Sodium fusidate freezing-dried powder injection
A technology of sodium fusidate and freeze-dried powder injection, which is applied in the field of freeze-dried powder injection of sodium fusidate, can solve the problems of affecting patient medication safety, unqualified product quality, poor long-term stability, etc. Risks, the effect of improving safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
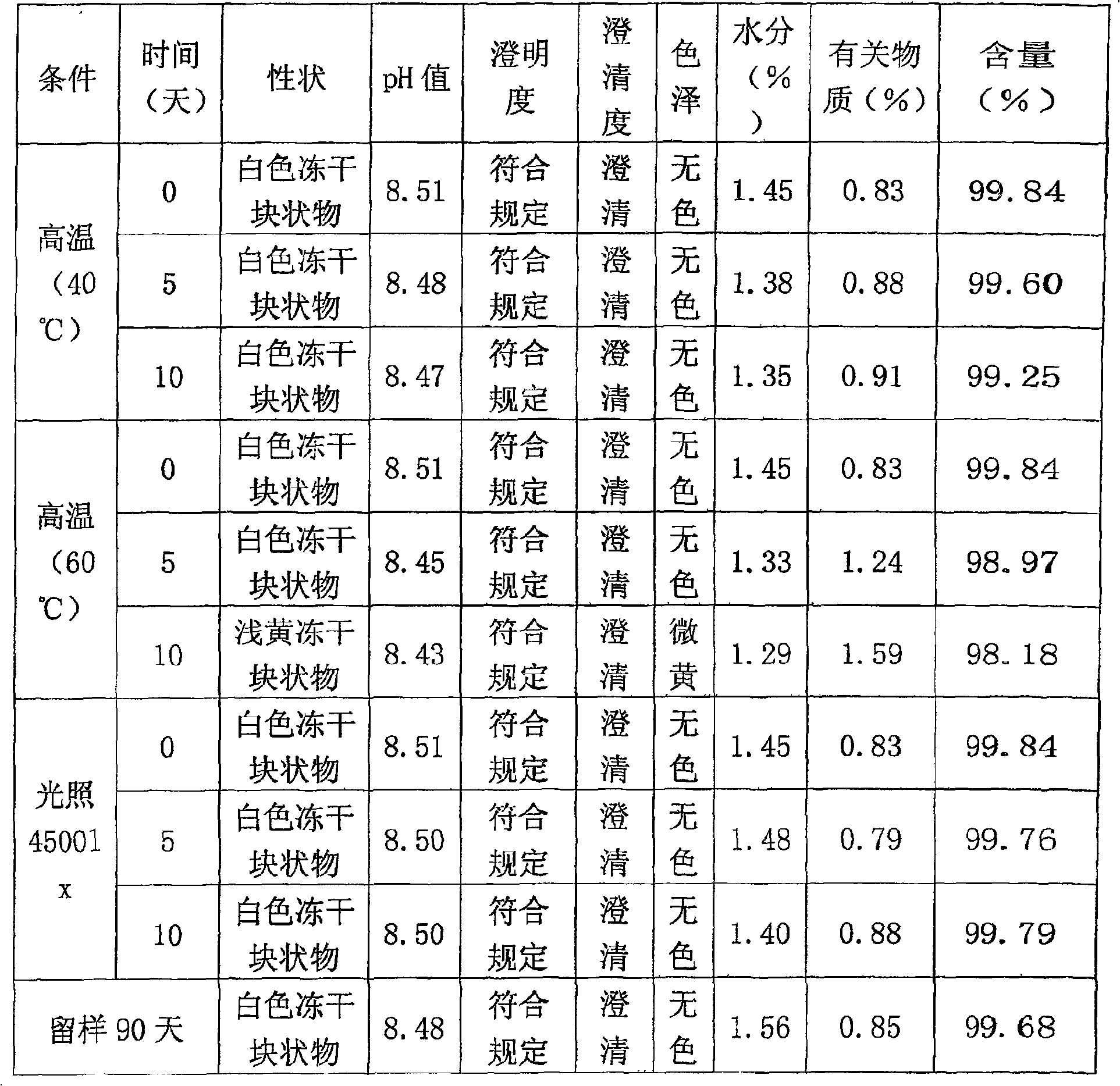
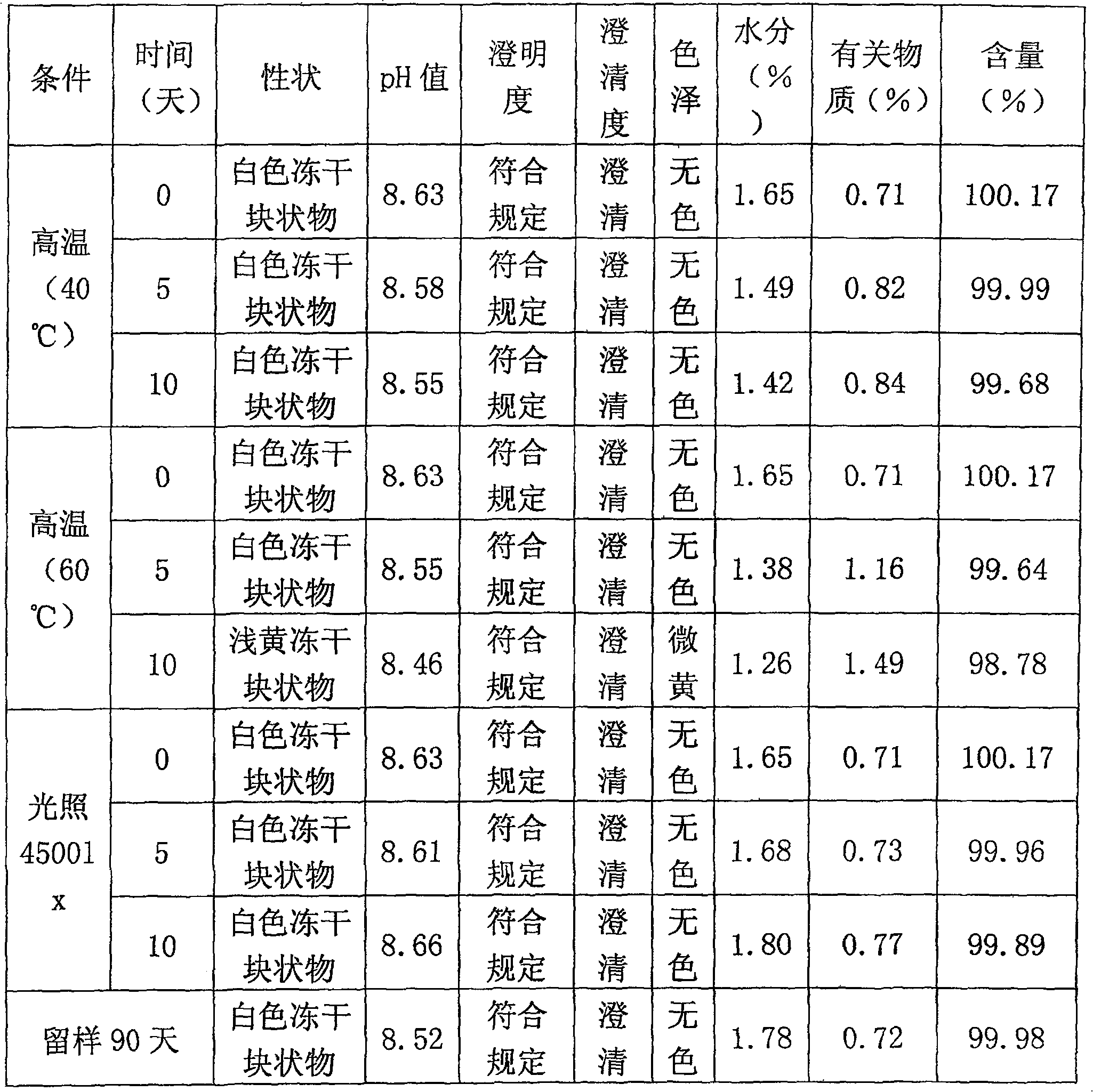
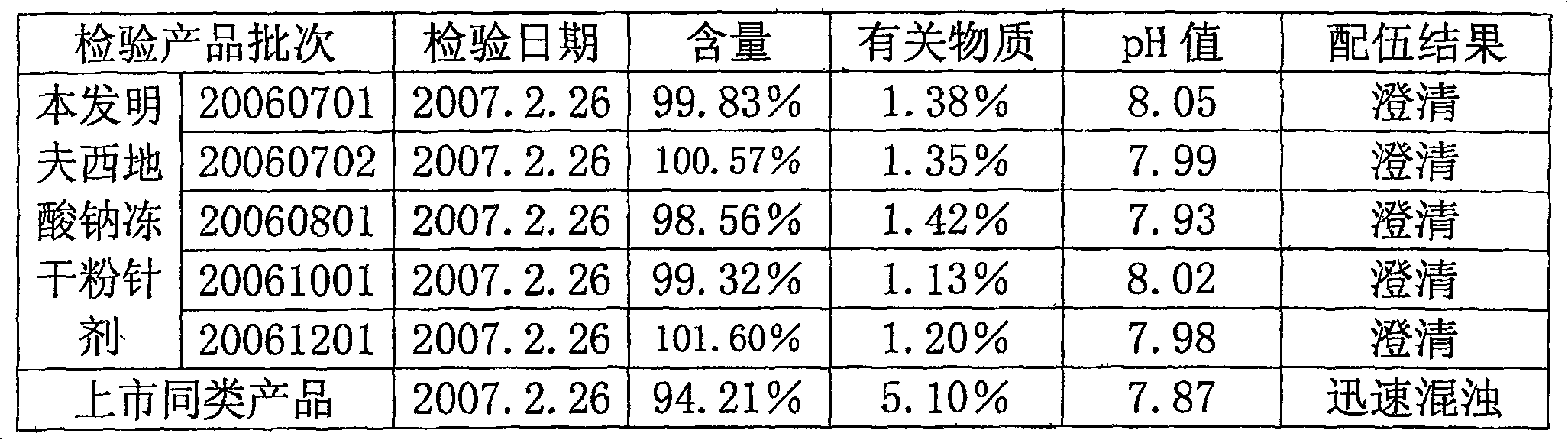
[0015] Take water for injection, let it cool to 30°C, add glycine, arginine, lactose and mannitol according to the prescription amount described in Table 1, and stir to dissolve. Adjust the pH of the solution to 8.0-9.0, add sodium fusidate in the prescription amount described in Table 1, stir to dissolve, and add water for injection to the full amount. Add special activated carbon for injection to the liquid medicine, stir for 15 minutes, decarbonize and filter. 0.22 ~ 0.45μm microporous membrane fine filter sterilization, filling liquid medicine, half cork, freeze-drying, out of the box, cork and cover. Redissolve in buffer solution (components and contents: disodium hydrogen phosphate 196mg, citric acid 10mg, EDTA-2Na 5mg, 10ml in total), and compatibility with 5% glucose injection and 0.9% sodium chloride injection results in stable selection The final dosage of agents and excipients. The research results are shown in Table 1:
[0016] Table 1 Stabilizer dosage selectio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com