Special injection for sodium fusidate freeze-dried powder injection, and preparation method of special injection
A technology for freeze-dried powder injection and sodium fusidate, which is applied in the field of pharmaceutical preparations, can solve the problems of change in appearance and properties, decrease in sodium fusidate content, decrease in pH value, etc., so as to improve stability and facilitate safe use. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
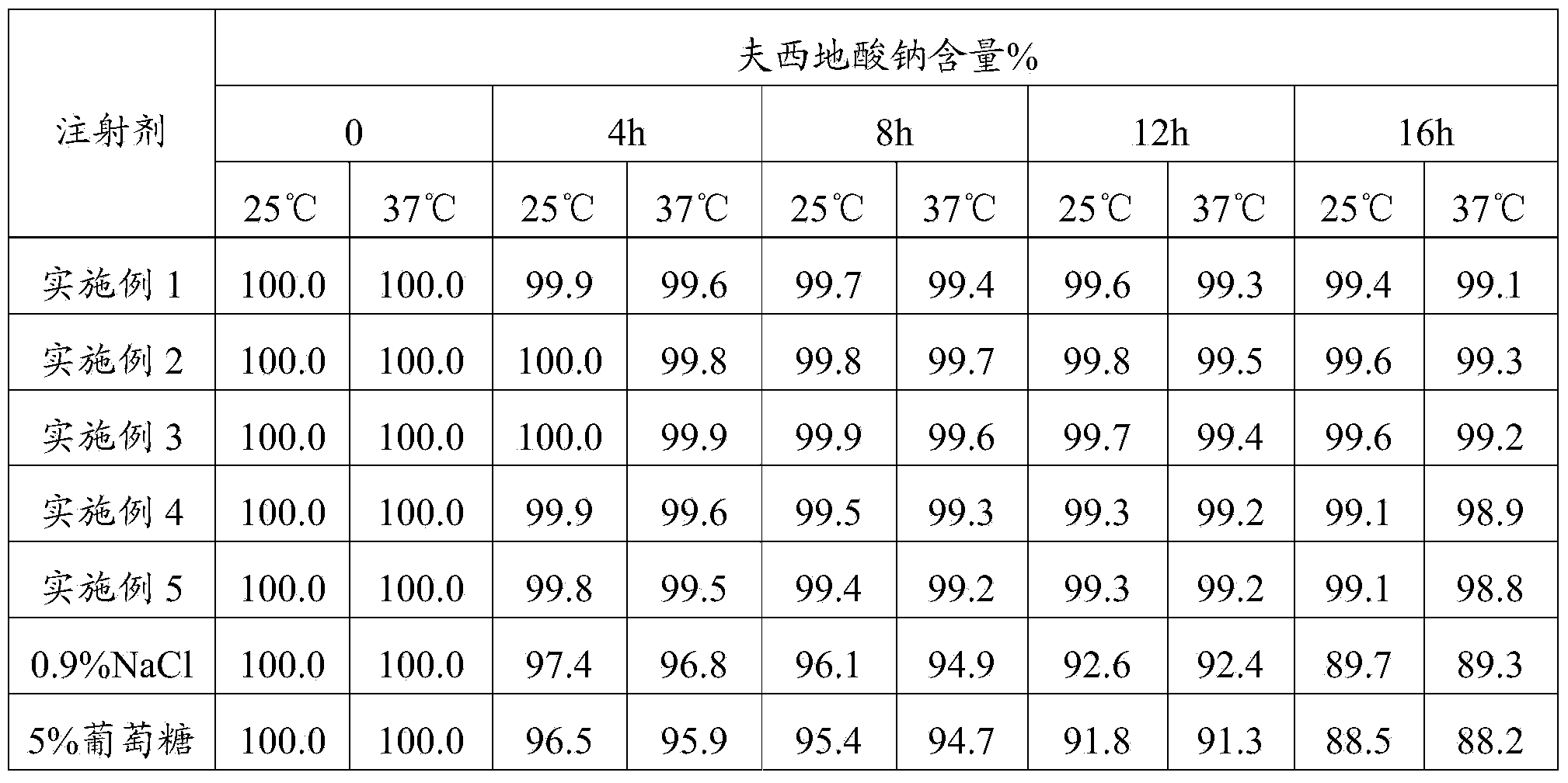
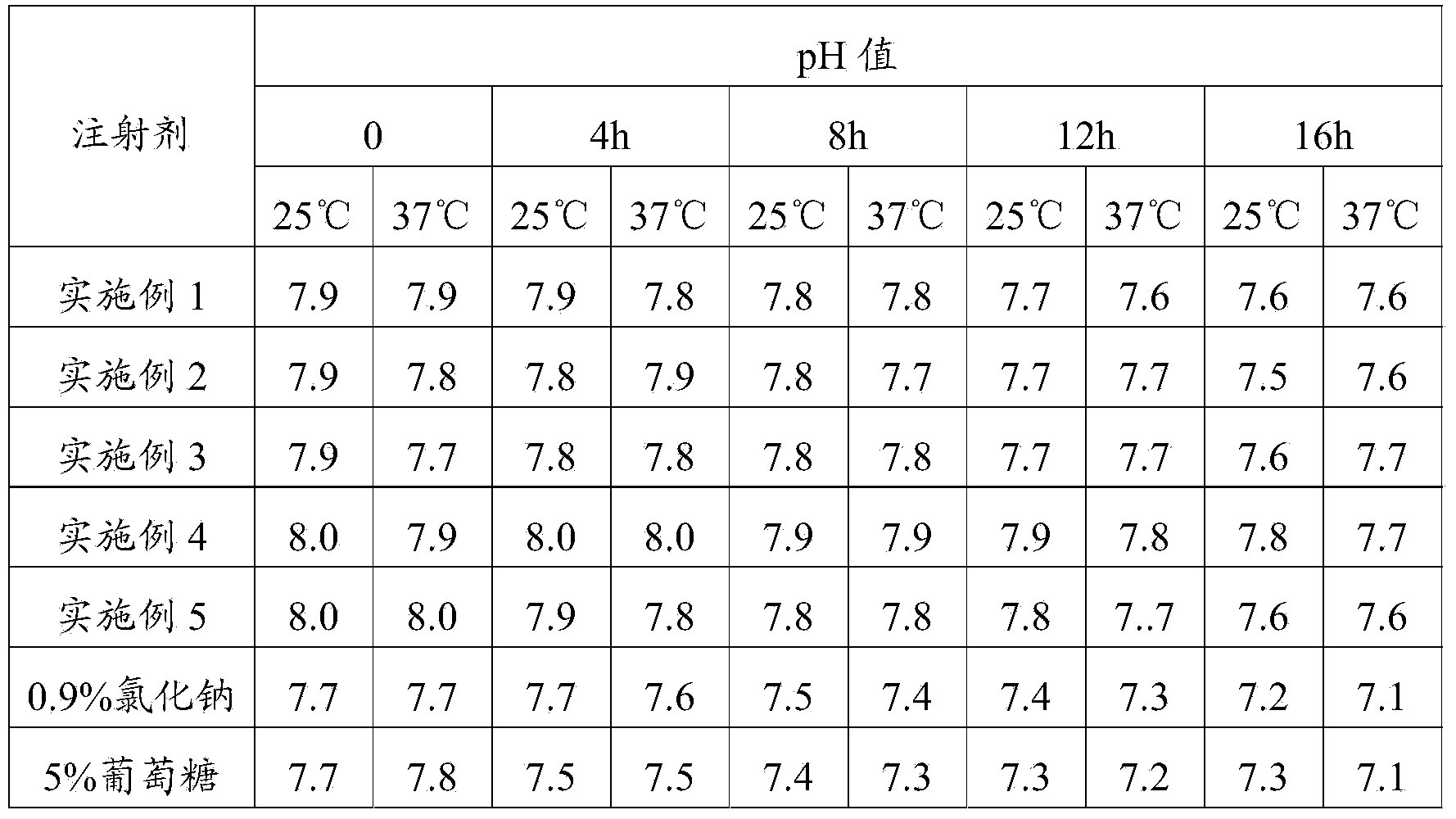
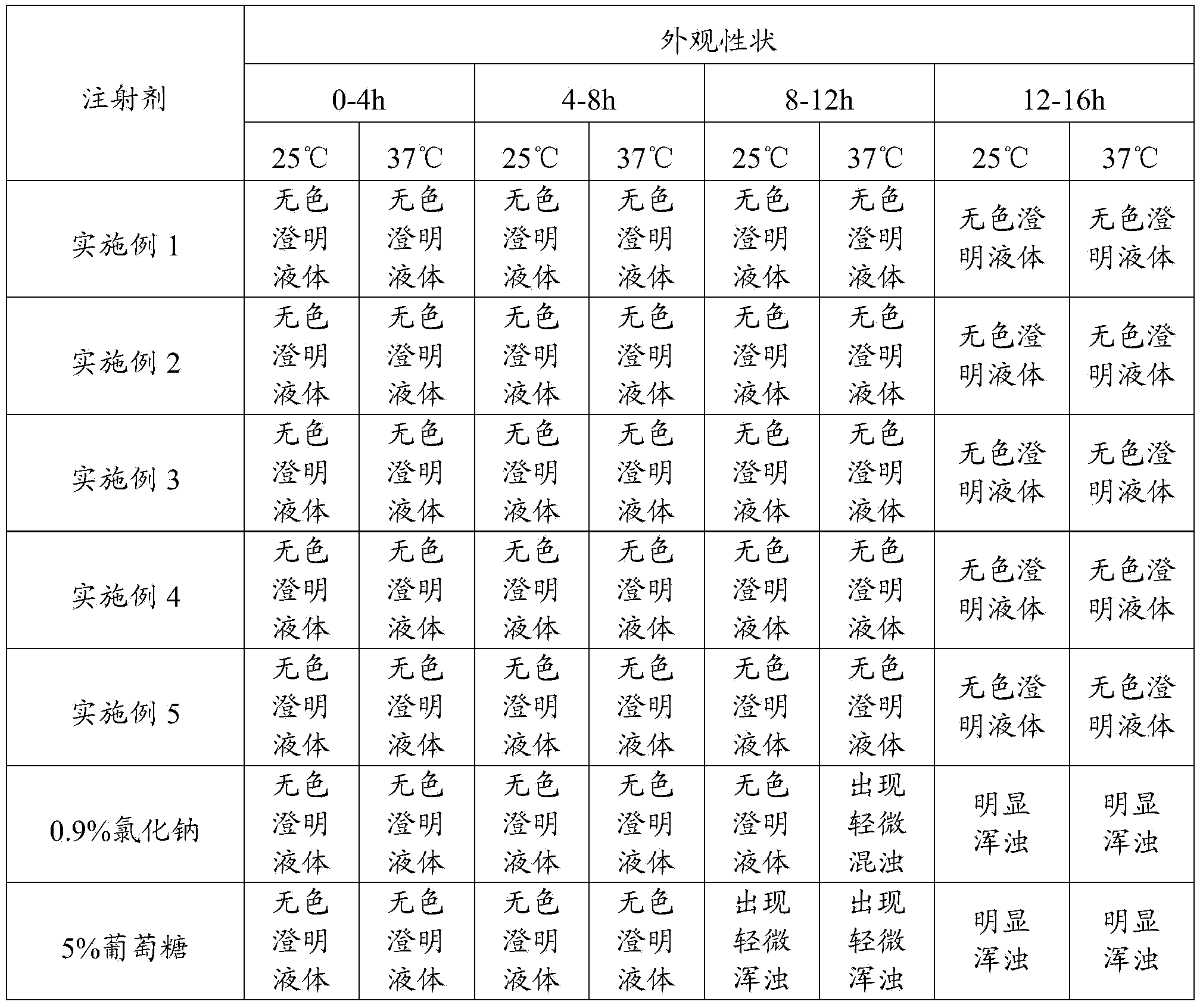
[0019] Embodiment 1: Preparation of special injection for sodium fusidate freeze-dried powder injection of the present invention
[0020] Weigh 5.0g of nitrilotriacetic acid, 5.0g of potassium sodium tartrate, 1.0g of sodium metabisulfite, and 10.0g of disodium hydrogen phosphate, stir and dissolve with 7000mL of water for injection, add 10g of methionine to adjust the pH value between 7.2 and 7.7, and then add activated carbon Stir for 15 minutes to decarbonize and filter, add water for injection to the filtrate to 10,000 mL, stir evenly, retest the pH value, fine filter, pot, and sterilize. The product of this embodiment was tested for abnormal toxicity, sterility, and bacterial endotoxin, and the test results all met the requirements.
Embodiment 2
[0021] Embodiment 2: Preparation of special injection for sodium fusidate freeze-dried powder injection of the present invention
[0022] Weigh 15.0g nitrilotriacetic acid, 10.0g potassium sodium tartrate, 2.0g sodium metabisulfite, 20.0g disodium hydrogen phosphate, stir and dissolve with 7000mL water for injection, add 20g methionine to adjust the pH value between 7.2 and 7.7, and then add activated carbon Stir for 15 minutes to decarbonize and filter, add water for injection to the filtrate to 10,000 mL, stir evenly, retest the pH value, fine filter, pot, and sterilize. The product of this embodiment was tested for abnormal toxicity, sterility, and bacterial endotoxin, and the test results all met the requirements.
Embodiment 3
[0023] Embodiment 3: Preparation of special injection for sodium fusidate freeze-dried powder injection of the present invention
[0024] Weigh 20.0g nitrilotriacetic acid, 15.0g potassium sodium tartrate, 5.0g sodium metabisulfite, 50.0g disodium hydrogen phosphate, stir and dissolve with 7000mL water for injection, add 30g methionine to adjust the pH value between 7.2 and 7.7, and then add activated carbon Stir for 15 minutes to decarbonize and filter, add water for injection to the filtrate to 10,000 mL, stir evenly, retest the pH value, fine filter, pot, and sterilize. The product of this embodiment was tested for abnormal toxicity, sterility, and bacterial endotoxin, and the test results all met the requirements.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com