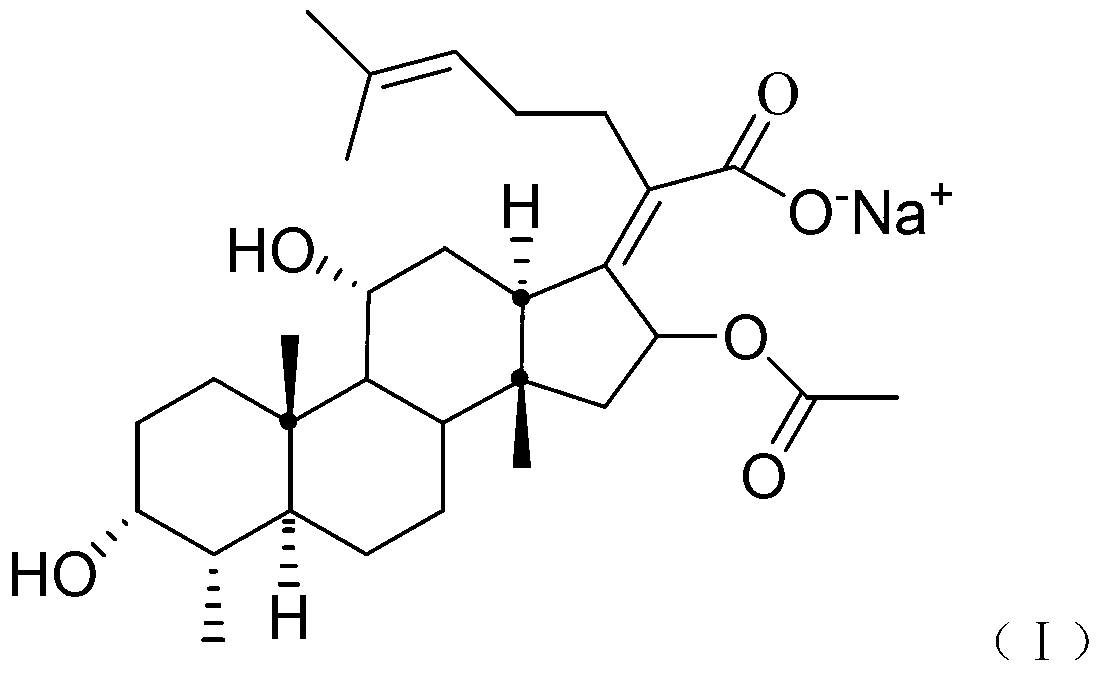
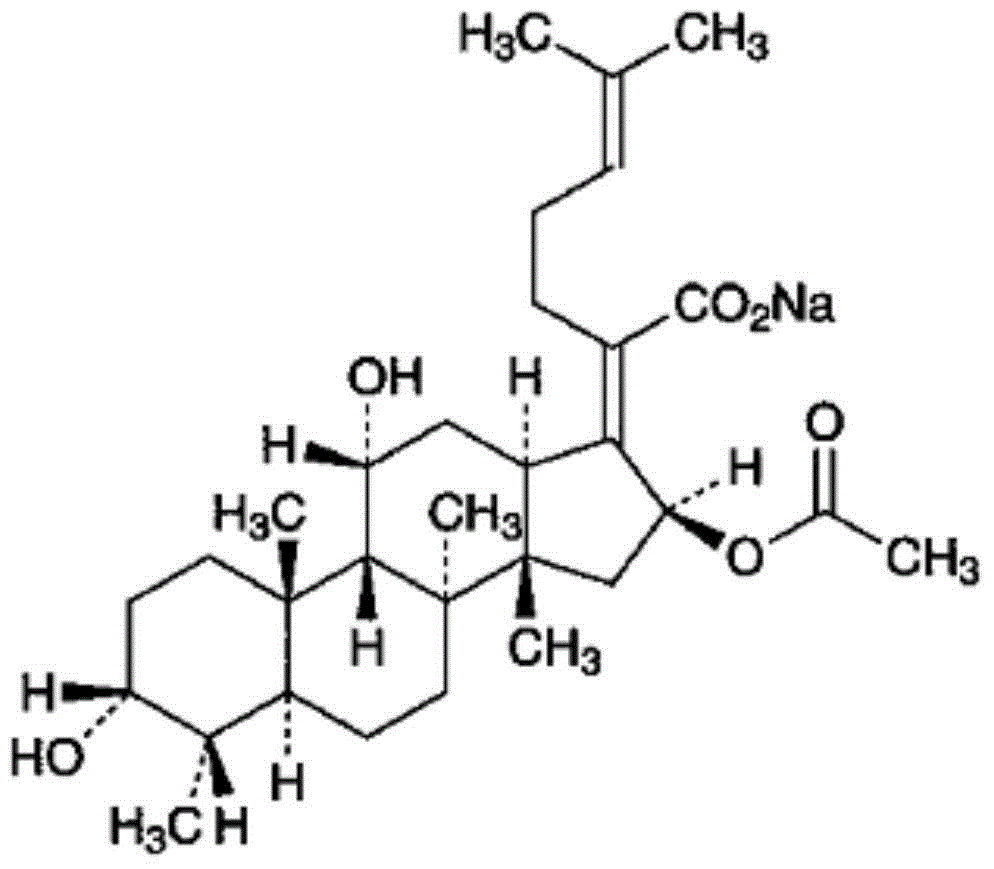
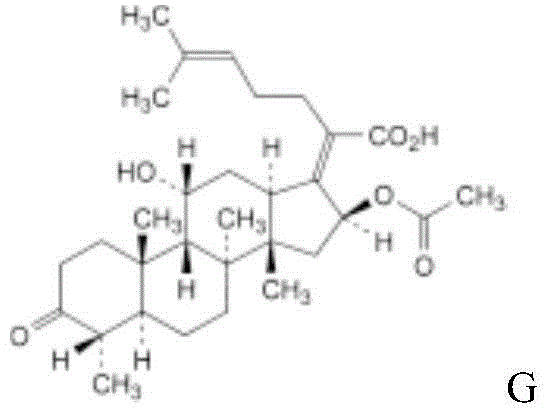
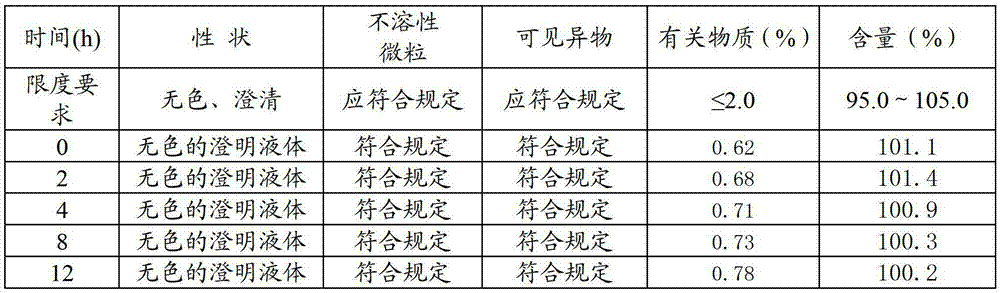
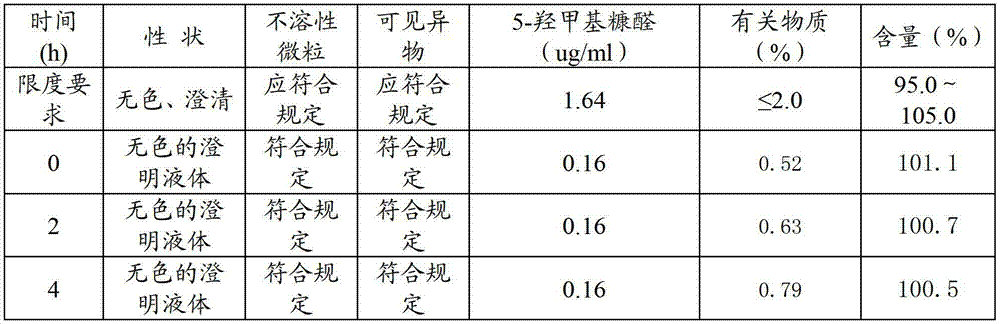
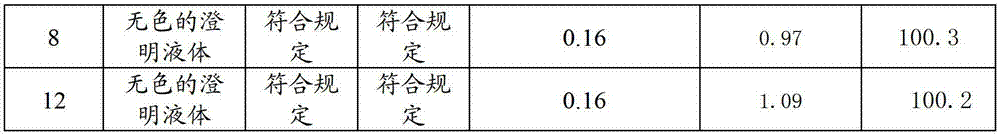
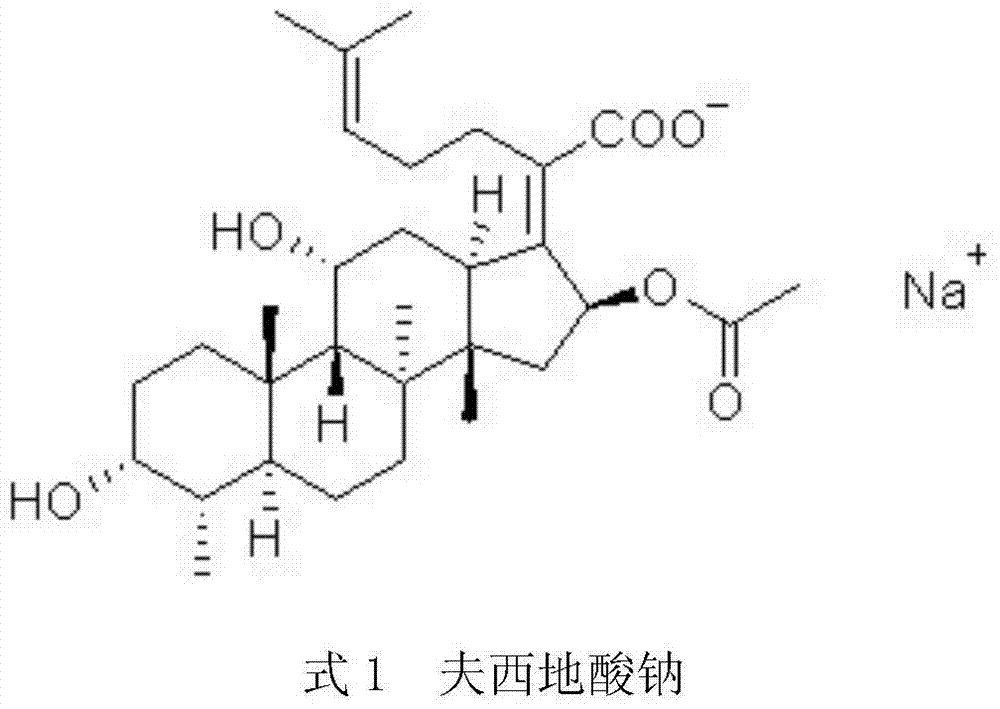
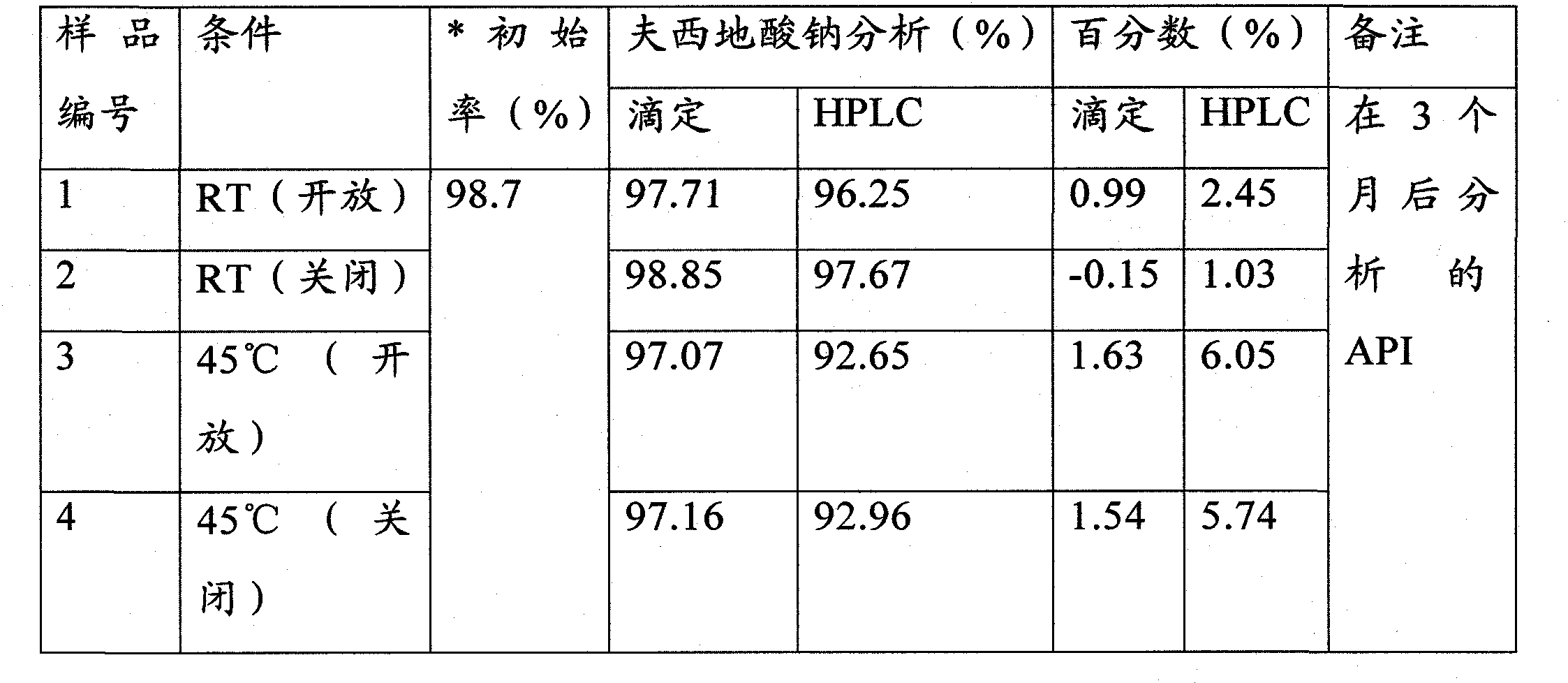
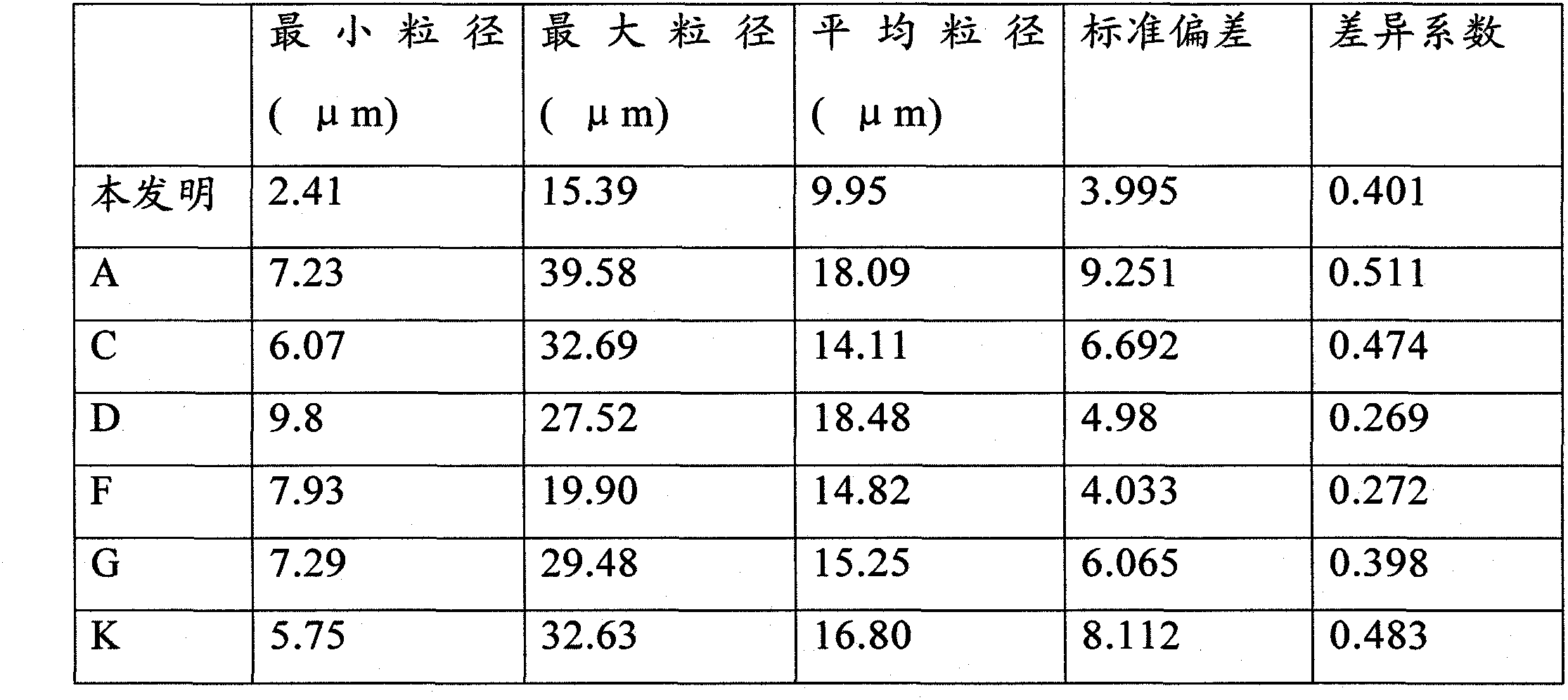
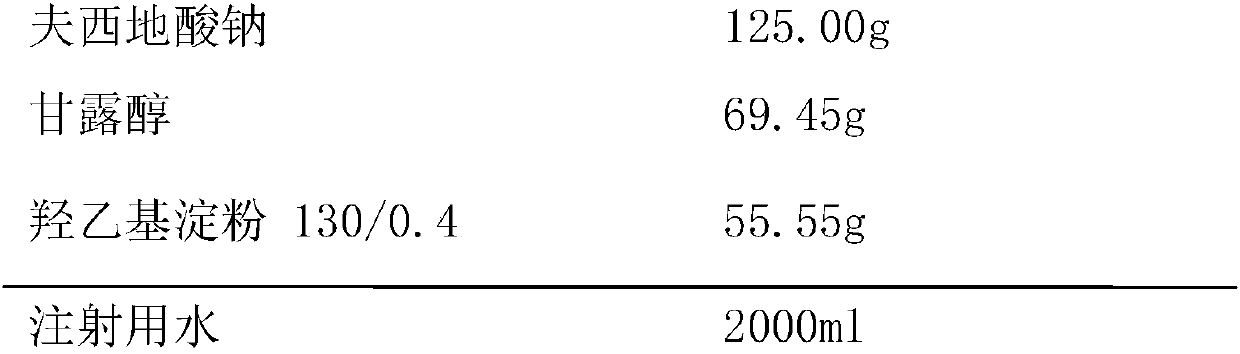
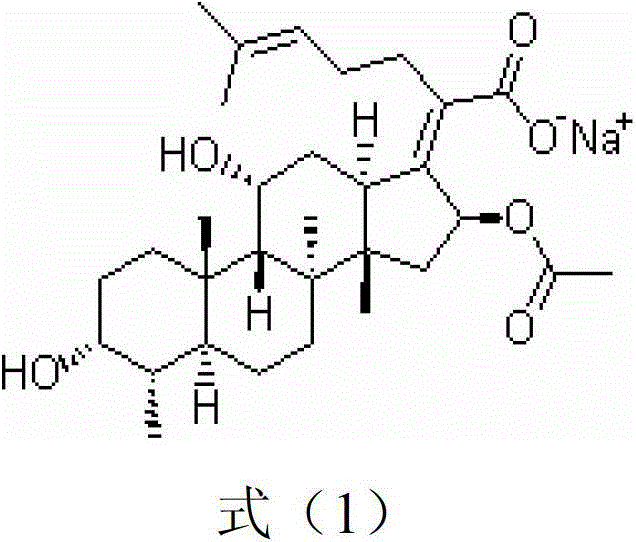
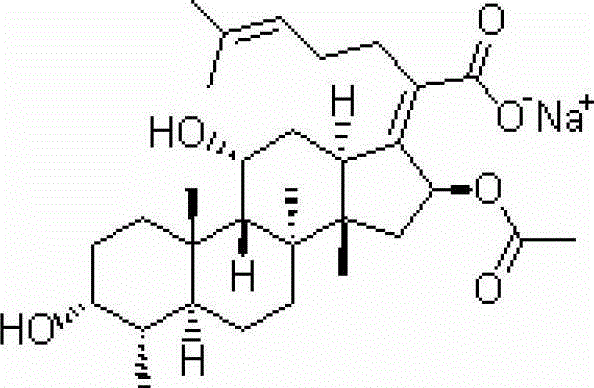
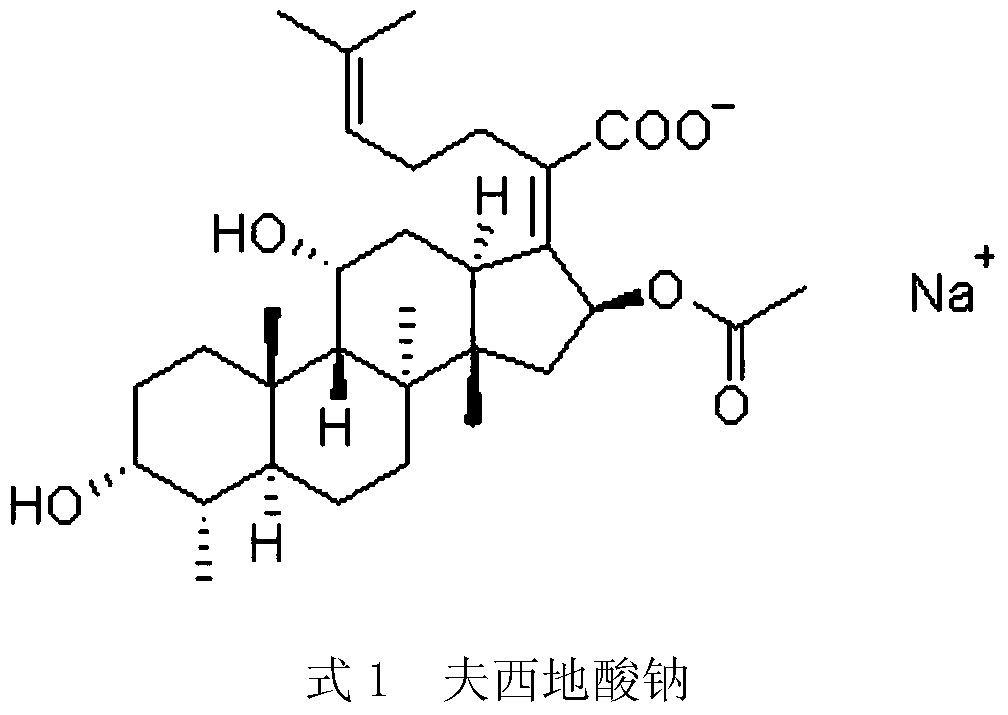
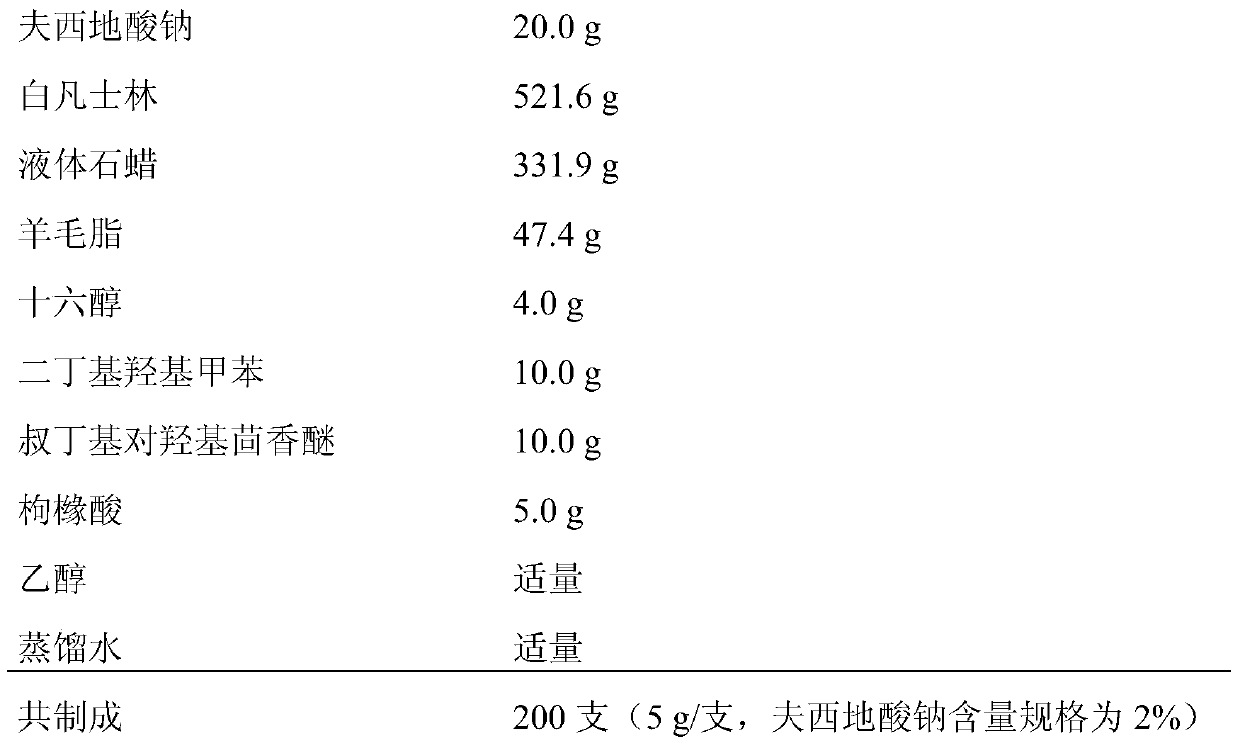
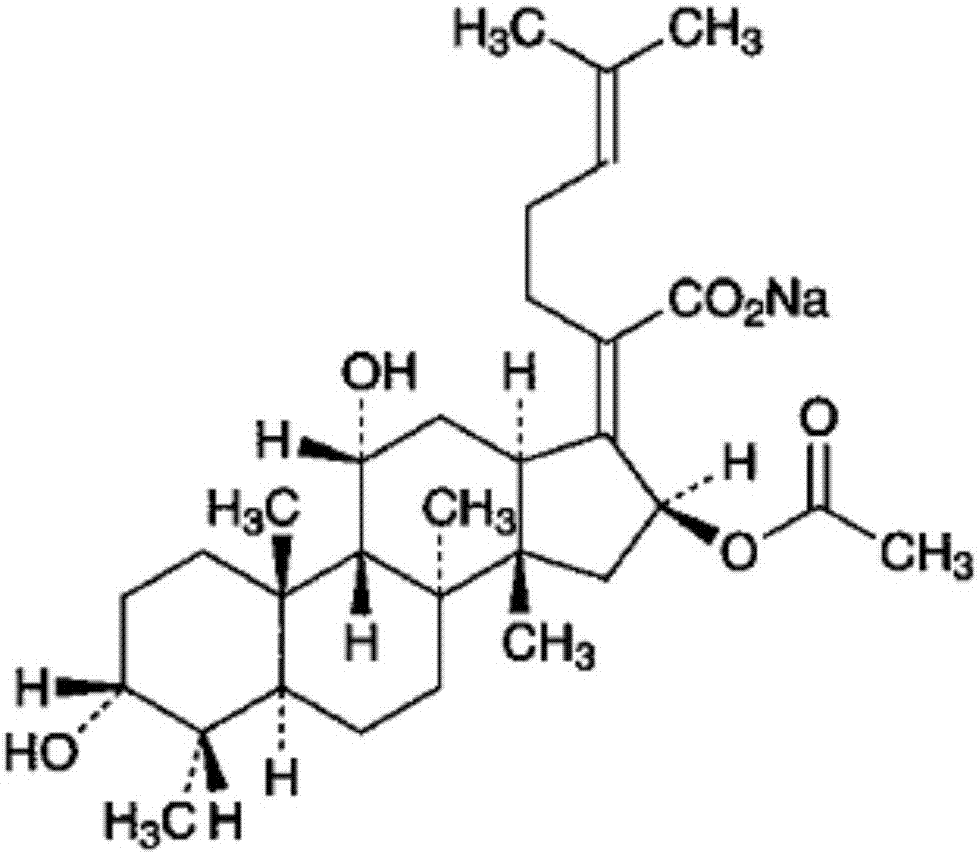
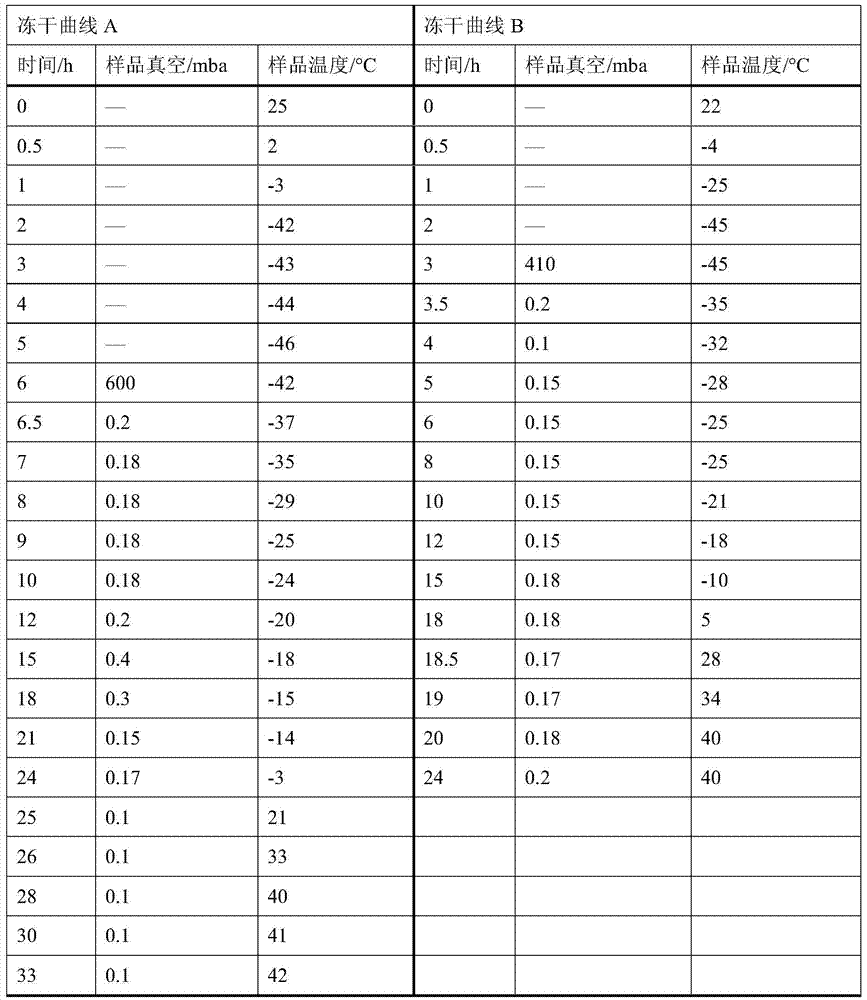
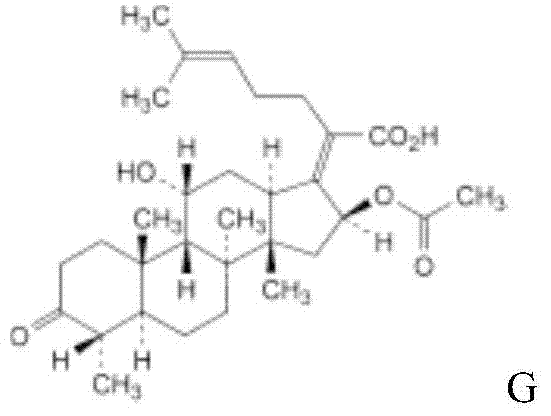
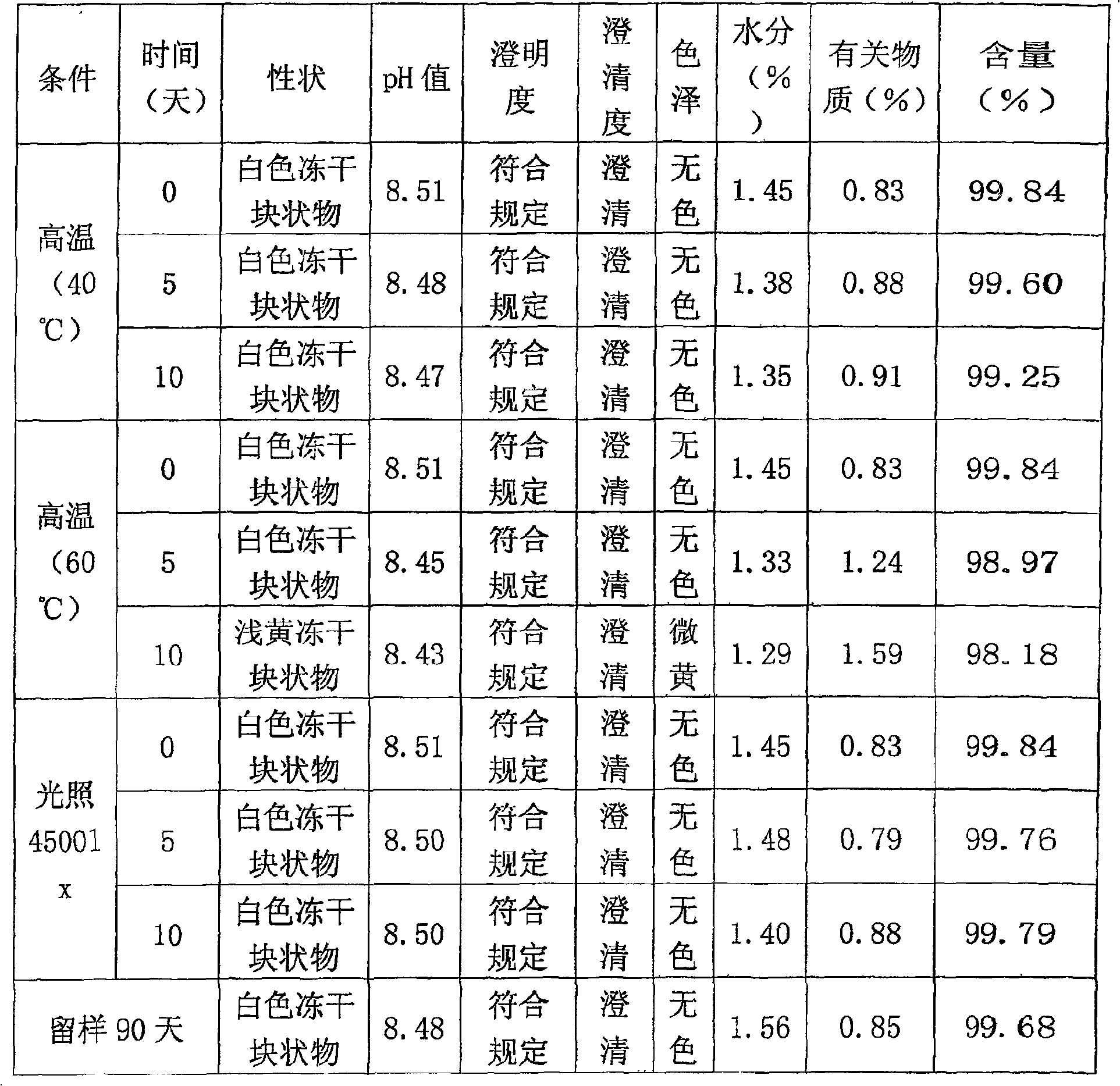
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
35 results about "Sodium fusidate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Proteinic drug delivery system using membrane mimetics
A mixed liposome pharmaceutical formulation with multilamellar vesicles, comprises a proteinic pharmaceutical agent, water, an alkali metal lauryl sulphate in a concentration of from 1 to 10 wt. / wt. %, at least one membrane-mimetic amphiphile and at least one phospholipid. The membrane-mimetic amphiphile is hyaluronic acid, pharmaceutically acceptable salts of hyaluronic acid, lauramidopropyl betain, lauramide monoisopropanolamide, sodium cocoamphopropionate, bishydroxypropyl dihydroxypropyl stearammonium chloride, polyoxyethylene dihydroxypropyl stearammonium chloride, dioctadecyldimethylammonium chloride, sulphosuccinates, stearamide DEA, gamma-linoleic acid, borage oil, evening of primrose oil, monoolein, sodium tauro dihydro fusidate, fusidic acid, alkali metal isostearyl lactylates, alkaline earth metal isostearyl lactylates, panthenyl triacetate, cocamidopropyl phosphatidyl PG-diammonium chloride, stearamidopropyl phosphatidyl PG-diammonium chloride, borage amidopropyl phosphatidyl PG-diammonium chloride, borage amidopropyl phosphatidylcholine, polysiloxy pyrrolidone linoleyl phospholipid, trihydroxy-oxo-cholanylglycine and alkali metal salts thereof, and octylphenoxypolythoxyethanol, polydecanol X-lauryl ether, polydecanol X-oleyl ether, wherein X is from 9 to 20, or combinations thereof. The phospholipid is phospolipid GLA, phosphatidyl serine, phosphatidylethanolamine, inositolphosphatides, dioleoylphosphatidylethanolamine, sphingomyelin, ceramides, cephalin, triolein, lecithin, saturated lecithin and lysolecithin, or a combination thereof. The amount of each membrane mimetic amphiphile and phospholipid is present 1 to 10 wt. / wt. % of the total formulation, and the total concentration of membrane mimetic amphiphiles and phospholipids is less than 50 wt. / wt. % of the formulation.
Owner:GENEREX PHARMA
Composition of sodium fusidafe as injection and preparing method thereof
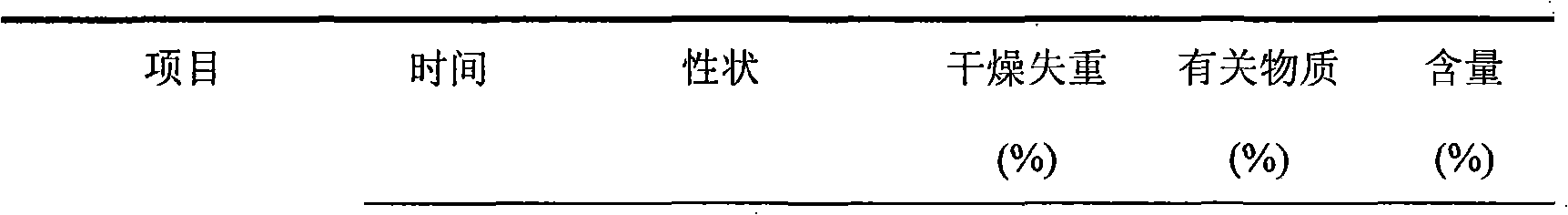
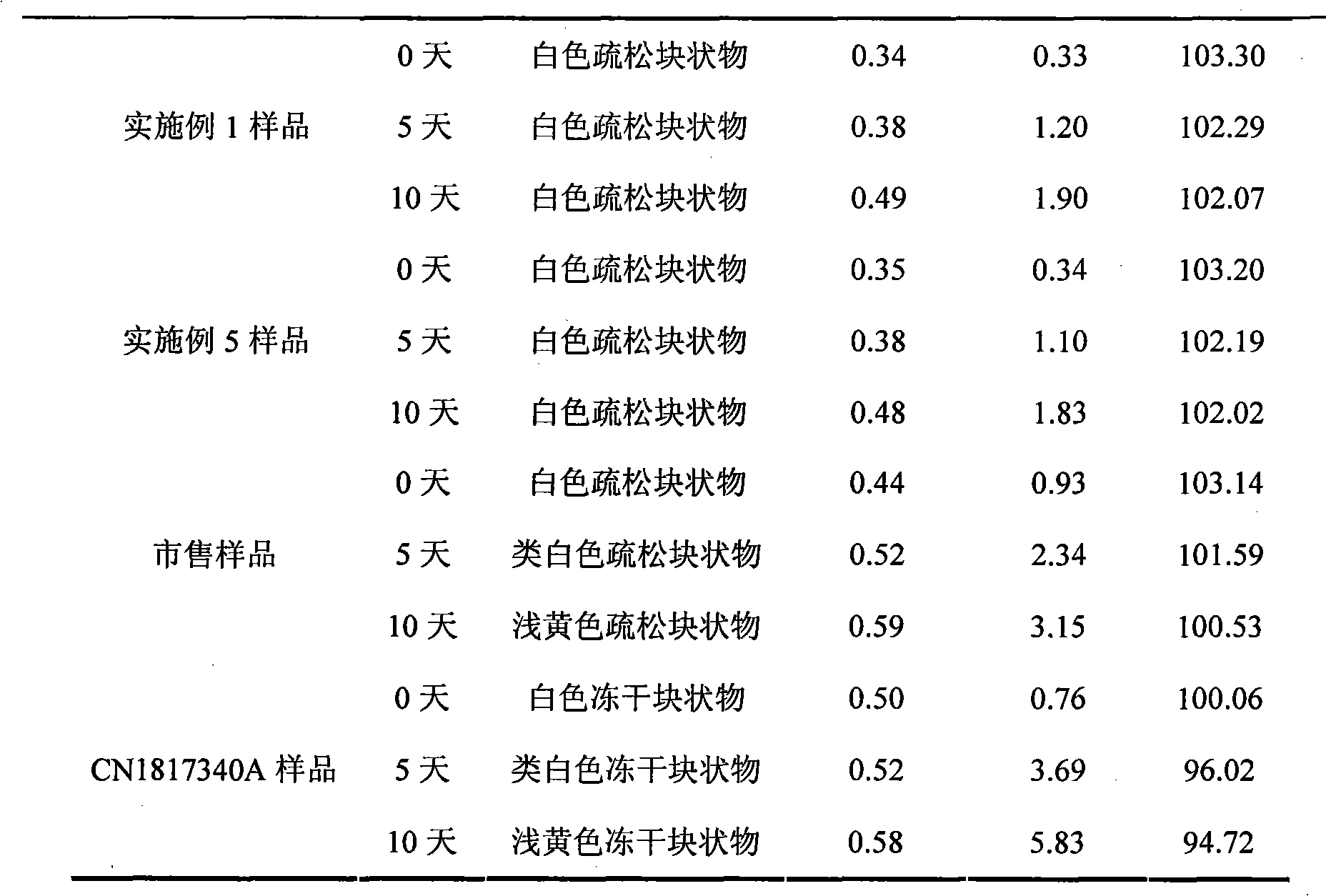
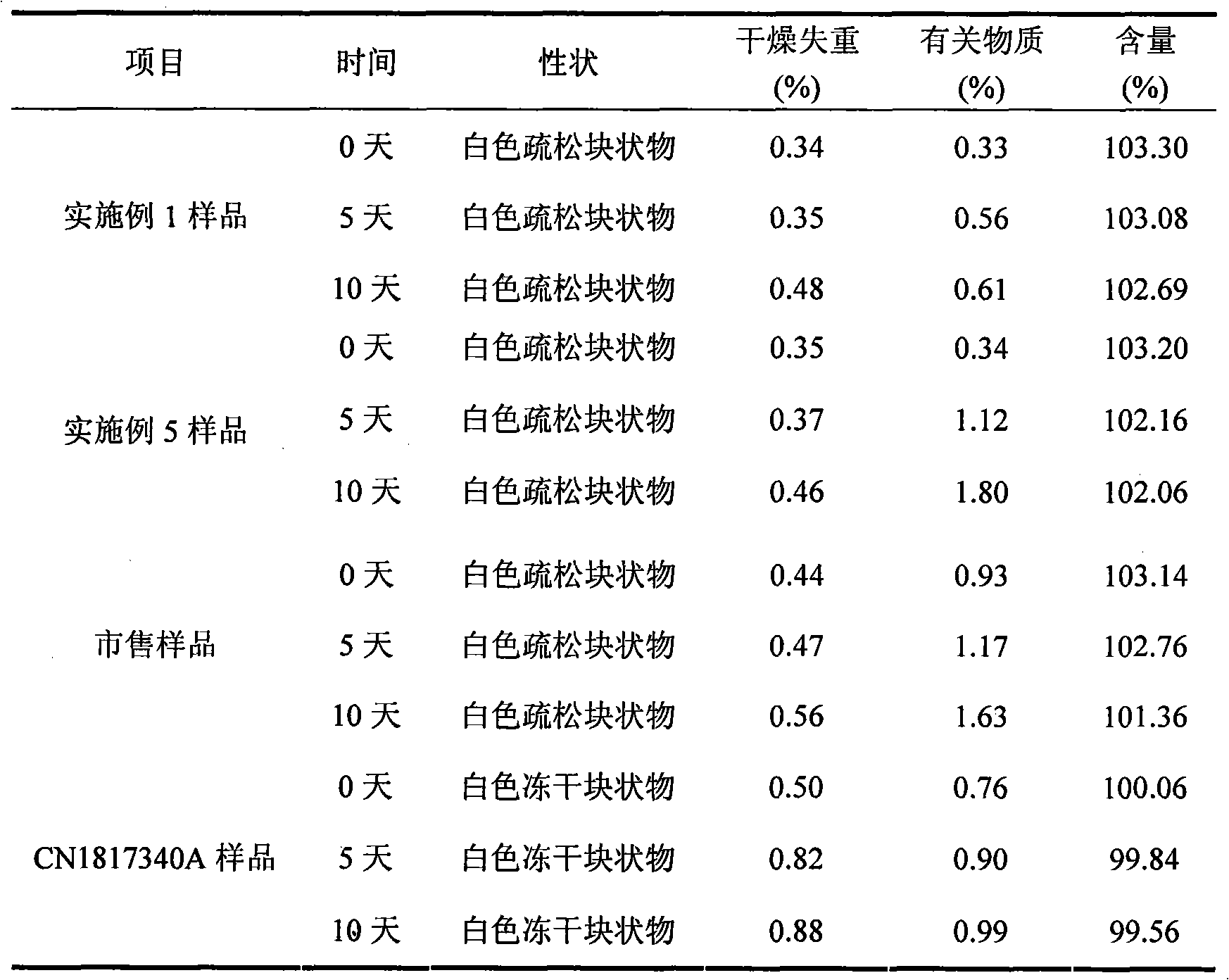
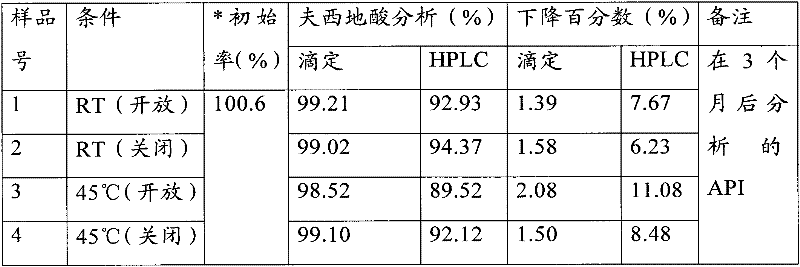
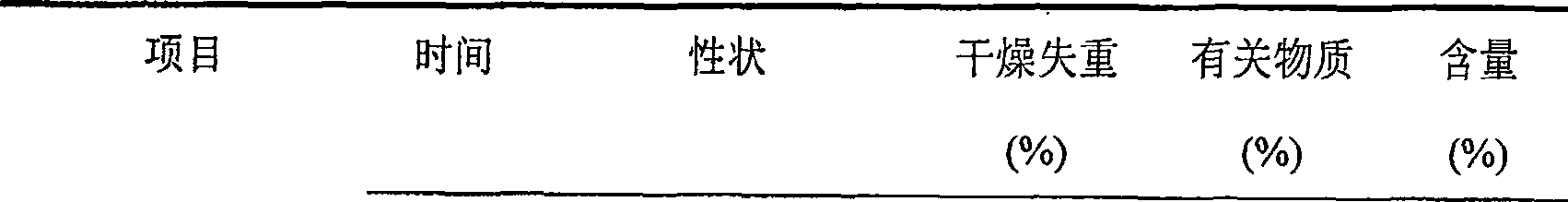
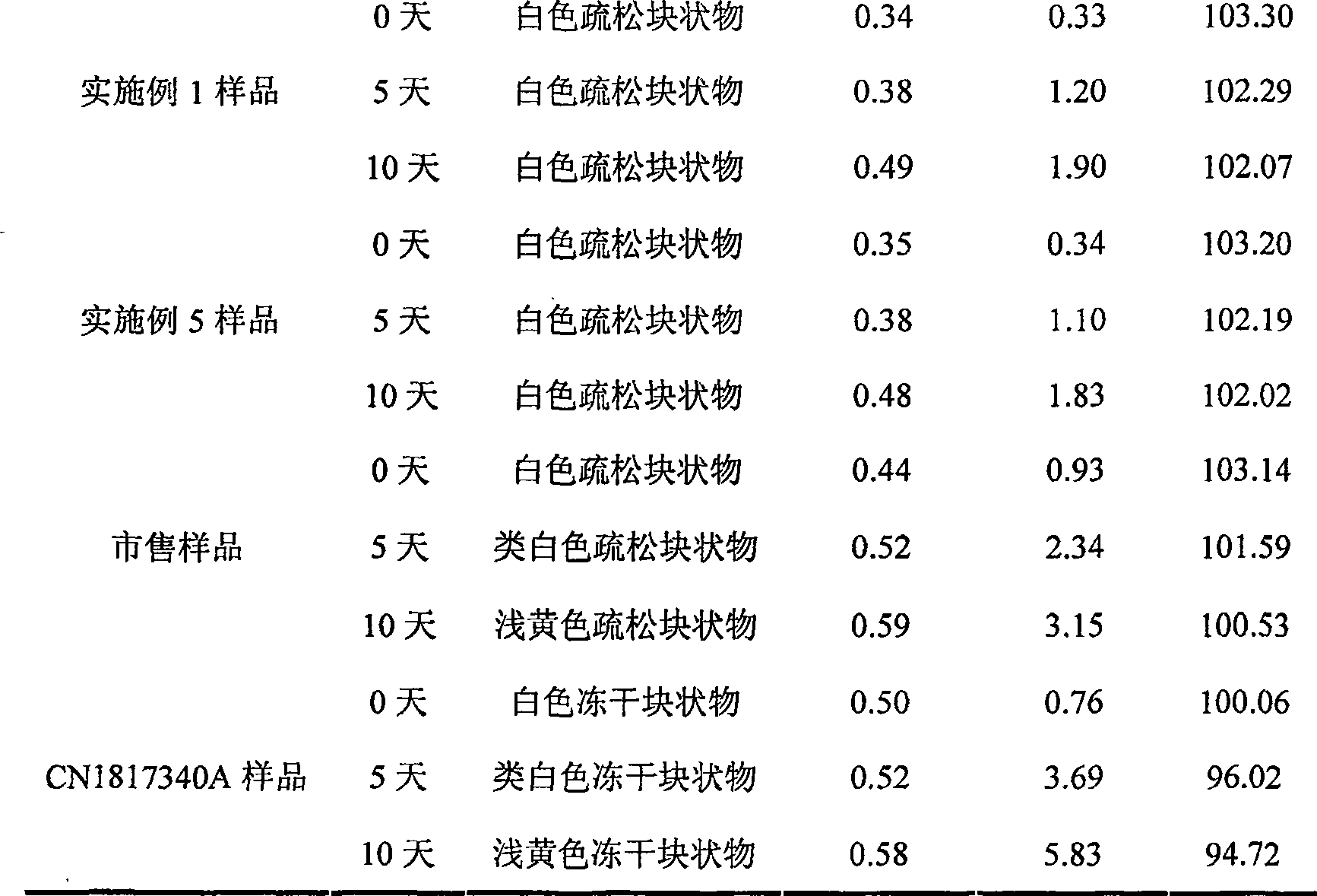
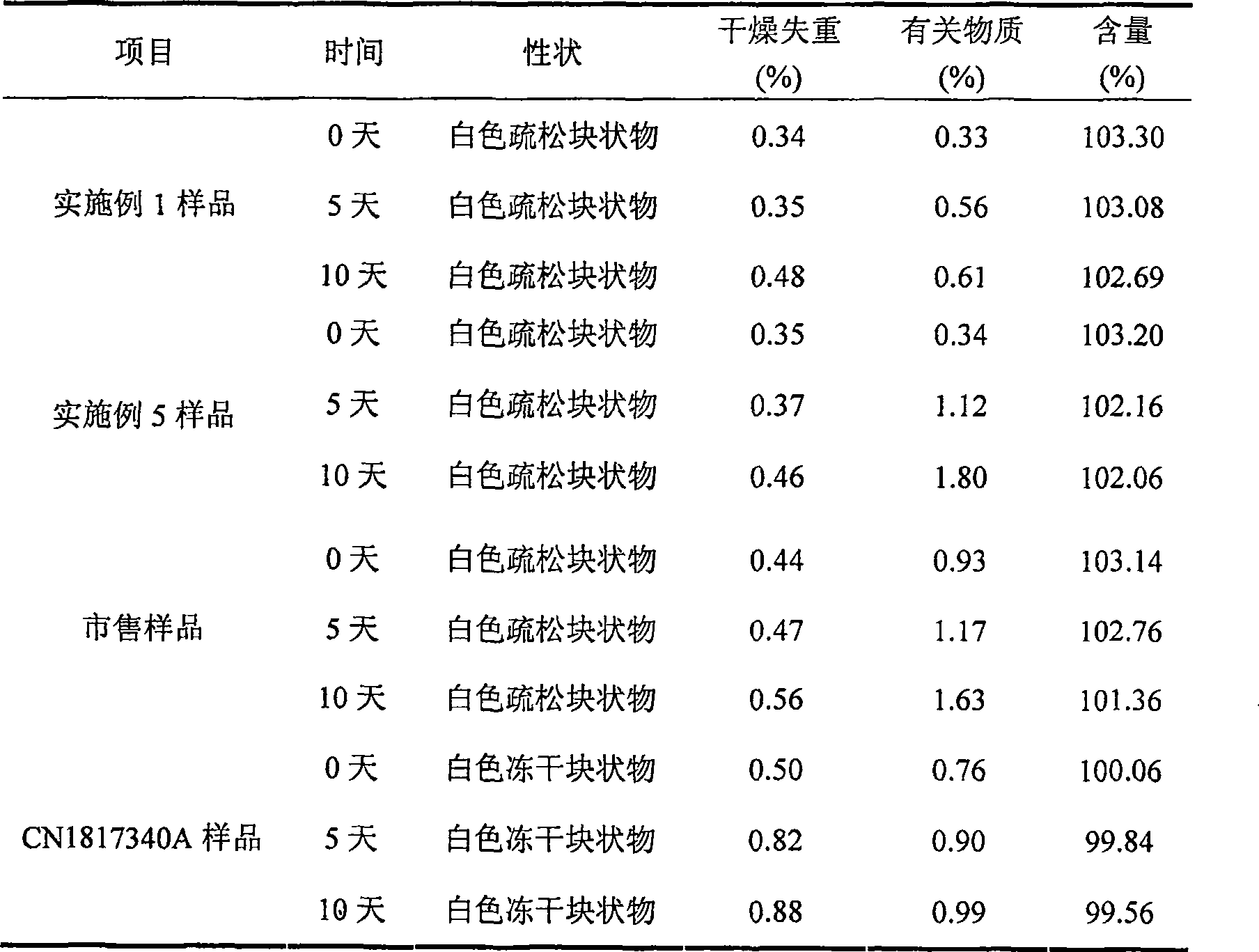
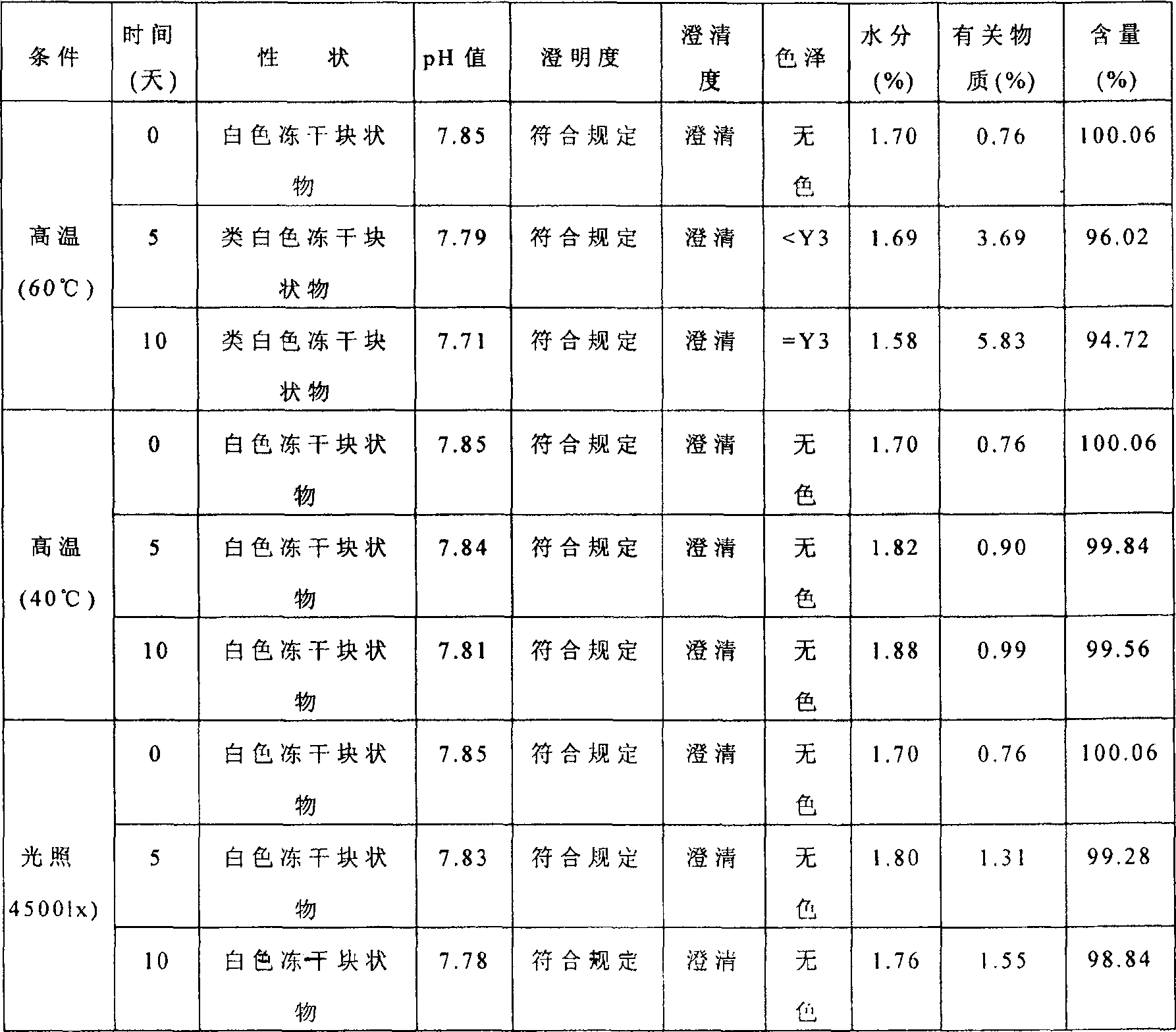
ActiveCN1817340AEasy to useImprove toleranceAntibacterial agentsPowder deliveryHydrogen phosphateFreeze-drying
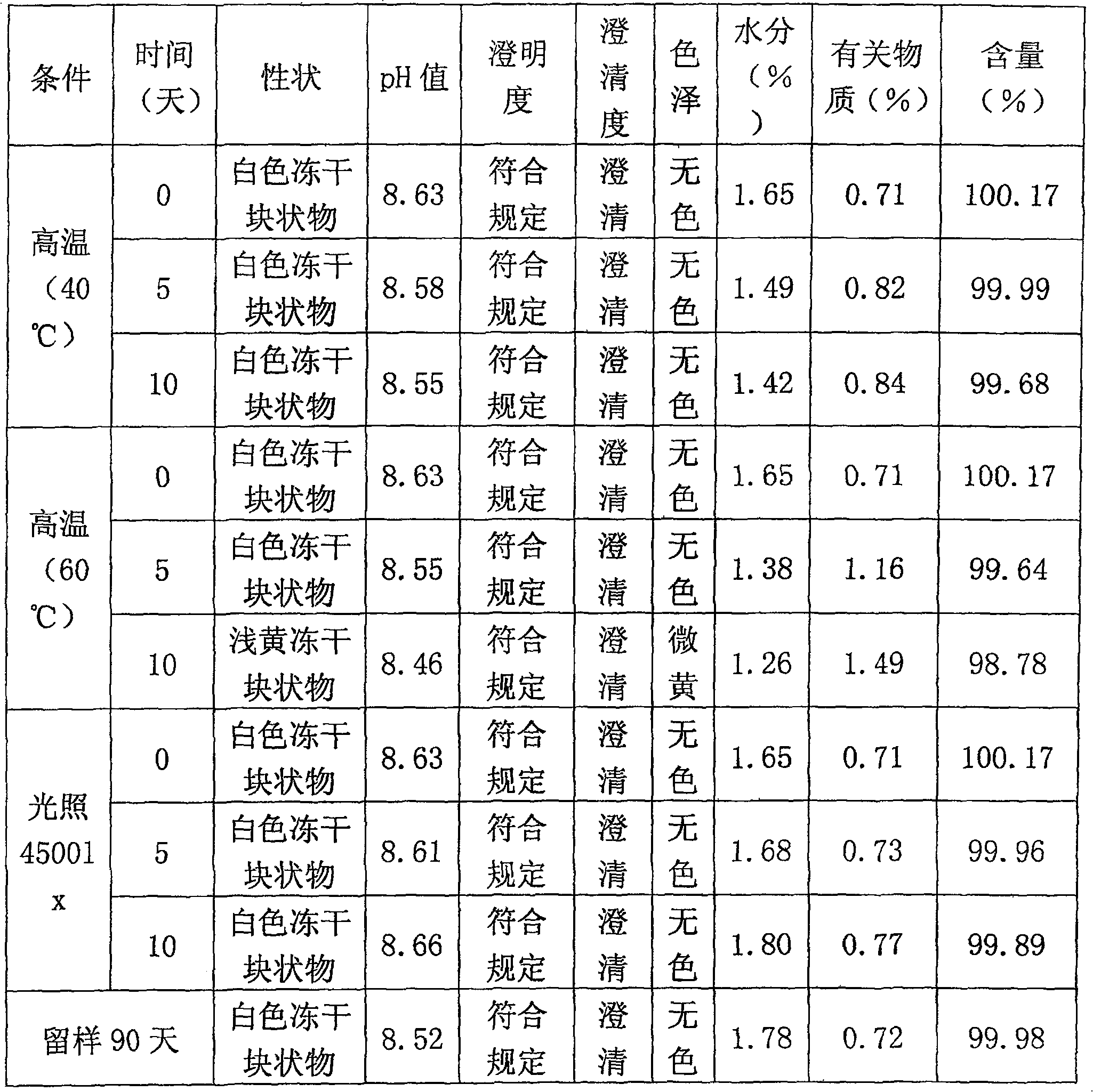
A freeze-dried powder injection of sodium fusidate composition for treating the serious Staphylococcus infection contains sodium fusidate, bisodium hydrogen phosphate and citric acid in weight ratio of 500: (78-113): (4-5.7). The water for injection is used as its solvent.
Owner:SHANDONG BESTCOMM PHARMA CO LTD
Fusidate sodium composition and preparation of its freeze-drying preparations
ActiveCN101264089AImprove stabilityEasy to useAntibacterial agentsOrganic active ingredientsArginineFreeze-drying
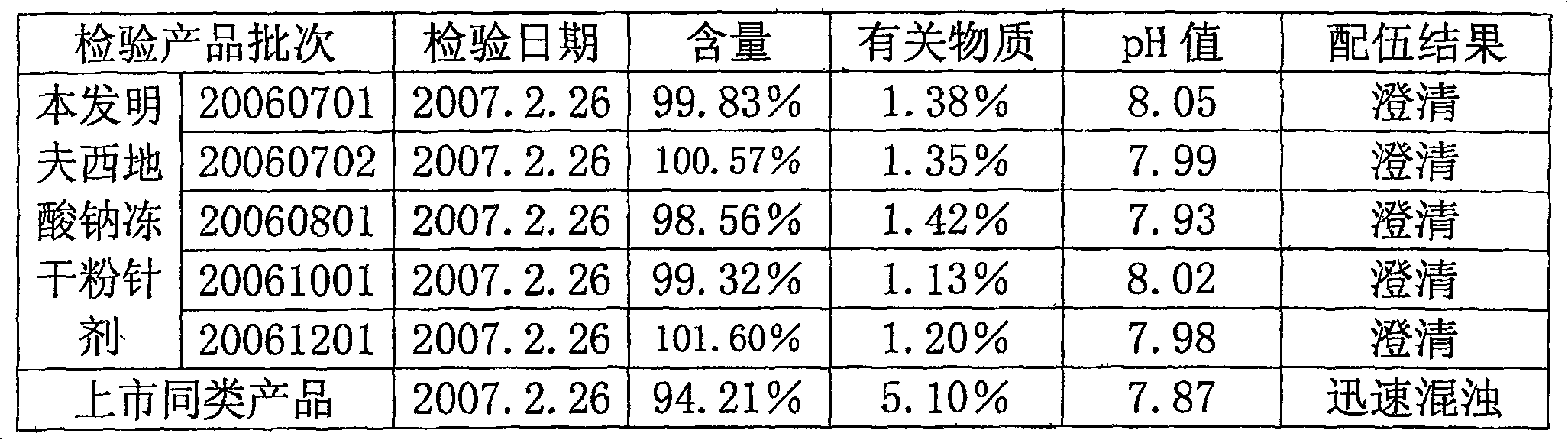
The invention relates to a sodium fusidate combination and the preparation method of freeze dried formulation. Weight ratio of the sodium fusidate, the excipient and the stabilizing agent / pH regulator in the sodium fusidate combination is 25 to 100 : 2 to 100 : 1 to 50, wherein, preferred excipient is glucose and / or mannitol; the stabilizing agent / pH regulator is one from arginine, ethylene diamine tetraacetic acid disodium and EDTA calcium disodium or the mixture. The preparation method is characterized in that: water is used as solvent; the sodium fusidate, the excipient and the stabilizing agent / pH regulator are made into clear and transparent solution before filtered and subpacked, thereby producing freeze dried formulation being suitable for requirement of injection through drying by sublimation, wherein, pH value of the formulation is 7.5 to 9.0 and the formulation of each unit dose comprises 125 to 500mg sodium fusidate. The sodium fusidate combination and the preparation method of freeze dried formulation have the advantages of good stability, convenient use, improvement of drugs tolerance upon patients and simple medical operation, strong practicability, simple technology and good stability of finished product.
Owner:四川阳光润禾药业有限公司
Novel Skin Cream Using Sodium Fusidate, Antifungals, and Steroids
InactiveCN102292080AStable manufacturingAvoid rapid degradationAntibacterial agentsAntimycoticsAntifungalSkin Cream
The present invention discloses a skin cream comprising an antifungal agent, a steroid and an antibacterial agent in the form of fusidic acid formed in situ with sodium fusidate as a starting material, wherein fusidic acid Sodium acid is converted to fusidic acid in the absence of oxygen. The cream of the present invention has higher shelf-life stability and finer API particle size than conventional creams containing fusidic acid. The cream of the present invention comprises as API fusidic acid and steroids which have been formed in situ from sodium fusidate in a cream base comprising acids, co-solvents, emulsifiers and waxy materials and Water, preferably purified water.
Owner:苏鲁・苏布拉马尼・瓦南加穆迪
Sodium fusidate crystallization method
The invention provides a sodium fusidate crystallization method which comprises the following steps: a, preparing a sodium fusidate solution by taking a acetone water solution as solvent; b, heating the sodium fusidate solution, feeding acetone while stirring, reducing the feeding rate when the sodium fusidate solution is turbid, and standing for crystallization after the feeding operation is finished; and performing solid-liquid separation, leaching the obtained solids with acetone, and then drying to obtain sodium fusidate crystals. According to the invention, the method is simple to operate and low in cost; and the sodium fusidate crystals prepared by the method are stable in crystal form and favorable in flowability.
Owner:NCPC NEW DRUG RES & DEV
Medicinal fusidic acid cream made using sodium fusidate and incorporating a biopolymer and a process to make it
ActiveUS20120040946A1Improve shelf life stabilityFine granularityAntibacterial agentsOrganic active ingredientsBiopolymerAllergy
The present invention is directed to a medicinal composition for treating bacterial skin infections and related wounds, and also other skin wounds including those caused by burns. The cream also causes skin rejuvenation through an epithelisation process. The cream comprises a) a biopolymer in the form of Chitosan, b) an Active Pharmaceutical Ingredient (API), in the form of fusidic, c) a cream base, and d) water. The invention also discloses a process to make the medicinal cream in which Fusidic acid which is formed in situ from Sodium Fusidate as the starting raw material, by converting it into Fusidic acid under oxygen-free environment created using inert gas, preferably nitrogen. The cream produced by the process of the present invention has greater shelf-life stability and the finer particle size of the API than the conventional creams containing Fusidic acid and found to be surprisingly superior for use against skin infections with allergy & itching, & wounds on human skin than alternative creams currently available.
Owner:SULUR VANANGAMUDI SUBRAMANIAM +4
Sodium fusidate crystal and preparation method thereof
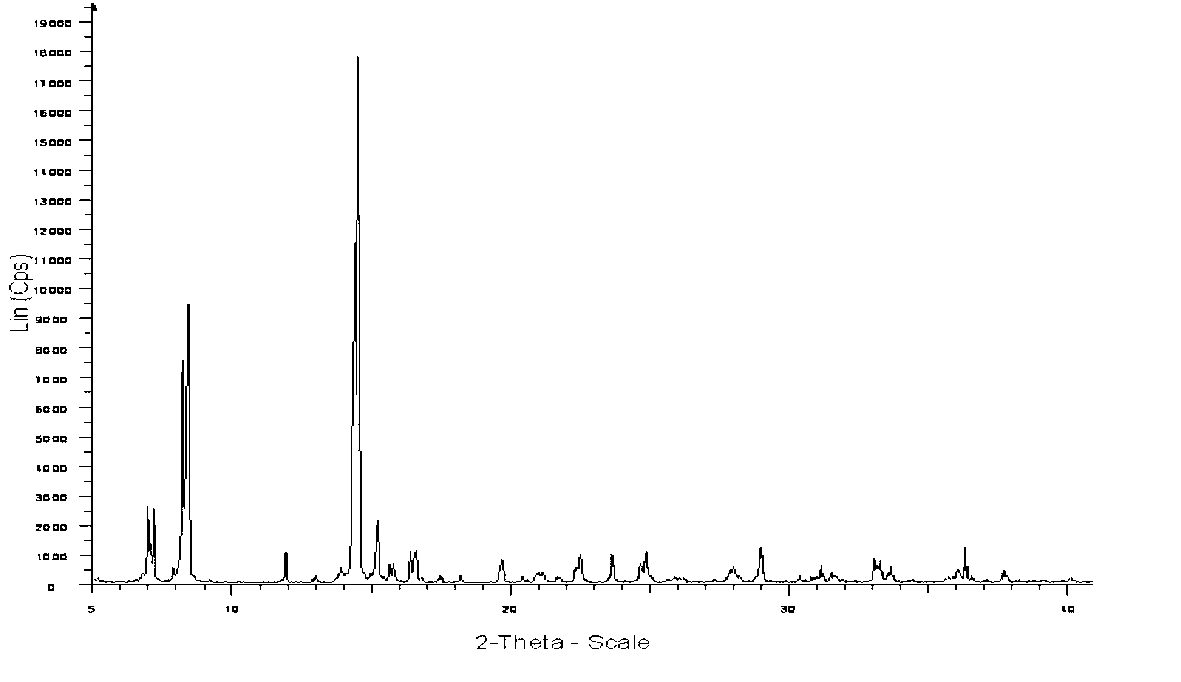
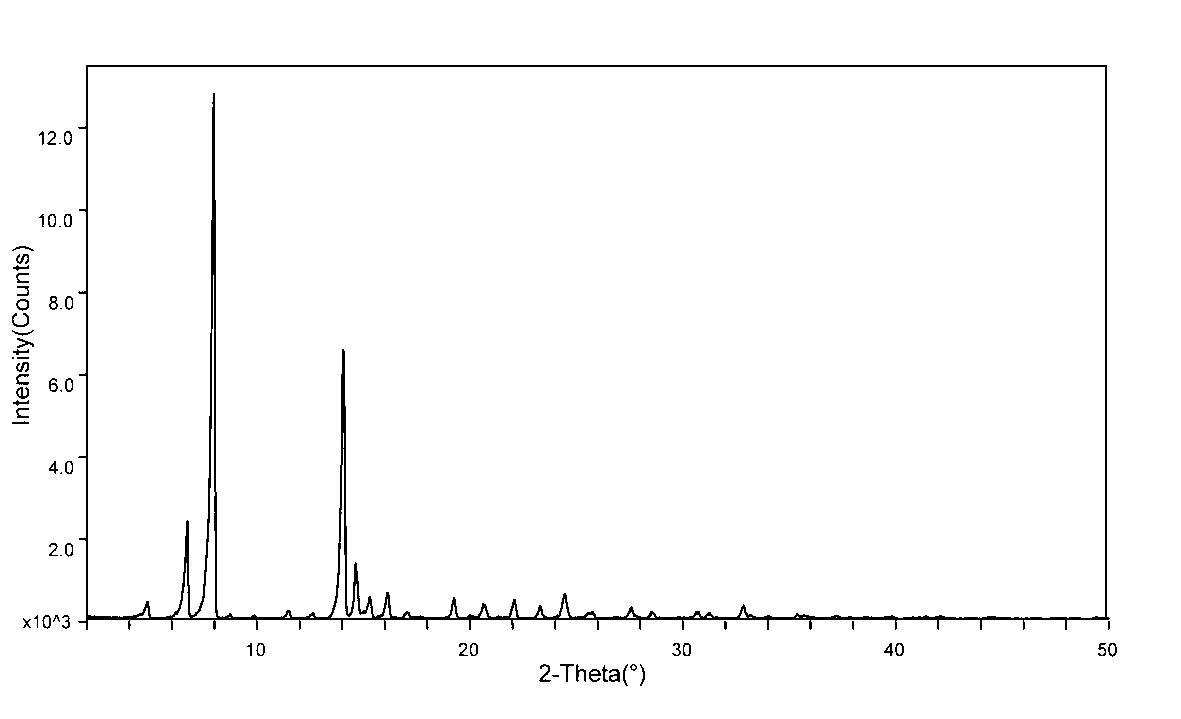
The invention relates to a sodium fusidate crystal and a preparation method thereof. The X-ray powder diffraction spectrum characteristic peaks of the sodium fusidate crystal newly provided by the invention, represented by 2theta+ / -0.2 degrees, are at 6.960, 7.117, 8.180, 8.355, 11.895, 14.460, 15.176, 16.389, 16.574, 24.837, 28.967 and 36.344. The preparation method comprises the following steps of: 1) dissolving fusidic acid in a low alcohol solution to obtain a sodium fusidate solution; 2) feeding an ethyl acetate solution into the sodium fusidate solution to crystallize sodium fusidate, and collecting a solid phase; and 3) drying the solid phase, thus obtaining a sodium fusidate crystal. The sodium fusidate crystal provided by the invention has the advantages of being glittering and translucent in appearance, uniform in granule, stable in crystal form, favorable for sub-package and storage, and the like. The method provided by the invention has the advantages that the prepared sodium fusidate crystal is good in stability, the process is simple in process and environment-friendly, and the adopted solvent is reusable and recoverable, low in production cost and the like.
Owner:NCPC NEW DRUG RES & DEV
Sodium fusidate powder-injection pharmaceutical composition for injection and preparation method
ActiveCN104352454AGood tissue permeabilityWidely distributedAntibacterial agentsPowder deliveryNeutral Amino AcidsFreeze-drying
The invention relates to a sodium fusidate powder-injection pharmaceutical composition for injection and a preparation method. Specifically, the invention belongs to the technical field of medicines, relates to a medicine which can be used for treating various infections such as osteomyelitis, sepsis, endocarditis, repeatedly infected cystic fibrosis, pneumonia, skin and soft tissue infections, surgical and traumatic infections and the like caused by various sensitive bacteria, especially staphylococcus, and particularly relates to a pharmaceutical composition such as a freeze-dried powder injection prepared by taking sodium fusidate as an active component. The invention also relates to a preparation method of the pharmaceutical composition. In an implementation scheme, the invention relates to a sodium fusidate powder-injection pharmaceutical composition for injection, which comprises sodium fusidate, a neutral amino acid and an alkaline amino acid. The pharmaceutical composition has excellent pharmaceutical properties.
Owner:CHENGDU TIANTAISHAN PHARMA
Sodium fusidate freeze-dried powder injection and preparation method thereof
ActiveCN103169673AImprove stabilityReduce contentAntibacterial agentsOrganic active ingredientsFreeze-dryingArginine
The invention relates to the field of pharmaceutical preparation, and particularly discloses sodium fusidate freeze-dried powder injection and a preparation method thereof. The sodium fusidate freeze-dried powder injection disclosed by the invention is prepared from sodium fusidate, dimercaprol dimercaptopropanol, erythorbic acid, cysteine hydrochloride, phenylalanine, arginine and injection water in a freeze-drying manner. Preferably, the arginine, the dimercaprol dimercaptopropanol, erythorbic acid, cysteine hydrochloride and phenylalanine are taken as auxiliary materials of the sodium fusidate freeze-dried powder injection; stable performance of the sodium fusidate freeze-dried powder injection is improved by synergistic effect; the content of related substances is reduced; the characters of a product are maintained in a normal requirement; and safe use and long-term storage of clinical drugs are facilitated.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD
Sodium fusidate novel crystal form and preparation method and application thereof
InactiveCN112979739AImprove liquidityEasy to packAntibacterial agentsOrganic active ingredientsDrug crystalsPharmaceutical Substances
The invention relates to the technical field of medicine crystals, in particular to a sodium fusidate novel crystal form and a preparation method and application thereof. In an X-ray powder diffraction spectrogram of the novel crystal form, the sodium fusidate novel crystal form has characteristic peaks at the positions of 9.646 degrees, 10.882 degrees, 11.034 degrees, 13.840 degrees, 14.046 degrees, 16.577 degrees, 17.318 degrees, 17.687 degrees, 18.072 degrees, 19.868 degrees, 23.036 degrees, 25.489 degrees and 27.400 degrees as shown in 2 theta+ / -0.2 degrees. The novel sodium fusidate crystal form prepared by the invention has the advantages of good fluidity, convenience in subpackage and preservation and the like, can be used for preparing a sodium fusidate injection, and is favorable for improving the medicine quality and the like.
Owner:BEIJING ZHENDONG GUANGMING PHARMA RES INST
Fusidate sodium composition and preparation of its freeze-drying formulation
ActiveCN100502878CImprove stabilityEasy to useAntibacterial agentsOrganic active ingredientsDisodium EdetateArginine
The invention relates to a sodium fusidate composition and a method for preparing a freeze-dried preparation thereof. In the sodium fusidate composition, the weight ratio of sodium fusidate, excipient, stabilizer / pH regulator is 25 ~100:2~100:1~50. Wherein the excipient is preferably glucose and / or mannitol, and the stabilizer / pH regulator is preferably one of arginine, disodium edetate and calcium sodium edetate or a mixture thereof. Its preparation method is to use water for injection as a solvent, make sodium fusidate, excipients, stabilizers / pH regulators into a clear and transparent solution, filter and subpackage, and freeze-dry to make a freeze-dried preparation that meets the requirements of injections . The pH value of the preparation is 7.5-9.0. The preparation per unit dose contains 125-500 mg of sodium fusidate. The freeze-dried powder preparation of the sodium fusidate composition of the present invention has good stability, is convenient to use, improves the drug tolerance of patients and the convenience of medical operation, and has strong practicability. The preparation method of the invention has the advantages of simple process and good stability of finished products.
Owner:北京鑫诺康桥药物研究有限公司
Composition of sodium fusidafe for injection and preparing method thereof
ActiveCN100493610CEasy to useImprove toleranceAntibacterial agentsPowder deliveryHydrogen phosphateFreeze-drying
A freeze-dried powder injection of sodium fusidate composition for treating the serious Staphylococcus infection contains sodium fusidate, bisodium hydrogen phosphate and citric acid in weight ratio of 500: (78-113): (4-5.7). The water for injection is used as its solvent.
Owner:SHANDONG BESTCOMM PHARMA CO LTD
Sodium fusidate ointment medicine composition and preparing method thereof
ActiveCN104758242ASolve the problem of being easily oxidized and degradedGuaranteed contentAntibacterial agentsOrganic active ingredientsAlcoholMedicine
The invention belongs to the technical field of medicines, and mainly relates to a sodium fusidate ointment medicine composition and a preparing method thereof. The composition comprises, by weight, 1%-10% of sodium fusidate, 80%-90% of ointment matrix, 0.2%-15% of emulgator, 0.1%-5% of antioxygen and the balanced distilled water and ethyl alcohol. The preparing method includes the steps of oil phase preparation, aqueous phase preparation, ointment mixing, ointment filling and the like. By means of the sodium fusidate ointment medicine composition and the preparing method, the problem that sodium fusidate is easily oxidized and degraded under the influences of the high temperature in the ointment preparation process is solved, generation of impurities is effectively controlled, the content of the sodium fusidate in the product can be stably kept, the product quality is improved, the local untoward effect of the skin during clinical medication is reduced, and the effectiveness and the safety of the medicine in clinic application are better guaranteed.
Owner:SICHUAN HAISCO PHARMA CO LTD
Novel dermaceutical cream made using sodium fusidate and steroids
ActiveUS20110281830A1Improve stabilityProcess stabilityOrganic active ingredientsBiocideCream baseAntibacterial agent
The invention discloses a dermaceutical cream containing steroids and an antibacterial agent in the form of Fusidic acid, which Fusidic acid is formed in situ from Sodium Fusidate as the starting raw material, wherein Sodium Fusidate is converted into Fusidic acid under oxygen-free environment. The cream of the present invention has greater shelf-life stability and the finer particle size of the API than the conventional creams containing Fusidic acid. The cream of the present invention contains Fusidic acid as the API that has been formed in situ from Sodium Fusidate, and steroids in a cream base comprising an acid, a co-solvent, an emulsifier and a waxy material along with water, preferably purified water.
Owner:SULUR VANANGAMUDI SUBRAMANIAM +4
Local anesthesia gel preparation and preparation method thereof
InactiveCN105616478APrevent corruptionExtended shelf lifeAnthropod material medical ingredientsHydroxy compound active ingredientsCelastrus orbiculatusGel preparation
The invention discloses local anesthesia gel preparation and a preparation method thereof. The local anesthesia gel preparation is prepared from, by weight, 8-20 parts of Celastrus orbiculatus, 8-20 parts of benzoin, 2-2.8 parts of bee venom, 0.1-2.0 parts of etamsylate, 0.3-1.0 part of benzoate, 3-5 parts of menthol, 2-3.6 parts of diclofenac, 1.0-1.5 parts of sodium fusidate, 1.5-3.5 parts of local anesthetic, 2.3-4.0 parts of viscous polymer, 0.05-0.3 part of preservative, 0.05-0.1 part of antioxidant, 0.2-0.6 part of surfactant and 10-16 parts of gel matrix. The local anesthesia gel preparation can be used for local administration of trauma or during small external surgery, can play a good role in controlling pain, resisting bacteria, diminishing inflammation and preventing wound infection, can reduce local damage, has high affinity with skin and is convenient to use and quick in action; anesthetic action generated by the local anesthesia gel preparation can quickly seep into collenchyme and can last for a long time, and the local anesthesia gel preparation is safe and nontoxic, simple in preparation process and low in cost.
Owner:卢连伟
Medicinal fusidic acid cream made using sodium fusidate and incorporating a biopolymer, a corticosteroid, and an antifungal agent, and a process to make it.
InactiveUS20120035144A1Improve shelf life stabilityFine granularityAntibacterial agentsBiocideBiopolymerOxygen
The present invention is directed to a medicinal composition for treating skin inflammations, fungal / bacterial skin infections and related wounds, and also other skin wounds including those caused by burns. The cream also causes skin rejuvenation through an epithelisation process. The cream comprises:a) a biopolymer in the form of Chitosan, b) active Pharmaceutical Ingredients (APIs), in the form of fusidic acid that has been generated in situ from sodium fusidate Hydrocortisone acetate & clotrimazole, c) a cream base containing primary and secondary emulsifiers, waxy materials, co-solvents, acids, preservatives, buffering agents, anti oxidants, chelating agents, and humectants and d) water. The invention also discloses a process to make medicinal cream containing Fusidic acid formed in situ from Sodium Fusidate by converting it into Fusidic acid under oxygen-free environment. The cream has greater shelf-life and the finer particle size of the API than the conventional creams containing Fusidic acid.
Owner:APEX LAB PRIVATE LTD
Novel dermaceutical cream made using sodium fusidate
InactiveUS20110257144A1Improve stabilityImprove shelf life stabilityAntibacterial agentsOrganic active ingredientsAdditive ingredientChemistry
The present invention relates to primary and secondary bacterial skin infections and in particular it relates to the treatment of these infections using a Fusidic acid cream that has been made using Sodium fusidate as the starting Active Pharmaceutical Ingredient (API). The invention discloses a dermaceutical cream containing Fusidic acid which is formed in situ from Sodium Fusidate as the starting raw material, wherein Sodium Fusidate is converted into Fusidic acid under oxygen-free environment. The cream of the present invention has greater shelf-life stability and the finer particle size of the API than the conventional creams containing Fusidic acid.
Owner:APEX LAB PRIVATE LTD
Dermaceutical gel made using sodium fusidate & process to make it
The invention discloses a process to make dermaceutical gel containing Fusidic acid which is formed in situ from Sodium Fusidate as the starting raw material, wherein Sodium Fusidate is converted into Fusidic acid under oxygen-free environment comprising an inert gas, preferably nitrogen. The gel produced by the process of the present invention has greater shelf-life stability and the finer particle size of the API than the conventional creams containing Fusidic acid. The gel also contains Fusidic acid as the API that has been formed in situ from Sodium Fusidate, in a gel base; said gel base comprising a natural, semi-synthetic or synthetic polymers, a preservative, an acid, an alkali, a co-solvent, along with water, preferably purified water. The gel produced by the process of the present invention further optionally contains an ingredient selected from a group comprising, an anti oxidant, a chelating agent, and a humectant, or any combination thereof.
Owner:苏鲁・苏布拉马尼・瓦南加穆迪
Sodium fusidate freeze-drying powder injection preparation with double auxiliary materials for injection
InactiveCN107811974ASimple preparation processImprove product qualityAntibacterial agentsOrganic active ingredientsHydroxyethyl starchAdjuvant
The invention provides a freeze-dried sodium fusidate powder for injection with double auxiliary materials, relates to the field of pharmaceutical preparations and preparation methods, and mainly solves the problem of excipients in the production formula of sodium fusidate freeze-dried powder for injection in the prior art The disadvantages of long freeze-drying time during production, high drying temperature, high energy consumption, and low pass rate of finished product clarity and solution color. The sodium fusidate freeze-dried powder for injection uses double excipients, the excipients include mannitol and hydroxyethyl starch 130 / 0.4, the main drug is sodium fusidate, and the weight ratio of the main drug sodium fusidate to the auxiliary materials 100:100‑100:120, the weight ratio between mannitol and hydroxyethyl starch 130 / 0.4 is 100:70‑100:90. The above main drug and auxiliary materials are used to prepare sodium fusidate freeze-dried powder for injection. The sodium fusidate freeze-dried powder for injection provided by the invention is stable in quality, safe and effective, simple in production process, short in freeze-drying time during production, low in drying temperature, low in energy consumption, high in the clarity of the finished product and high pass rate of solution color .
Owner:刘兴付
Sodium fusidate crystallization method
The invention provides a sodium fusidate crystallization method which comprises the following steps: a, preparing a sodium fusidate solution by taking a acetone water solution as solvent; b, heating the sodium fusidate solution, feeding acetone while stirring, reducing the feeding rate when the sodium fusidate solution is turbid, and standing for crystallization after the feeding operation is finished; and performing solid-liquid separation, leaching the obtained solids with acetone, and then drying to obtain sodium fusidate crystals. According to the invention, the method is simple to operate and low in cost; and the sodium fusidate crystals prepared by the method are stable in crystal form and favorable in flowability.
Owner:NCPC NEW DRUG RES & DEV
Special injection for sodium fusidate freeze-dried powder injection, and preparation method of special injection
ActiveCN103405392AImprove stabilityEasy to useAntibacterial agentsOrganic active ingredientsFreeze-dryingSodium potassium tartrate tetrahydrate
The invention relates to the field of pharmaceutical preparations, and particularly discloses a special injection for a sodium fusidate freeze-dried powder injection, and a preparation method of the special injection. The special injection for the sodium fusidate freeze-dried powder injection comprises nitrilotriacetic acid, methionine, sodium potassium tartrate tetrahydrate, sodium metabisulfite, disodium hydrogen phosphate and water for injection. Preferably, the nitrilotriacetic acid, methionine, sodium potassium tartrate tetrahydrate, sodium metabisulfite and disodium hydrogen phosphate are adopted as compositions of the special injection for the sodium fusidate freeze-dried powder injection and are synergized so as to improve the stability performance of the dissolved sodium fusidate freeze-dried powder injection, maintain the content of sodium fusidate and the product character within the normal requirement, and be beneficial for safety use of clinical pharmaceuticals.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD
Novel dermaceutical cream made using sodium fusidate, antifungals and steroids
The invention discloses a dermaceutical cream containing antifungal agents, steroids and an antibacterial agent in the form of Fusidic acid, which Fusidic acid is formed in situ from Sodium Fusidate as the starting raw material, wherein Sodium Fusidate is converted into Fusidic acid under oxygen-free environment. The cream of the present invention has greater shelf-life stability and the finer particle size of the API than the conventional creams containing Fusidic acid. The cream of the present invention contains Fusidic acid as the API that has been formed in situ from Sodium Fusidate, and steroids in a cream base comprising an acid, a co-solvent, an emulsifier and a waxy material along with water, preferably purified water.
Owner:APEX LAB PRIVATE LTD
Dermaceutical gel made using sodium fusidate and a process to make it
InactiveUS20110301137A1Improve shelf life stabilityFine granularityAntibacterial agentsPowder deliveryOxygenAnti oxidant
The invention discloses a process to make dermaceutical gel containing Fusidic acid which is formed in situ from Sodium Fusidate as the starting raw material, wherein Sodium Fusidate is converted into Fusidic acid under oxygen-free environment comprising an inert gas, preferably nitrogen. The gel produced by the process of the present invention has greater shelf-life stability and the finer particle size of the API than the conventional creams containing Fusidic acid. The gel also contains Fusidic acid as the API that has been formed in situ from Sodium Fusidate, in a gel base; said gel base comprising a natural, semi-synthetic or synthetic polymers, a preservative, an acid, an alkali, a co-solvent, along with water, preferably purified water. The gel produced by the process of the present invention further optionally contains an ingredient selected from a group comprising, an anti oxidant, a chelating agent, and a humectant, or any combination thereof.
Owner:APEX LAB PRIVATE LTD
Sodium fusidate lipidosome injection
Owner:海南路易丹尼生物科技有限公司
Medicinal fusidic acid cream made using sodium fusidate and incorporating a biopolymer and a process to make it
ActiveUS8895542B2Improve stabilityEfficient infectionAntibacterial agentsOintment deliveryCream baseBiopolymer
Owner:SULUR VANANGAMUDI SUBRAMANIAM +4
Sodium fusidate pharmaceutical composition for injection, and preparation method thereof
ActiveCN111012747ASimple recipeLow costAntibacterial agentsOrganic active ingredientsFreeze-dryingSodium Chloride Injection
Owner:SICHUAN HAISCO PHARMA CO LTD
Process to make fusidic acid cream
Owner:SULUR VANANGAMUDI SUBRAMANIAM +3
A kind of sodium fusidate ointment pharmaceutical composition and preparation method thereof
ActiveCN104758242BSolve the problem of being easily oxidized and degradedGuaranteed contentAntibacterial agentsOrganic active ingredientsAlcoholDistilled water
The invention belongs to the technical field of medicines, and mainly relates to a sodium fusidate ointment medicine composition and a preparing method thereof. The composition comprises, by weight, 1%-10% of sodium fusidate, 80%-90% of ointment matrix, 0.2%-15% of emulgator, 0.1%-5% of antioxygen and the balanced distilled water and ethyl alcohol. The preparing method includes the steps of oil phase preparation, aqueous phase preparation, ointment mixing, ointment filling and the like. By means of the sodium fusidate ointment medicine composition and the preparing method, the problem that sodium fusidate is easily oxidized and degraded under the influences of the high temperature in the ointment preparation process is solved, generation of impurities is effectively controlled, the content of the sodium fusidate in the product can be stably kept, the product quality is improved, the local untoward effect of the skin during clinical medication is reduced, and the effectiveness and the safety of the medicine in clinic application are better guaranteed.
Owner:SICHUAN HAISCO PHARMA CO LTD
Pharmaceutical composition and preparation method of sodium fusidate powder for injection
ActiveCN104352454BGood tissue permeabilityWidely distributedAntibacterial agentsOrganic active ingredientsNeutral Amino AcidsRecurrent infections
The invention relates to a pharmaceutical composition and a preparation method of sodium fusidate powder for injection. Specifically, the present invention belongs to the field of medical technology, and relates to a method that can be used to treat various infections caused by various sensitive bacteria, especially staphylococcus, such as osteomyelitis, sepsis, endocarditis, cystic fibrosis with repeated infections , pneumonia, skin and soft tissue infections, surgical and traumatic infections, etc., especially related to a pharmaceutical composition made of sodium fusidate as an active ingredient such as freeze-dried powder injection. The invention also relates to a preparation method of the pharmaceutical composition. In one embodiment, the present invention relates to a pharmaceutical composition of sodium fusidate powder for injection, which comprises sodium fusidate, neutral amino acids and basic amino acids. The pharmaceutical composition has excellent pharmaceutical properties.
Owner:CHENGDU TIANTAISHAN PHARMA
Sodium fusidate freezing-dried powder injection
ActiveCN100566704CImprove securityConducive to long-term stabilityAntibacterial agentsPowder deliveryGlycineFreeze-drying
The invention discloses a sodium fusidate freeze-dried powder injection. It comprises the following components and contents (parts by weight): 450-550 parts of sodium fusidate, 30-500 parts of glycine and 40-600 parts of arginine. The sodium fusidate freeze-dried powder injection has good long-term stability due to the addition of a certain amount of stabilizer, which improves the safety of patients when taking medicine and reduces the risk of taking medicine.
Owner:HAISCO PHARMA GRP INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com