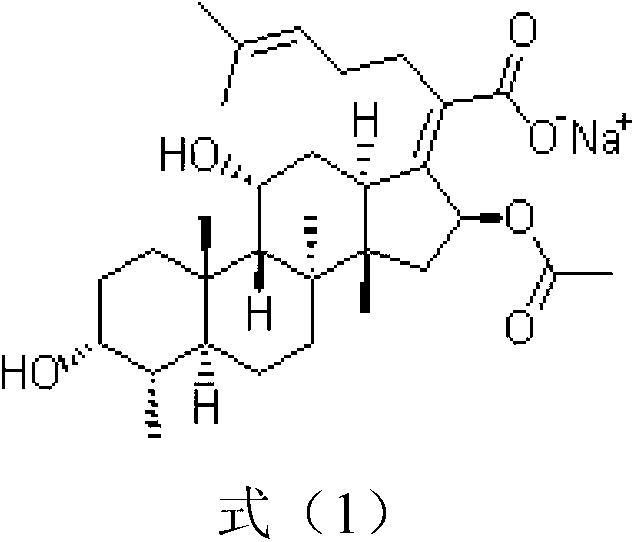
Sodium fusidate crystallization method
A technology of sodium fusidate and crystallization, applied in the directions of steroids, organic chemistry, etc., can solve the problems of poor stability of sodium fusidate crystals, unfavorable preservation and sub-packaging, and high production costs, and achieves a crystal clear appearance, The effect of uniform particles and good fluidity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Example 1 Preparation of high-quality sodium fusidate crystals by sodium fusidate solid
[0019] a. Weigh 7 g of sodium fusidate solid, and dissolve it in an aqueous solution of acetone with an acetone concentration of 80% (by volume ratio) at room temperature, and stir for 0.5-3 hours to obtain 21 mL of sodium fusidate solution;
[0020] b. Heat the above-mentioned sodium fusidate solution to 40°C and keep it warm, add acetone while stirring, and the flow rate is 20mL / h. The acceleration rate is reduced to 10mL / h. When the feeding amount of acetone is 210mL, stop feeding and let stand for 18h (crystal growth); after standing, filter and separate solid and liquid;
[0021] c. The filter cake was rinsed with acetone and dried at 40-45°C for 24 hours to obtain 6.5 g of sodium fusidate crystals, which were determined by HPLC with a purity of 99.8% and a yield of 93.2%. The obtained sodium fusidate crystal has a stable crystal form and good fluidity (measured value of spec...
Embodiment 2
[0022] Example 2 Preparation of high-quality sodium fusidate crystals from fusidic acid solids
[0023] a. Weigh 7g of fusidic acid solid, and at normal temperature, dissolve it in an aqueous solution of 50% acetone with acetone volume ratio concentration, stir until dissolved, add dropwise 4M aqueous sodium hydroxide solution under stirring, the added hydrogen The mol ratio of sodium oxide and fusidic acid is 0.8:1, obtains sodium fusidic acid solution 25mL;
[0024] b. Heat the above-mentioned sodium fusidate solution to 30°C and keep it warm, add acetone while stirring, and the flow rate is 22mL / h. When the sodium fusidate solution becomes turbid, reduce the flow rate to 6mL / h h, when the feeding amount of acetone is 250mL, stop feeding and let stand for 16h;
[0025] c. After standing still, filter with suction, rinse the filter cake with acetone and dry it at 40-42°C for 20 hours to obtain 6.3g of sodium fusidate crystals. The purity is 99.8% and the yield is determined ...
Embodiment 3
[0026] Example 3 Preparation of high-quality sodium fusidate crystals from fusidic acid solids
[0027] a. Weigh 7g of fusidic acid solid, and at normal temperature, dissolve it in an aqueous solution of acetone with acetone volume ratio concentration of 90%, stir until dissolved, then add dropwise 4M aqueous sodium hydroxide solution under stirring, and the The mol ratio of sodium oxide and fusidic acid is 1.2:1, obtains sodium fusidic acid solution 30mL;
[0028] b. Heat the above-mentioned sodium fusidate solution to 60°C and keep it warm, add acetone while stirring, and the flow acceleration rate is 24mL / h. When the sodium fusidate solution becomes turbid, reduce the flow acceleration rate to 18mL / h h, when the feeding amount of acetone is 450mL, stop feeding and let stand for 20h;
[0029] c. After standing still, filter with suction, rinse the filter cake with acetone and dry it at 44-45°C for 40h to obtain 6.6g of sodium fusidate crystals. The purity is 99.7% and the y...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com