin, retains superior adhesion even when adhered for a long period of time and has causes little skin irritation. A nail and / or skin patch for preventing or treating mycoses, which contains an antimycotic, having an octanol / water partition coefficient in the form of a logKo / w value of 4 or more, in a dissolved state in an acrylic-based pressure-sensitive adhesive layer or silicone-based pressure-sensitive adhesive layer.
Owner:NICHIBAN KK
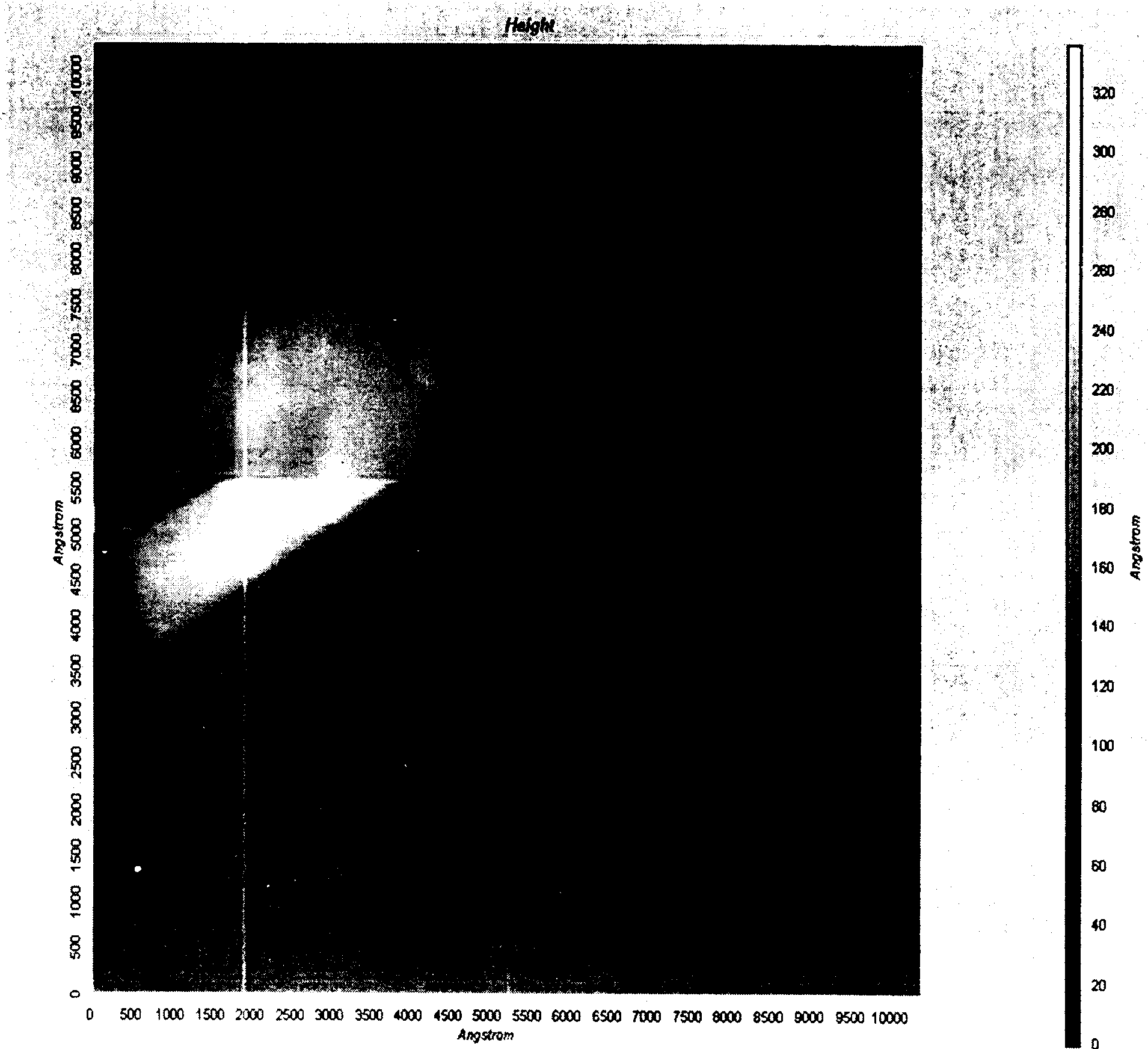
Drug crystal structure landscape analysis system and landscape analysis method thereof
ActiveUS20210265022A1Molecular entity identificationCheminformatics data warehousingStructure analysisDrug crystals
The invention belongs to the technical field of drug crystal analysis, and particularly relates to a drug crystal structure landscape analysis system and a landscape analysis method thereof. The drug crystal structure landscape analysis system calls a cloud computing interface to calculate the energy of input crystals through an algorithm deployed in the cloud in advance, and an energy-density space group landscape array diagram of the crystals is generated according to the computation results returned; and analysis is selectively carried out as needed, result reports arc analyzed and summarized as a final report, and the final report is converted into a text document. The drug crystal structure landscape analysis system and the landscape analysis method thereof satisfy the drug crystal structure analysis requirements in the new technology background, and can analyze a large quantity of crystals which are formed by a certain drug molecule and have different structures.
Owner:SHENZHEN JINGTAI TECH CO LTD
Non-steroidal anti-inflammatory drug ultra-fine powder and preparation method thereof
InactiveCN105198747AHigh drug loadingImprove bioavailabilityPowder deliveryOrganic compound preparationRheumatismNon steroid anti inflammatory drug
The invention relates to non-steroidal anti-inflammatory drug ultra-fine powder and a preparation method thereof. A non-steroidal anti-inflammatory drug has the functions of resisting inflammations and rheumatism, relieving pain, bringing down a fever and resisting blood coagulation and is widely used for alleviating osteoarthritis, rheumatoid arthritis, various fevers and various painful symptoms in clinic. The method for preparing the non-steroidal anti-inflammatory drug ultra-fine powder comprises the steps that non-steroidal anti-inflammatory drug crystals are rapidly obtained by exerting ultrasonic waves with the frequency ranging from 10 kHz to 500 kHz, the power ranging from 1 mW to 5000 W and the sound intensity ranging from 0.1 mW / cm<2> to 500 w / cm<2> into a homogeneous solution containing the non-steroidal anti-inflammatory drug, and then the non-steroidal anti-inflammatory drug ultra-fine powder is directly obtained after normal operations such as solid collection, washing and drying are conducted. The non-steroidal anti-inflammatory drug ultra-fine powder is free of substrate materials and has the advantages that the drug-loading rate is high, the dissolution velocity is high, higher bioavailability is easy to achieve, and the stability and the safety are achieved, so that the requirements for improving the bioavailability of the drug, decreasing the drug dosage and reducing adverse reactions are met, and a wide application prospect in clinic is achieved.
Owner:WUXI XINRENTANG PHARMA TECH +1
Drug balloon catheter as well as preparation method and application thereof
The invention provides a medicine balloon catheter as well as a preparation method and application thereof. The medicine balloon catheter comprises a balloon catheter as well as a medicine coating and a phospholipid excipient layer which are sequentially coated on the outer surface of the balloon catheter. The phospholipid excipient layer effectively balances the bonding force among the drug coating, the balloon catheter and the blood vessel wall, drug loss in the conveying process can be reduced, the drug utilization rate can be increased, and the release rate of the drug at the diseased region and the immediate absorption rate of the blood vessel to the drug can be increased; in addition, drug crystals in the drug coating are more uniform in size, and the stability of the drug balloon catheter is higher.
Owner:上海畅德医疗科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com