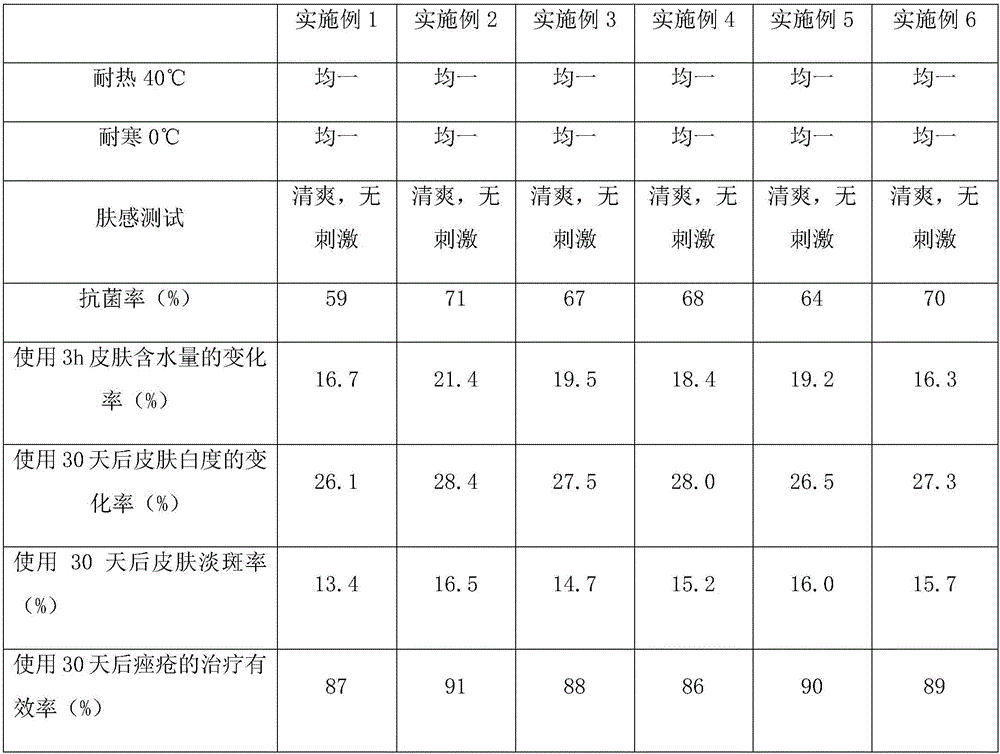
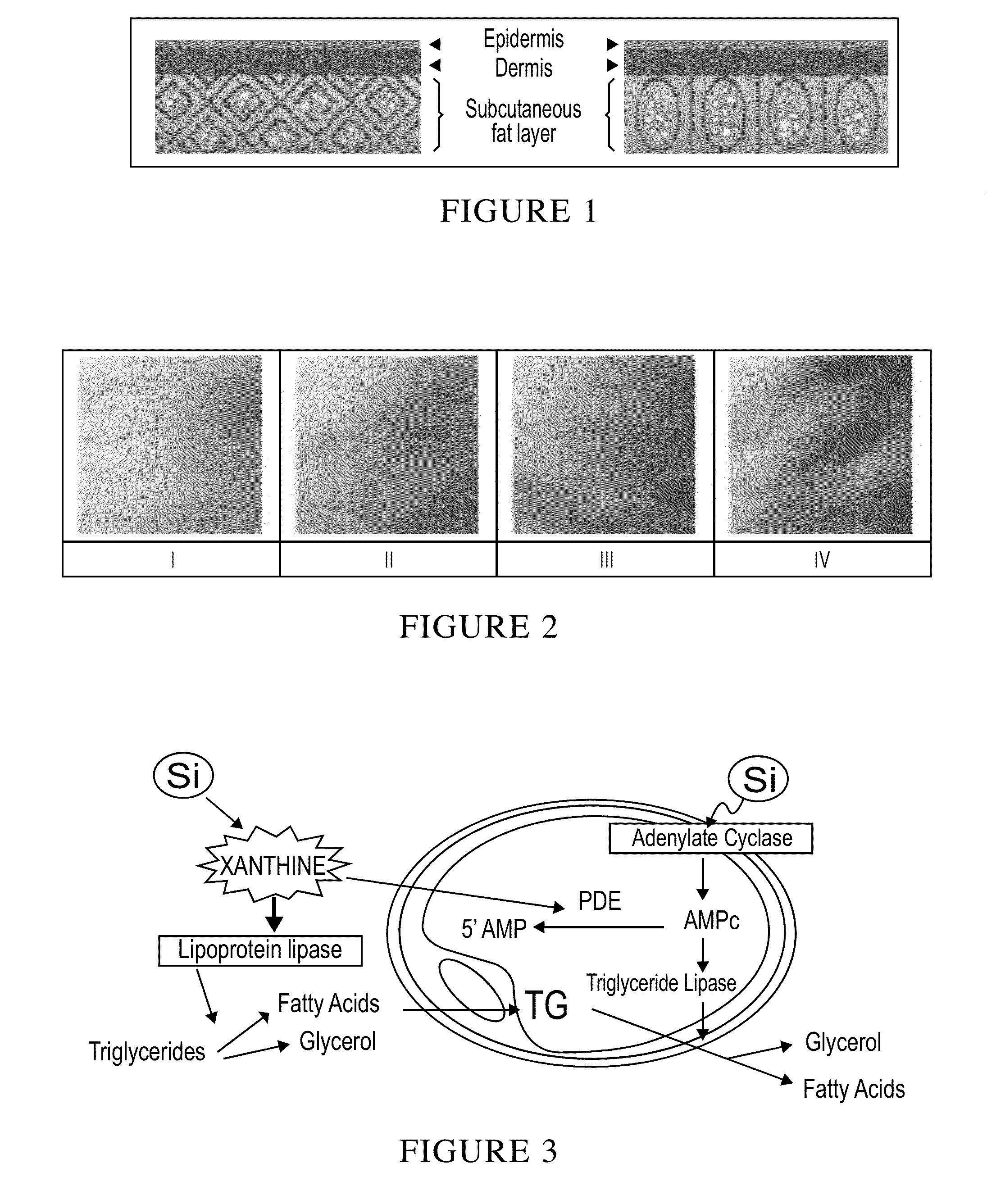
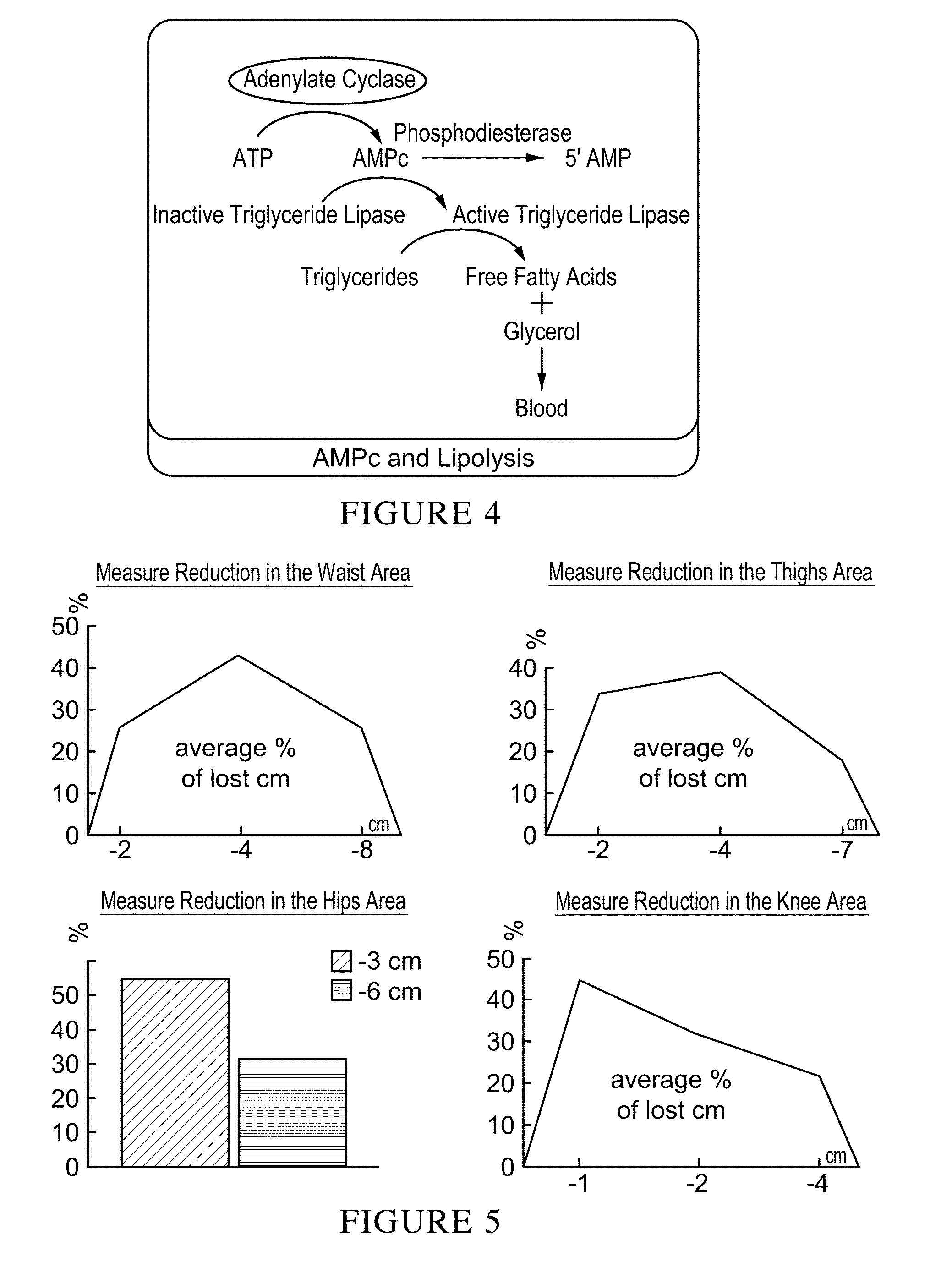
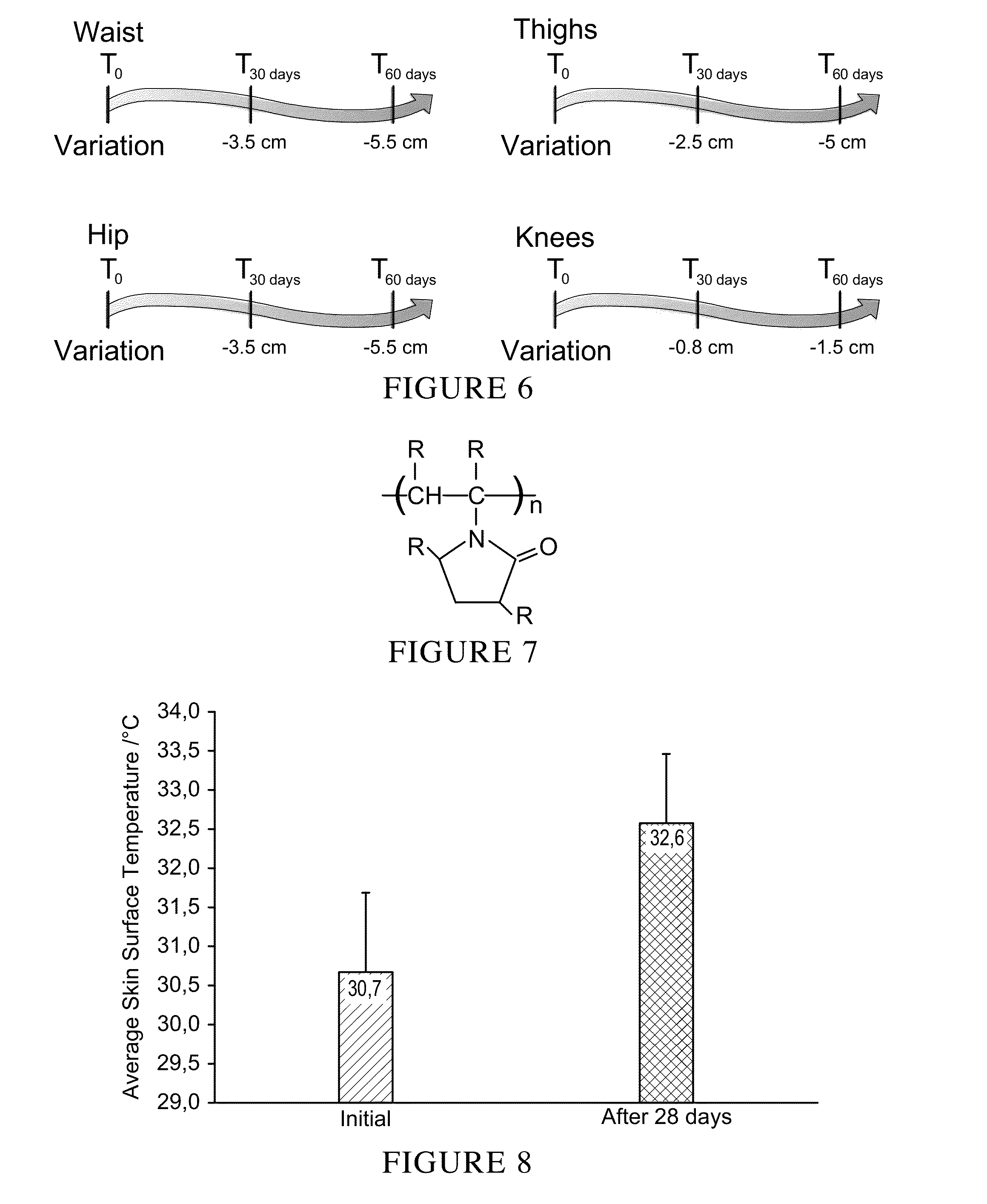
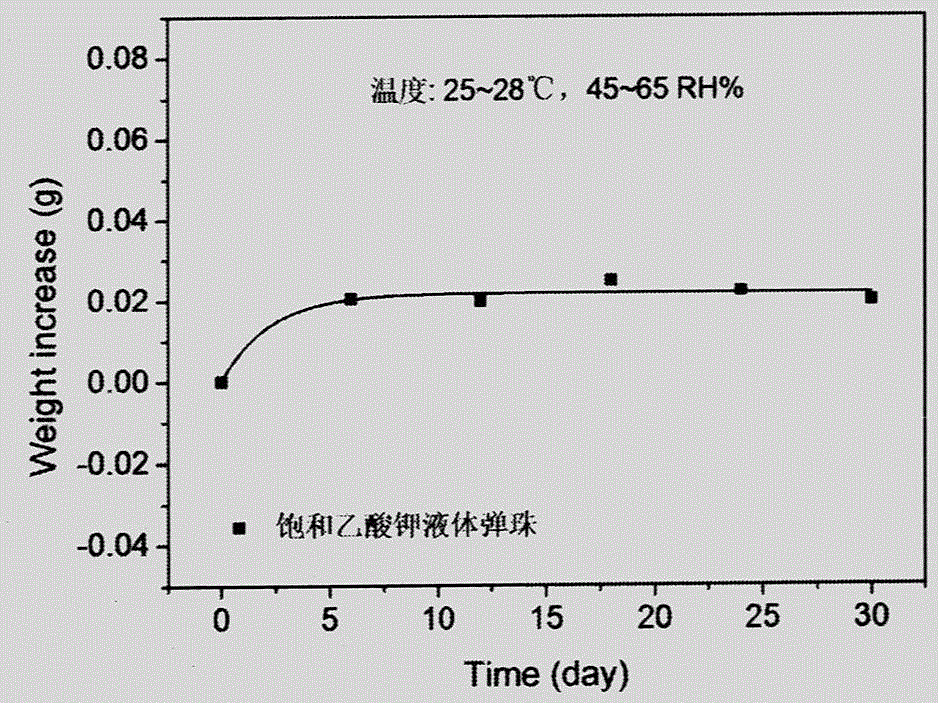
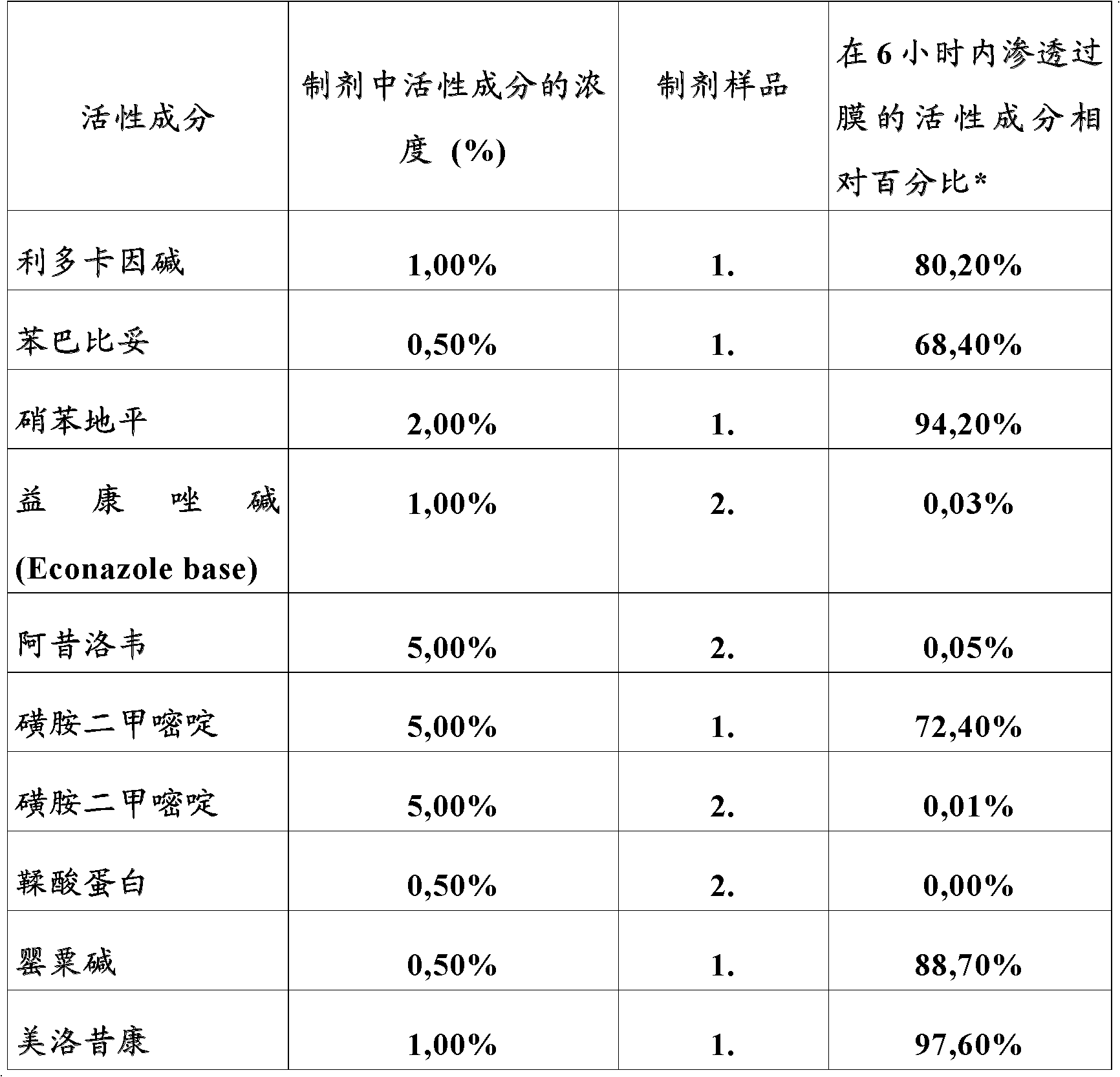
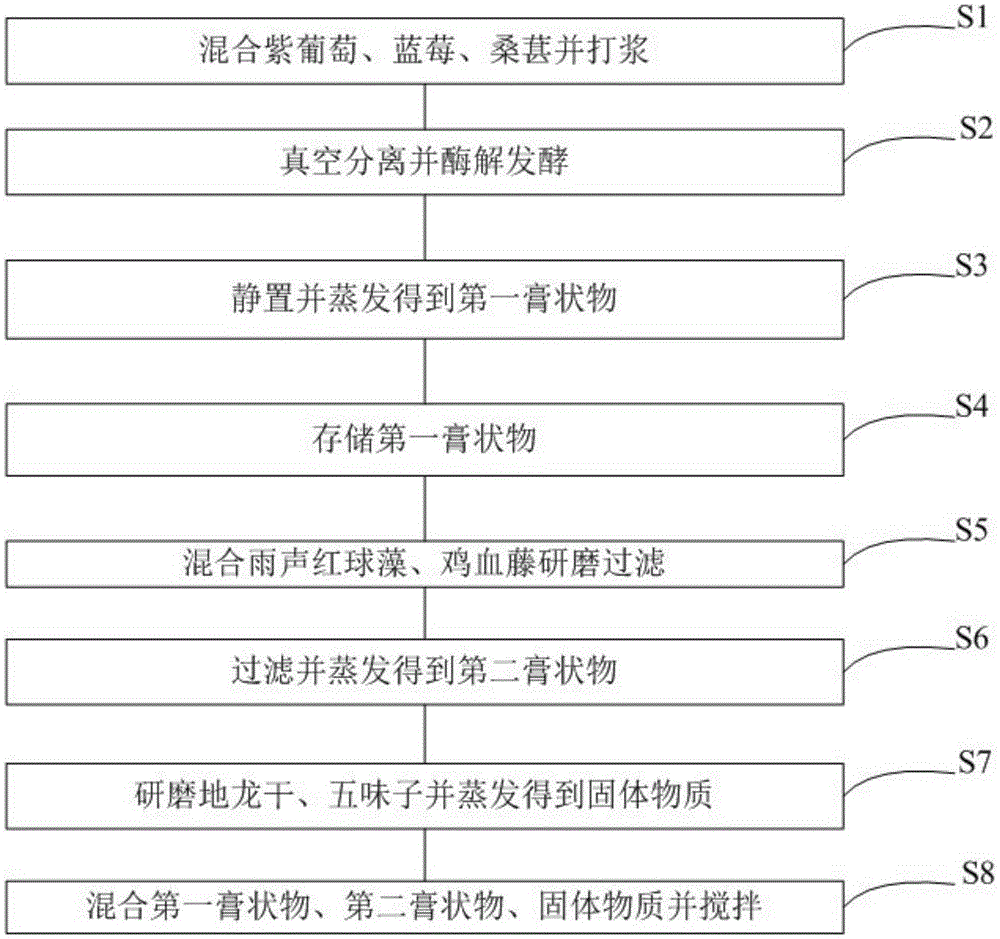
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
99 results about "Cream base" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
How to use Cream Base. Use this product as directed. Some products require priming before use. Follow all directions on the product package. If you are uncertain about any of the information ...
Transdermal hormone composition and combined static-cyclic delivery
InactiveUS20140031323A1Facilitates prescribing and patient educationSimulating the temporal variationsBiocideOrganic active ingredientsCream baseRegimen
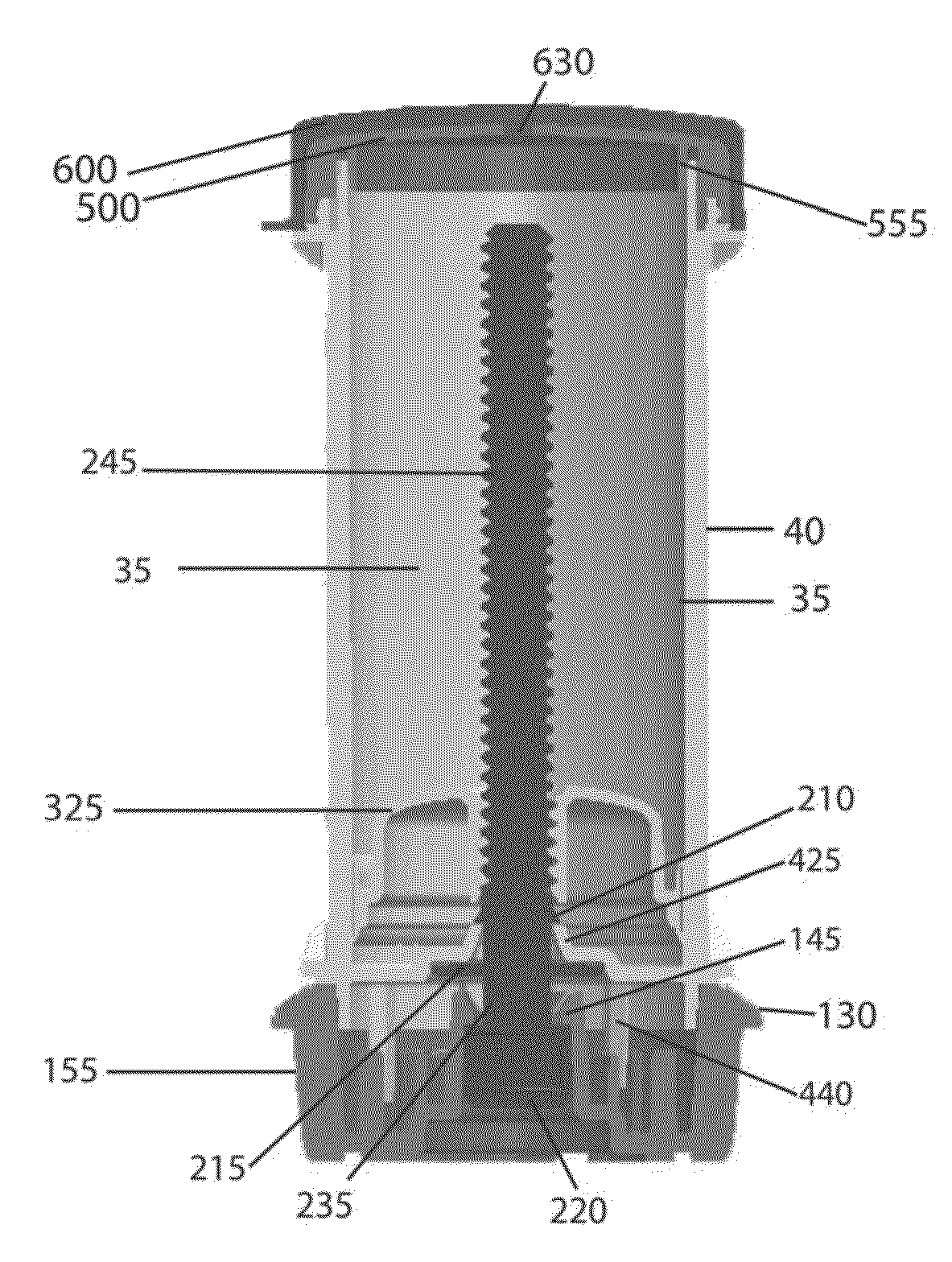
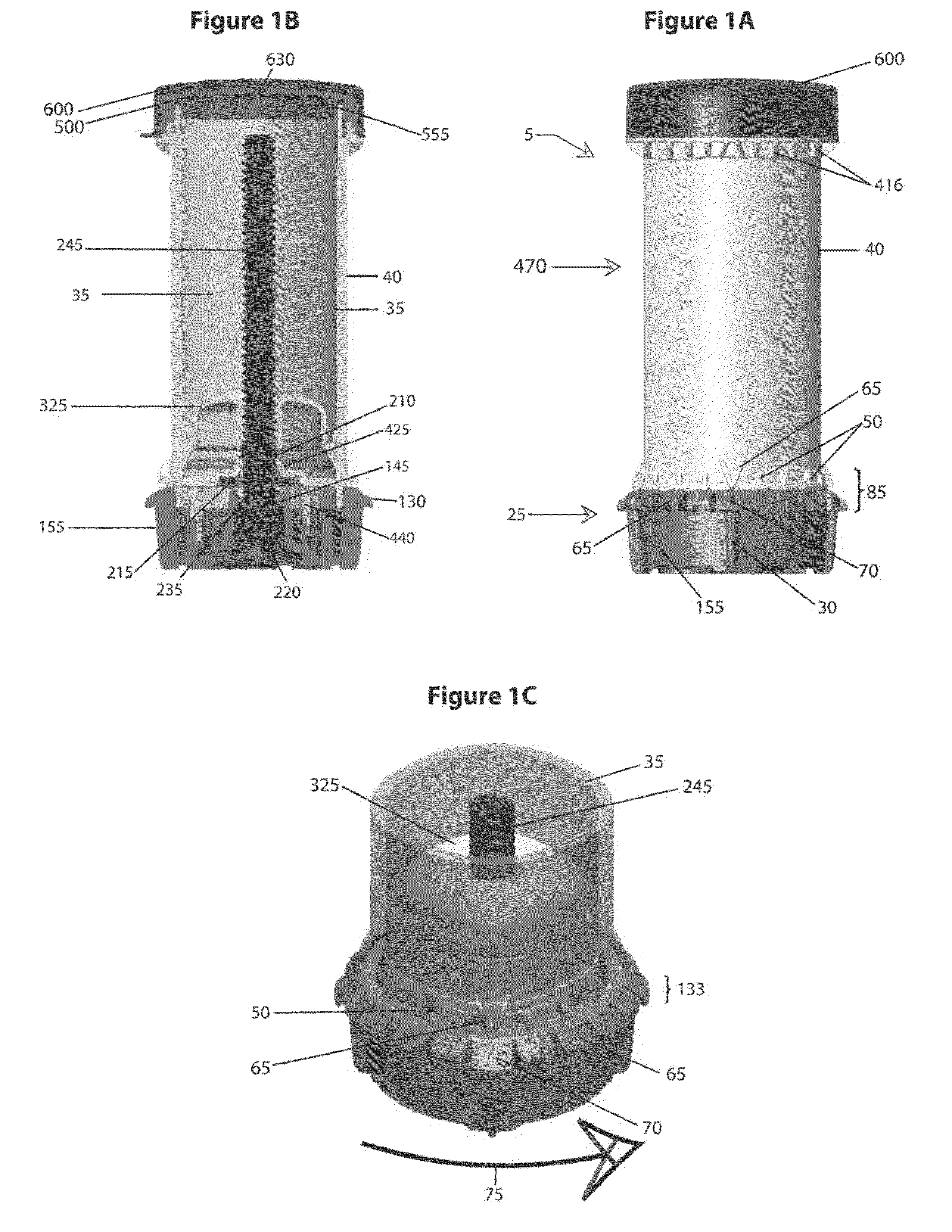
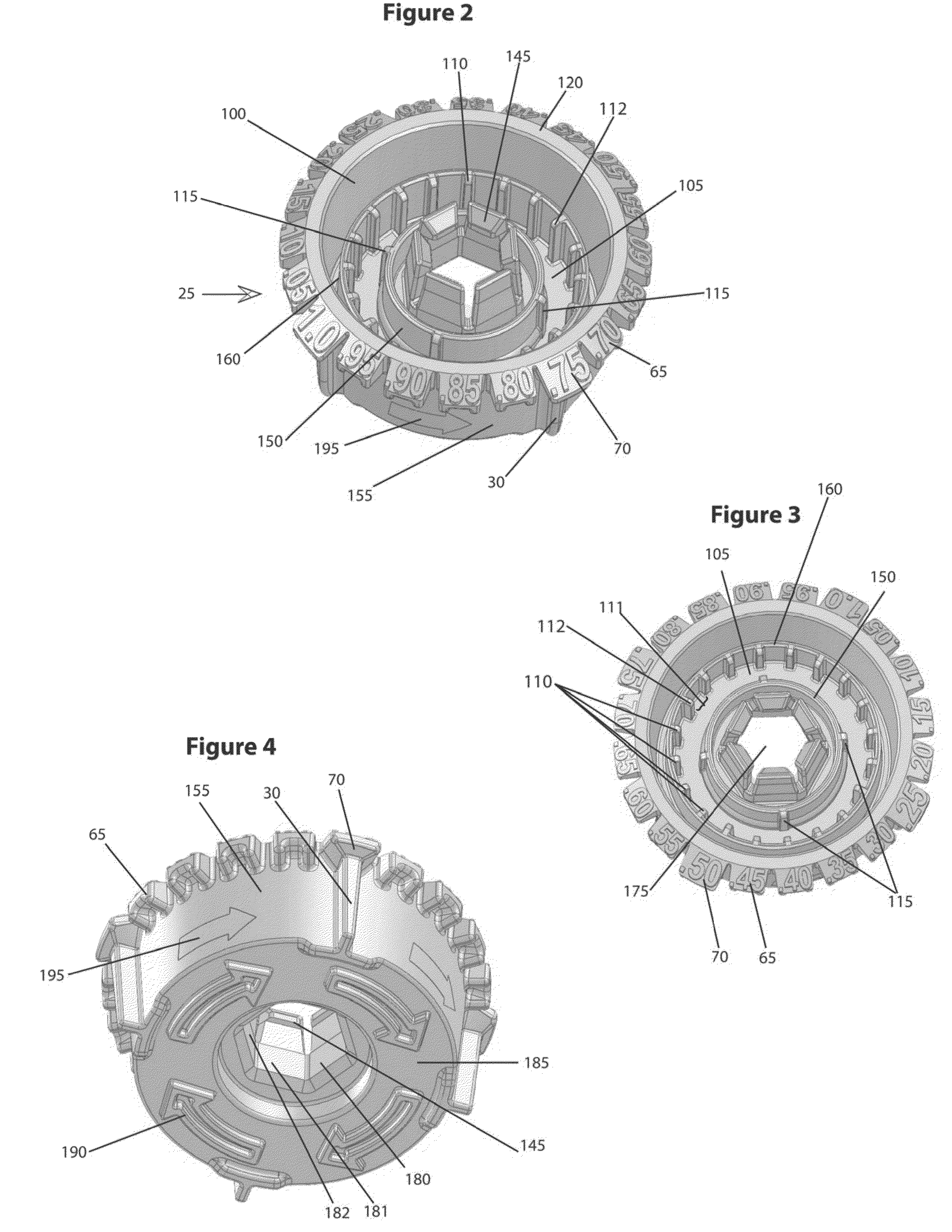
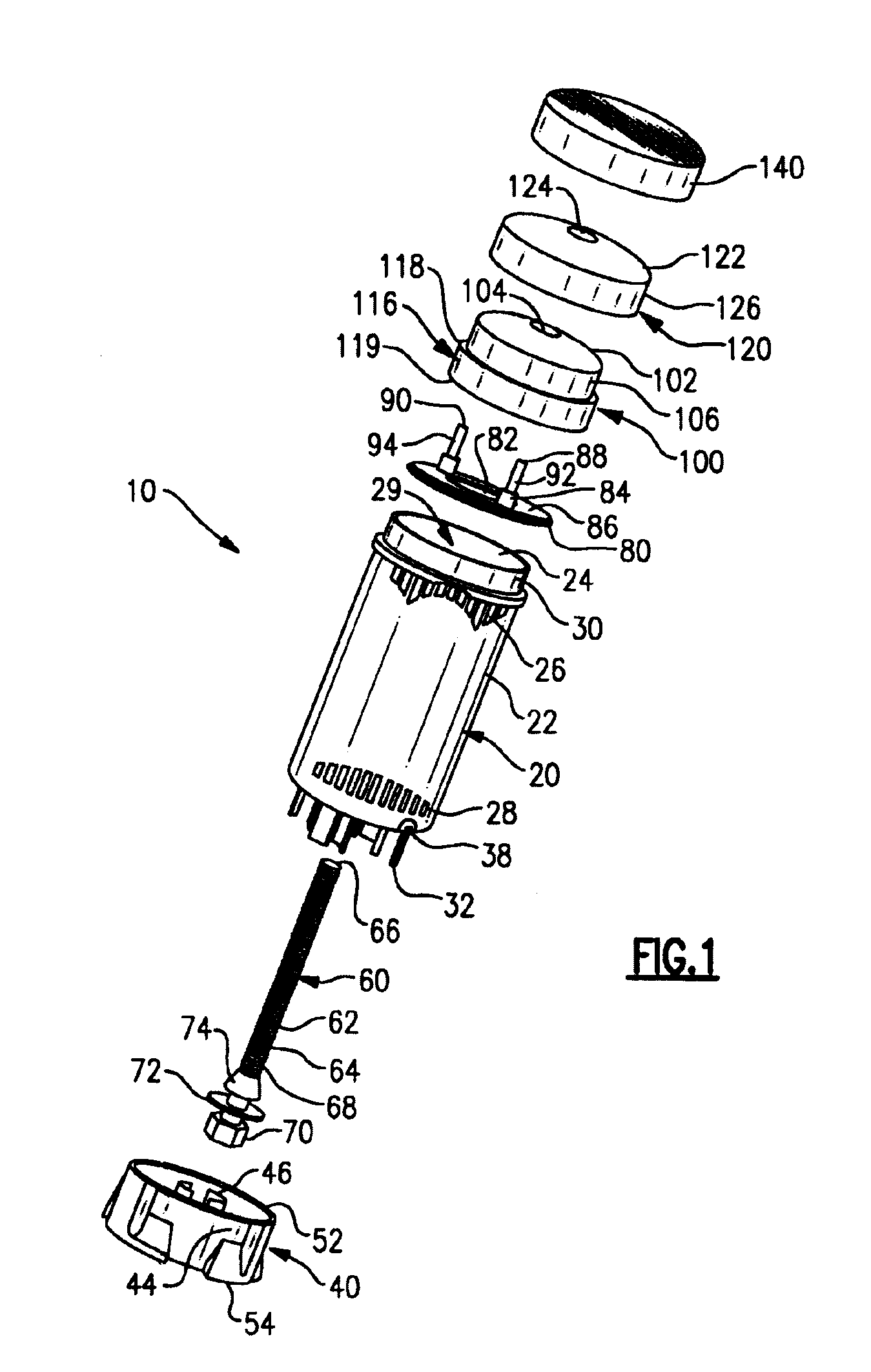
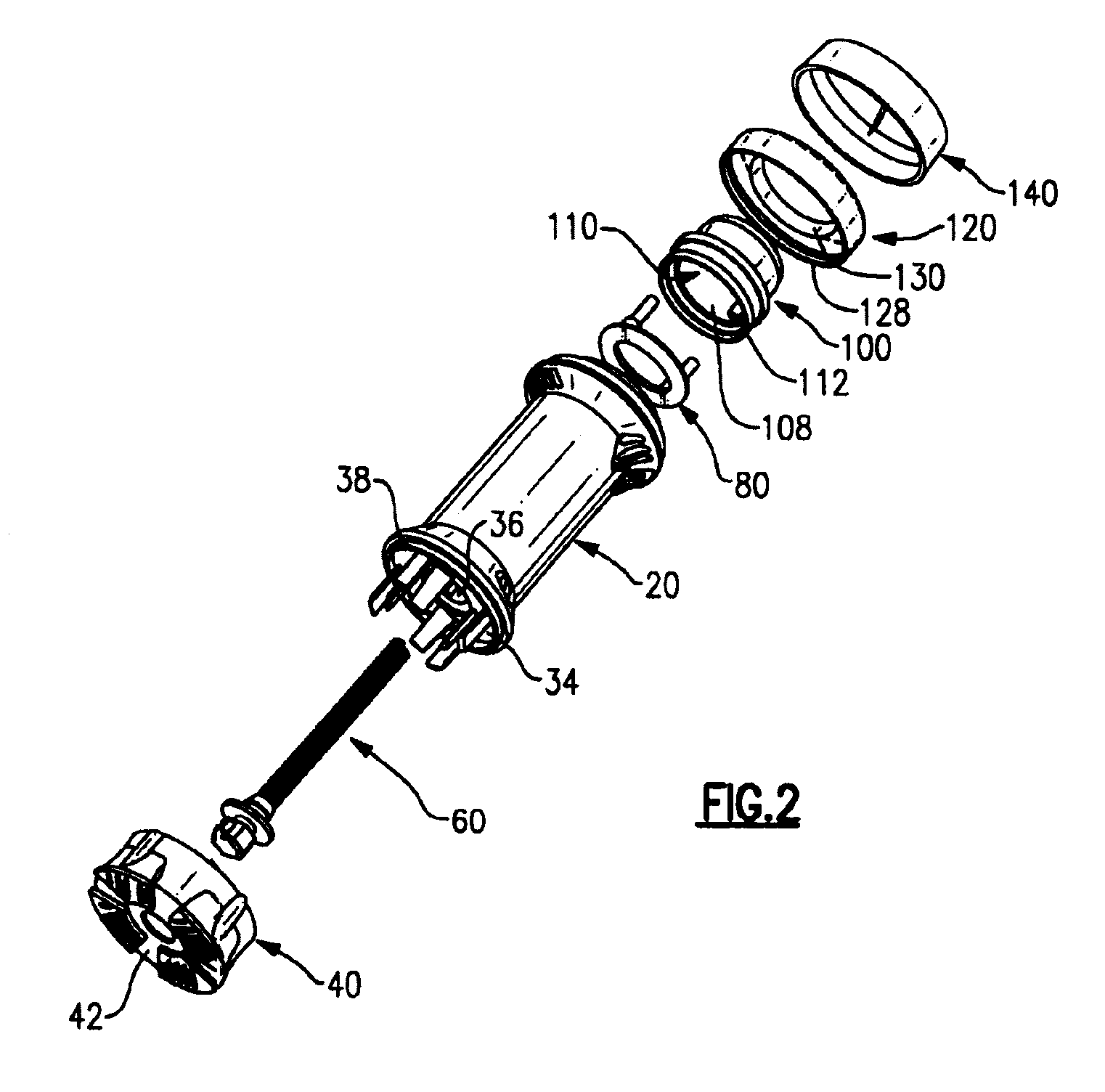
The various embodiments of the present invention relate to a human identical flowable hormone composition dispersed in a cream or gel contained in the novel “Smart” metered dose applicator, which facilitates numerous patient specific dosing options, dose titration, as well as continuous and variable dosing. The composition is designed for transdermal usage for consumers who follow a specified dosage protocol resulting in static and cyclic variations on serum hormone levels over a 28 day cycle stretched over the span of a month. The regimen is modeled after nature, in an attempt to replicate temporal variations in serum progesterone and estradiol hormone levels of premenopausal females with healthy hormone cycles. A dispenser is also disclosed for flowable cream-base medicaments, specifically; a unidirectional rotatable platform attached to a screw that is slipped into a barrel where it is secured in place and it joins the said components that house an elevator.
Owner:TICKERWORKS INC
Dosing dispenser for cream-based medicines
ActiveUS7213994B2Rule out the possibilityReduce bubble and pocketPropelling pencilsMedical applicatorsCream baseThreaded rod
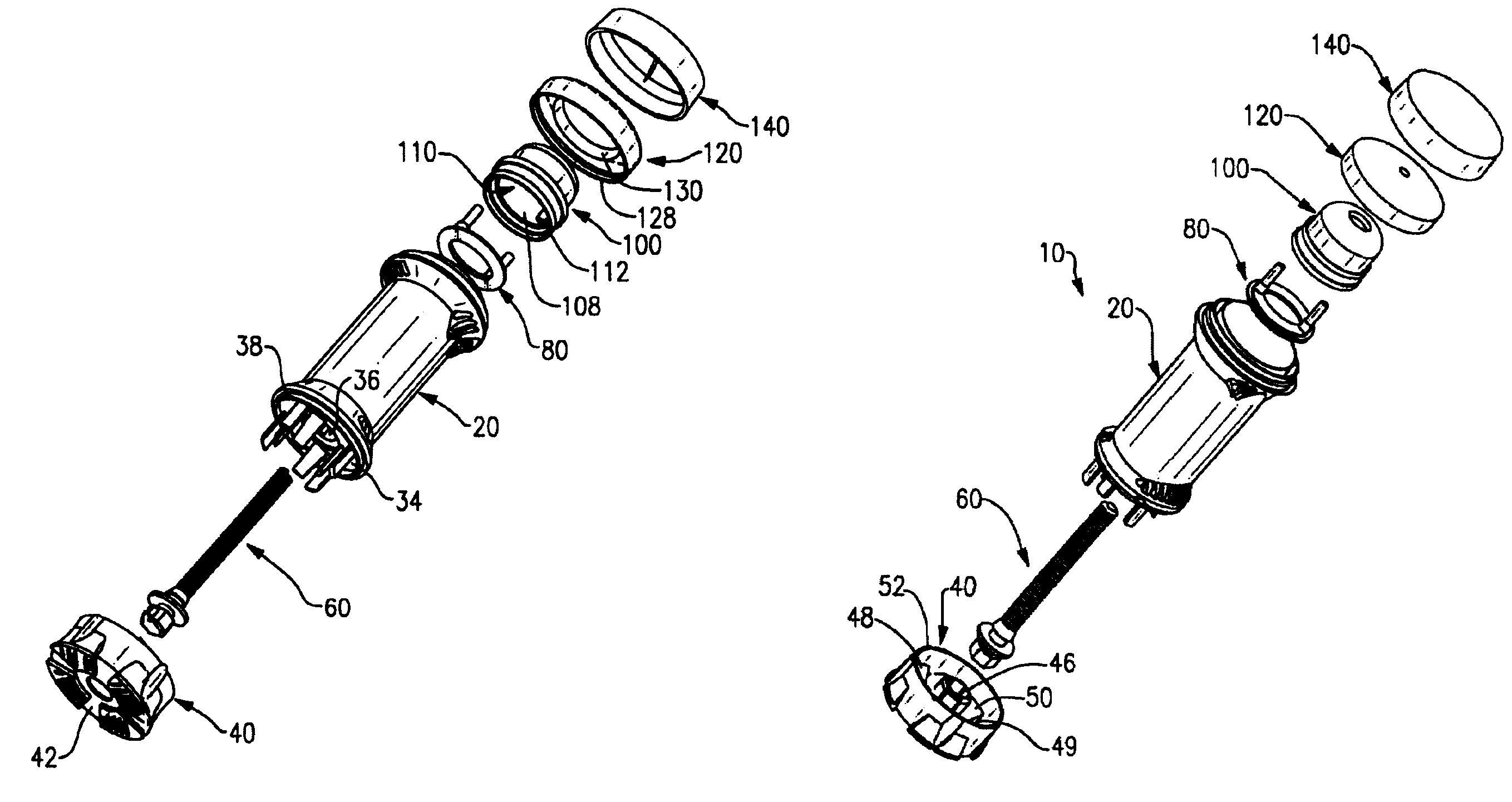
A dispenser for metered dosing of cream-based medicines comprising a barrel, a base having a threaded rod extending therefrom, a riser having at least one flexible seal which engages the barrel, an applicator cap having apertures therein for spreading dispensed cream onto a user's skin. The user positively knows when a metered amount of cream has been dispensed by tactile and audible feedback.
Owner:DOSELOGIX LLC
Dosing dispenser for cream-based medicines
ActiveUS20070000946A1Rule out the possibilityReduce bubblingPropelling pencilsMedical applicatorsCream baseEngineering
A dispenser for metered dosing of cream-based medicines comprising a barrel, a base having a threaded rod extending therefrom, a riser having at least one flexible seal which engages the barrel, an applicator cap having apertures therein for spreading dispensed cream onto a user's skin. The user positively knows when a metered amount of cream has been dispensed by tactile and audible feedback.
Owner:DOSELOGIX LLC
Topical skin care composition
InactiveUS20060099173A1Pleasing-colored productBiocidePeptide/protein ingredientsCream baseHydroquinone Compound
A cream base for the topical application of skin care therapeutics and a process for making the cream base. In one embodiment, the therapeutic is tretinoin, hydroquinone and fluocinolone acetonide for the treatment of hyperpigmented skin conditions, such as melasma.
Owner:GALDERMA SA
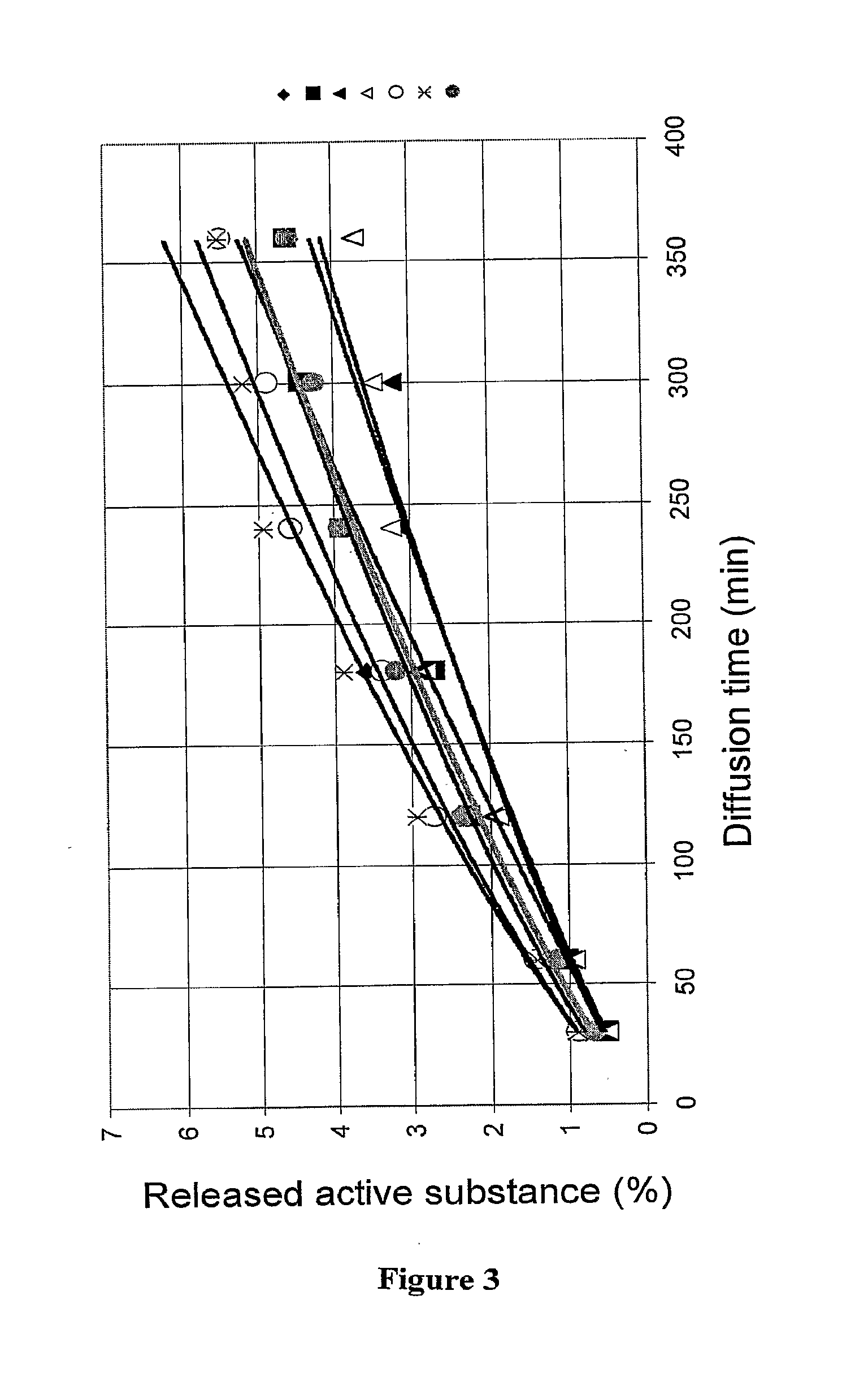
Visual, bi-audible, and bi-tactile metered-dose transdermal medicament applicator
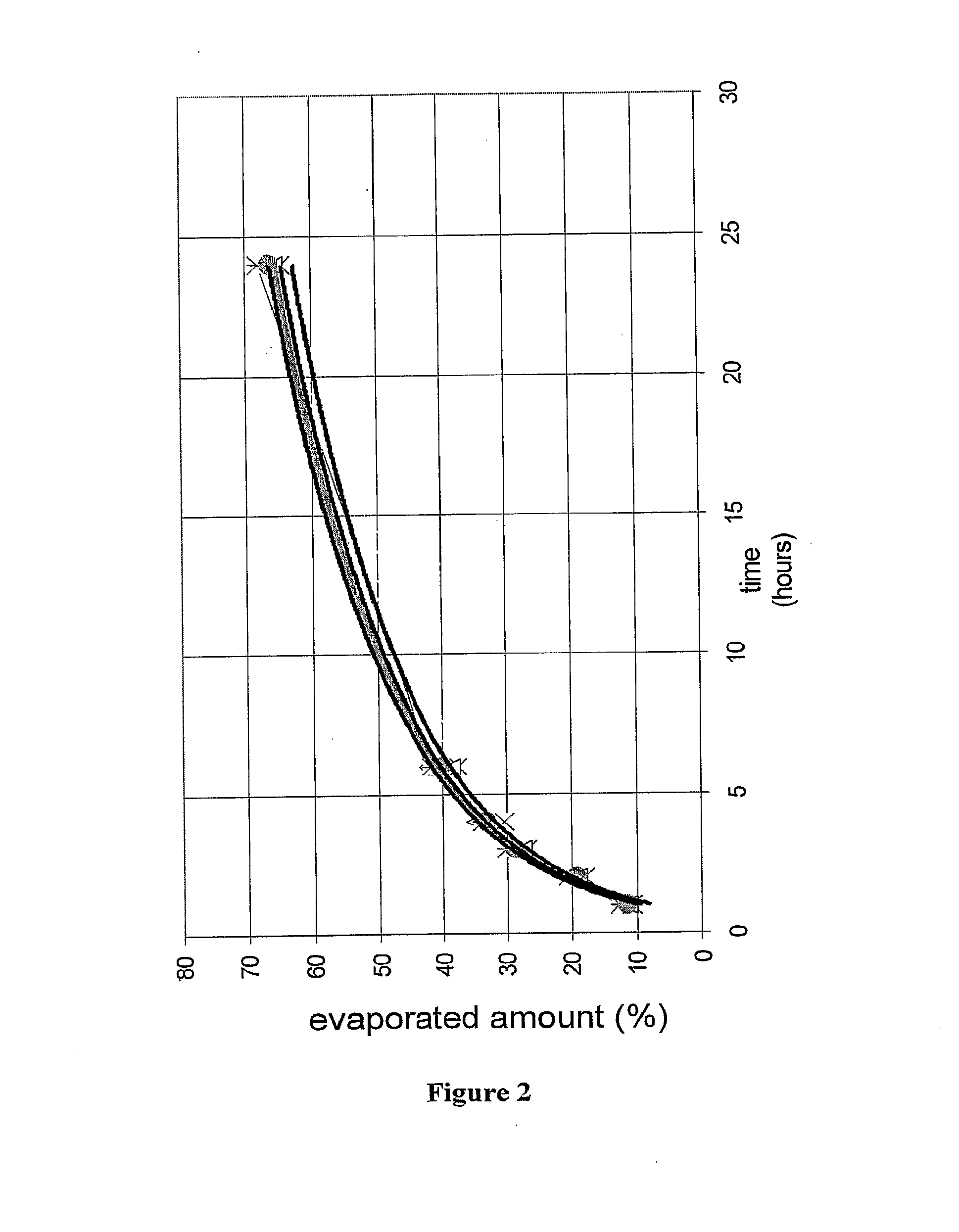
ActiveUS20120205393A1Reduce evaporationVolume measurement and fluid deliveryMedical applicatorsCream baseTactile sensation
A bi-audible, bi-tactile, and visual transdermal delivery apparatus for delivering specific desired quantities of cream-base medicament or any flowable composition; comprising: a revolving platform with equispaced side tabs stemming from an outer and inner base rim responsible for yielding said bi-audible and bi-tactile sensations upon interaction with ticker tabs projecting form the bottom exterior wall of the house; a threaded screw-complex that interacts with an elevator to transport the medicament upwards; a house that confines an inner chamber to store the medicament; an applicator pad with a center outlet where the composition exits the chamber; and a safety cap. Equispaced digit tabs on the outer side wall of the rotatable platform indicate the amount to be delivered. The platform rotates 18° clockwise per actuation against equispaced line demarcations on the house delivering roughly a 1 / 20th of a milliliter of composition.
Owner:PEREZ RAMIRO M
Multifuntional green environmental protection nanometer titania coating and preparing method thereof
InactiveCN1583907ASolve technical problems that are easy to reuniteGood weather resistanceCoatingsSilicic acidTalc
This invention is that a kind of multi-functional green nanometer two of environmental protection is oxidized Titanium coating and method of preparing, this coating can not merely be solved now. There is coating that is able to bear staining difference, is able to bear waiting difference, not the skills, such as environmental protection, etc. Skill question, it is clean to give the coating at the same time, disinfect and disinfect, Purify new functions, such as air, etc., it is the green environmental protection of more than one kind of kinetic energy Coating. Coating this by titanium white powder, exceed detailed silicic acid aluminium, nanometer Oxidize the titanium and disperse the liquid two times, the calcium carbonate of light quality, the calcium carbonate of heavy quality, Silicon lime powder, lithopone, talc powder, dispersant is moist. It is the dispersant, thickener, disappear and steep and pharmaceutical, become membrane auxiliary, flow flat pharmaceuticla, water, the cream base material, etc. make up, it is simple to prepare the craft. The cost performance is high, have very good economic benefits and social result Benefit.
Owner:RESEARCH INSTITUTE OF TSINGHUA UNIVERSITY IN SHENZHEN
Citrus-derived cosmetic and medicinal composition, and nutritional food ingredient and associated methods of application
InactiveUS20050079236A1Function increaseImprove consistencyCosmetic preparationsBiocideNaringinBase cream
A method to encompass a citrus-based cream or gel composition that is highly stable and all-natural from components in situ by removing undesired components from citrus, producing such cream or gel with desired components and texture according to intended use, either as a cosmetic or medicinal lotion, a cream base, or as a food ingredient with nutraceutical and / or binding properties for use in as example analogue meats. The method comprises the steps of grinding whole citrus fruit or peel adjusted via rate of heating and pH to optimize gel consistency and desired residual bioflavonoids rather than maximizing pectin production. Citrus-derived components such as, but not limited to, d-limonene, naringin, and psoralen are added to and blended with the gel for applications which require concentrations higher than found in vivo.
Owner:AHRENS JASON ROBERT
Composition for female sexual arousal
Compositions for female sexual arousal stimulating vaginal lubrication, vaginal and clitorial engorgement; said compositions contain aphrodisiacs, spermicidal and fungicidal components, may be applied intravaginally, extravaginally, and transdermally, and as a condom lubricant; and said compositions are contained in a water miscible cream base.
Owner:LONG JOSEPH F
Composition and Methods for Treating Hair Loss
InactiveUS20120258972A1Accelerated and robust effectPromotes hair growthCosmetic preparationsBiocideCream basePhysiology
A method for treating hair loss caused by androgenic alopecia and / or male pattern baldness. The method, which not only slows hair loss but causes hair re-growth, includes approximately daily application to a subject's scalp of a novel composition comprising finasteride (Propecia® or Proscar®), dutasteride (Avodart®), and minoxidil (Rogaine®) as active ingredients in a hypoallergenic cream-based vehicle, preferably coupled with daily ingestion of 1 mg per day of finasteride (Propecia® or Proscar®), application to the scalp of 5% minoxidil (Rogaine®) foam approximately once per day, and use of a ketoconazole-containing shampoo (e.g., Nizoral®) approximately 2-3 times per week. The method described herein also resolves scalp dermatitis in atopic subjects suffering therefrom. A method for making the novel composition is also provided.
Owner:RAFI ASIF +1
Medicinal cream made using neomycin sulphate, betamethasone valerate, and chitosan, and a process to make the same
The present invention is directed to a composition for treating bacterial skin infections & skin inflammation, along with skin rejuvenation. More particularly, the present invention relates to a pharmaceutical cream comprising a biopolymer, a corticosteroid and an antibacterial active ingredient. It discloses a composition for treating bacterial skin infections & skin inflammation, along with skin rejuvenation containing a) a biopolymer in the form of chitosan, b) a combination of active pharmaceutical ingredients (APIs), neomycin sulphate & betamethasone valerate used in treating bacterial skin infections & skin inflammations, c) a cream base containing primary and secondary emulsifiers, waxy materials, co-solvents, acids, preservatives, buffering agents, anti oxidants, chelating agents, and humectants and d) water. The active ingredients, namely chitosan, a corticosteroid Betamethasone Valerate, and an antibacterial agent Neomycin Sulphate, are incorporated in cream base for use in treating bacterial skin infections and skin inflammation due to allergy & itching, & wounds on human skin involving contacting human skin with the above identified composition.
Owner:SULUR VANANGAMUDI SUBRAMANIAM +4
Medicinal Cream Made Using Fluticasone Propionate And Chitosan And A Process To Make The Same
InactiveUS20120028943A1Antibacterial agentsOrganic active ingredientsFluticasone propionateBiopolymer
The present invention relates to a composition for treating skin inflammation, along with skin rejuvenation. More particularly, the present invention relates to a pharmaceutical cream comprising a biopolymer, and a corticosteroid. It discloses a composition for treating skin inflammation, along with skin rejuvenation containing a) a biopolymer in the form of chitosan, b) an active pharmaceutical ingredient (API) composition in the form of fluticasone propionate, used in treating skin inflammation c) a cream base containing primary and secondary emulsifiers, waxy materials, co-solvents, acids, preservatives, buffering agents, anti oxidants, chelating agents, and humectants and d) water. The active ingredients, namely chitosan, and a corticosteroid in the form of fluticasone propionate, are incorporated in cream base for use in treating skin inflammation due to allergy & itching & wounds on human skin involving contacting human skin with the above identified composition.
Owner:APEX LAB PRIVATE LTD
Topical skin care composition
ActiveUS20050101580A1Pleasing-colored productDisappear quicklyBiocideCosmetic preparationsCream baseHydroquinone Compound
A cream base for the topical application of skin care therapeutics and a process for making the cream base. In one embodiment, the therapeutic is tretinoin, hydroquinone and fluocinolone acetonide for the treatment of hyperpigmented skin conditions, such as melasma.
Owner:GALDERMA SA
Wound-Healing Pharmaceutical Compositions in the Form of a Cream Based on Amino Acids and Sodium Hyaluronate
InactiveUS20080287392A1Easy to eliminateFast regenerationBiocideOrganic active ingredientsCream baseWound healing
Owner:PROFESSIONAL DIETETICS SPA +1
Compound dexamethasone acetate nano-cream and preparation method therefor
InactiveCN105616426AImprove bioavailabilityImprove smudge effectHydroxy compound active ingredientsAntipyreticCream baseDexamethasone acetate
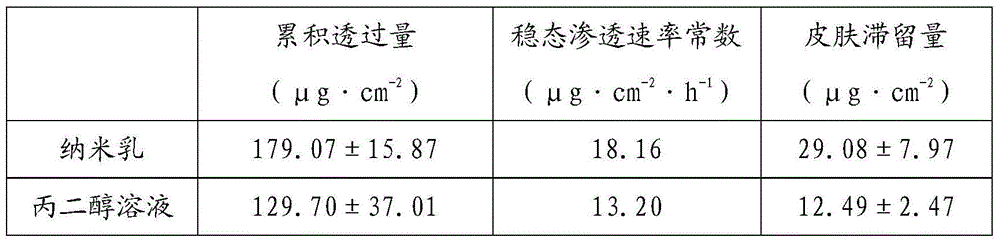
The invention discloses compound dexamethasone acetate nano-cream and a preparation method therefor. The cream comprises the following raw materials: dexamethasone acetate, camphor, menthol, a surfactant, a cosurfactant, an oil phase, a cream base material and water. The preparation method for the compound dexamethasone acetate nano-cream comprises the following steps: dissolving dexamethasone acetate in the oil phase, the surfactant and the cosurfactant, and uniformly mixing with the water to obtain nano (micro) emulsion; dispersing camphor, menthol and the nano-emulsion in the cream base and mixing uniformly to obtain the compound dexamethasone acetate nano-cream. The compound dexamethasone acetate nano-cream has the advantages of good smearing property and good transdermal effect.
Owner:GUANGZHOU BAIYUNSHAN PHARM CO LTD
Anti-acne cream based on high-purity herbal extract, and preparation method of anti-acne cream
ActiveCN105662962ABroad-spectrum antibacterialEnhanced inhibitory effectCosmetic preparationsToilet preparationsCream baseReflux extraction
The invention provides anti-acne cream based on high-purity herbal extract, and a preparation method of the anti-acne cream. The preparation method concretely comprises the following steps: (1) cleaning natural herbaceous plants, drying the cleaned herbaceous plants in the air, then cutting the dried herbaceous plants and grinding into powder, mixing the powder of the herbaceous plants according to proportion, adding an ethanol water solution into the mixture, carrying out ultrasonic dispersion, carrying out heat reflux extraction for twice, and carrying out rotary evaporation and concentration on filtrate until the filtrate is free from alcohol taste to obtain extract of the natural herbaceous plants; (2) centrifuging the extract of the natural herbaceous plants and taking supernatant of the extract, putting the supernatant into dry macroporous resin, standing still and carrying out suction filtration; after absorption, transferring the macroporous resin into a 80-90% of ethanol water solution, standing still and carrying out suction filtration; taking out the macroporous resin, carrying out rotary evaporation on filtrate until the filtrate is free from alcohol taste to obtain purified extract of the natural herbaceous plants; (3) mixing auxiliary agents, heating mixture up to 80-90 DEG C, stirring the heated mixture until the mixture is completely dissolved, cooling to 45-50 DEG C, adding the purified extract of the natural herbaceous plants into the mixture, evenly stirring, carrying out heat preservation, and then carrying out rotary evaporation until the mixture is in a cream state to obtain the anti-acne cream based on the high-purity herbal extract.
Owner:GUANGZHOU BAICAOTANG PHARMA
Treatment of vulvovaginitis with spirostanol enriched extract from Tribulus Terrestris
InactiveUS20050112218A1Good effectBlock cancer cells from growingBiocideUnknown materialsDiseaseSpirostanols
Therapeutic compositions made from the herb Tribulus terrestris and methods of making and using the same are provided. The therapeutic compositions include an enriched extract having an increased spirostanol saponin content that is prepared from the harvested Tribulus terrestris L. The enriched extract is prepared using discrete hydrolysis, separation and enrichment steps. The resulting therapeutic may be combined with a cream base and is useful for treating bacterial, fungal, and viral infections, particularly gynecologic infections. This product was also found to be very successful in suppository form for the treatment of vulvo-vaginal, vulvo-hemorrhoidal and colonic conditions.
Owner:ALEXIS BRIAN
Mesotherapy Cream
InactiveUS20080058287A1Promotes breakdown and leachingTo promote metabolismBiocidePhosphorous compound active ingredientsCholic acidBULK ACTIVE INGREDIENT
The present disclosure concerns a cream-based composition and method which reduces fat deposits upon topical application to the skin. The active ingredients of the composition include the active ingredients deoxycholic acid and phosphatidyl choline which are mixed together with a cream composition. The active ingredients may be combined with any suitable pharmaceutical vehicle to provide the fat-reducing composition claimed herein. The composition is topically applied to a skin area over a period of days or months in order to reduce fat deposits that have collected beneath the dermis.
Owner:NATURAL DESIGNS
Ozonization oil complex and preparing method and application
ActiveCN107028977AAddresses technical shortcomings of quick releasesLow costInorganic active ingredientsAntiinfectivesCream baseOzone
The invention provides an ozonization oil complex and a preparing method and application. The ozonization oil complex is obtained by mixing, by mass, 50-120 parts of ozonization oil, 30-80 parts of cream base and 10-20 parts of water under a vacuum closed condition, and then conducting standing at 10-15 DEG C. By the adoption of the preparing method, the cost is low, ozone can enter matrix molecules to form the ozonization oil complex which has a closed and steady structural state, the technique defect that ozone molecules are released quickly under high temperature is overcome, and a slow release effect is realized.
Owner:HUBEI JINGGENG BIOLOGICAL ENG CO LTD
Cosmetic composition, formulation in gel-cream and its use
InactiveUS20150005255A1Reduce appearance problemsAvoid problemsBiocideCosmetic preparationsCream baseAntioxidant
The present invention aims to provide reduction of localized fat, particularly for the treatment, prevention or alleviation of gynoid lypodystrophy.The cosmetic composition of the invention comprises:(a) from 4.0 to 27.0% by weight of active ingredients comprising:(i) at least one localized fat reducing agent selected from the group consisting of natural, modified and synthetic substances,(ii) at least one alkaloid,(iii) at least one oil or vegetable extract,(iv) at least one antioxidant, and(v) at least one film forming agent;(b) 6.0 to 32.0% by weight of cosmetically acceptable additives; and(c) q.s.p. 100% by weight of hydrophilic gel-cream base.The invention also comprises a gel-cream formulation for localized fat reduction, as well as the use of said composition or formulation for manufacturing of a cosmetic for the treatment, prevention or alleviation of gynoid lypodystrophy.
Owner:EMS
Medicinal fusidic acid cream made using sodium fusidate and incorporating a biopolymer and a process to make it
ActiveUS20120040946A1Improve shelf life stabilityFine granularityAntibacterial agentsOrganic active ingredientsBiopolymerAllergy
The present invention is directed to a medicinal composition for treating bacterial skin infections and related wounds, and also other skin wounds including those caused by burns. The cream also causes skin rejuvenation through an epithelisation process. The cream comprises a) a biopolymer in the form of Chitosan, b) an Active Pharmaceutical Ingredient (API), in the form of fusidic, c) a cream base, and d) water. The invention also discloses a process to make the medicinal cream in which Fusidic acid which is formed in situ from Sodium Fusidate as the starting raw material, by converting it into Fusidic acid under oxygen-free environment created using inert gas, preferably nitrogen. The cream produced by the process of the present invention has greater shelf-life stability and the finer particle size of the API than the conventional creams containing Fusidic acid and found to be surprisingly superior for use against skin infections with allergy & itching, & wounds on human skin than alternative creams currently available.
Owner:SULUR VANANGAMUDI SUBRAMANIAM +4
Multifunctional sun cream based on liquid beads and preparation method of multifunctional sun cream
InactiveCN105560070AFast preparation methodThe preparation method is simple and gentleCosmetic preparationsToilet preparationsCream baseEngineering
The invention provides a multifunctional sun cream based on liquid beads and a preparation method of the multifunctional sun cream and belongs to the field of colloid and interface science and cosmetics. The multifunctional sun cream is of a structure shown as follows. The preparation method includes following steps: adding hydrophobic powder and a functional liquid into a stirrer according to a certain proportion; stirring at a high speed for a certain time to obtain fluffy liquid beads, namely the multifunctional sun cream. The multifunctional sun cream is simple and mild in preparation process, has excellent stability and water retaining, moisture preserving and sun screening characteristics, can effectively block ultraviolet rays and timely replenish moisture of skin and is conducive to industrialized production.
Owner:JIANGNAN UNIV
Transdermal pharmaceutical preparations
The present invention relates to semisolid transdermal pharmaceutical preparation having enhanced stability and bioavailability, wherein the particles are coated by a volatile silicon oil component and the thus obtained suspension is dispersed in a gel or cream base.
Owner:EGIS GYOGYSZERGYAR NYILVANOSAN MUKODO RESZVENYTARSASAG (EGIS PHARMA PLC)
Wound-healing pharmaceutical compositions in the form of a cream based on amino acids and sodium hyaluronate
ActiveUS8404661B2Easy to eliminateFast regenerationOrganic active ingredientsBiocideCream baseWound healing
Owner:PROFESSIONAL DIETETICS SPA +1
Whitening cream based on natural extracts, and preparation method thereof
The invention provides a whitening cream based on natural extracts. The whitening cream comprises, by weight, 0.4 to 0.6 part of superoxide dismutase, 1 to 3 parts of anthocyanin, 0.2 to 0.25 part of fructus schisandrae chinensis, 0.30 to 0.35 part of lumbricus peptide, 0.3 to 0.4 part of catalase, 1 to 3 parts of astaxanthin, 3 to 5 parts of resveratrol oligomer, 6 to 7 parts of formononetin, and 15 to 18 parts of an emulsifier. The invention also provides a preparation method of the whitening cream based on natural extracts.
Owner:FOSHAN WIN COSMETIC
Traditional Chinese medicine and western medicine-combined formula for radically treating chloasma and preparation process thereof
InactiveCN102058777AMaintain micro-ecological balanceLeave no traceOrganic active ingredientsAnthropod material medical ingredientsCream baseWestern medicine
The invention discloses a traditional Chinese medicine and western medicine-combined formula for removing chloasma at one time and a preparation process thereof. A product prepared according to the formula comprises a liquid medicine A and an ointment B, which are used respectively. The liquid medicine A is prepared from 10 to 50g of white silkworm, 10 to 50g of white poria, 10 to 50g of angelica dahurica, 10 to 50g of thunberg fritillary bulb, 10 to 20ml of bletilla, 10 to 50g of largehead atractylodes rhizome and a proper amount of 75 percent ethanol, and the liquid medicine A is prepared by the following steps of: crushing the white silkworm, the white poria, the angelica dahurica, the thunberg fritillary bulb, the bletilla and the largehead atractylodes rhizome, adding the proper amount of 75 percent ethanol, soaking for 3 to 10 days, removing dregs, and adding the 75 percent ethanol until the total volume is 1,000ml so as to obtain the liquid medicine A; and the ointment B is prepared from 0.03 to 0.1 mg of neomycin, 0.3 to 1 mg of clobetasol propionate, 5 to 15 mg of ketoconazole, a proper amount of glycerol and a proper amount of cream base, and the ointment B is prepared by the following steps of: adding the proper amount of the glycerol into the neomycin, the clobetasol propionate and the ketoconazole and grinding into paste, adding the proper amount of the cream base, and uniformly grinding to prepare the ointment B. The chloasma removing technology is that: the liquid medicine A is applied to an affected part until the chloasma basically disappears, the ointment B is applied to the affected part, and the chloasma can be cured within 20 to 50 days. The invention has a good chloasma removing effect, and ensures no recurrence and no side effect.
Owner:费小平
Medicinal Cream Made Using Hydrocortisone Acetate and A Process To Make The Same
The present invention relates to a composition for treating skin inflammation, along with skin rejuvenation. More particularly, the present invention relates to a pharmaceutical cream comprising a biopolymer, and a corticosteroid. It discloses a composition for treating skin inflammation, along with skin rejuvenation containing a) a biopolymer in the form of chitosan, b) an active pharmaceutical ingredient (API) composition in the form of hydrocortisone acetate, used in treating skin inflammation c) a cream base containing primary and secondary emulsifiers, waxy materials, co-solvents, acids, preservatives, buffering agents, anti oxidants, chelating agents, and humectants and d) water. The active ingredients, namely chitosan, and a corticosteroid in the form of hydrocortisone acetate, are incorporated in cream base for use in treating skin inflammation due to allergy & itching & wounds on human skin involving contacting human skin with the above identified composition.
Owner:APEX LAB PRIVATE LTD
Medicinal Cream Made Using Neomycin Sulphate, Betamethasone Valerate, And Chitosan, And A Process To Make The Same
The present invention is directed to a composition for treating bacterial skin infections & skin inflammation, along with skin rejuvenation. More particularly, the present invention relates to a pharmaceutical cream comprising a biopolymer, a corticosteroid and an antibacterial active ingredient. It discloses a composition for treating bacterial skin infections & skin inflammation, along with skin rejuvenation containing a) a biopolymer in the form of chitosan, b) a combination of active pharmaceutical ingredients (APIs), neomycin sulphate & betamethasone valerate used in treating bacterial skin infections & skin inflammations, c) a cream base containing primary and secondary emulsifiers, waxy materials, co-solvents, acids, preservatives, buffering agents, anti oxidants, chelating agents, and humectants and d) water. The active ingredients, namely chitosan, a corticosteroid Betamethasone Valerate, and an antibacterial agent Neomycin Sulphate, are incorporated in cream base for use in treating bacterial skin infections and skin inflammation due to allergy & itching, & wounds on human skin involving contacting human skin with the above identified composition.
Owner:SULUR VANANGAMUDI SUBRAMANIAM +4
Medicinal cream made using silver sulphadiazine and chitosan and a process to make it
The present invention is directed to a composition for treating bacterial skin infections, along with skin rejuvenation. More particularly, the present invention relates to a pharmaceutical cream comprising a biopolymer, and an antibacterial active ingredient. It discloses a composition for treating fungal skin infections along with skin rejuvenation containing a) a biopolymer in the form of chitosan, b) an active pharmaceutical ingredient (API) composition in the form of silver sulphadiazine used in treating bacterial skin infections, c) a cream base containing primary and secondary emulsifiers, waxy materials, co-solvents, acids, preservatives, buffering agents, anti oxidants, chelating agents, and humectants and d) water. The active ingredients, namely chitosan, and an anti bacterial agent in the form of silver sulphadiazine, are incorporated in cream base for use in treating bacterial skin infections with allergy & itching, & wounds on human skin involving contacting human skin with the above identified composition.
Owner:SUBRAMANIAM VANANGAMUDI SULUR +3
Anti-dermatophytic preparation and use thereof
InactiveUS20050181081A1SkinReduce absorptionBiocideHydroxy compound active ingredientsIn vivoSynergistic combination
The present invention provides an improved preparation based on the synergistic action of garlic extract and essential oil of M. spicata var. Ganga or cinnamon oil against dermatophytic fungus. More particularly, the present invention relates to the synergistic enhancement of activity of a combination by menthyl acetate or Geraniol. The invention also provides a method of preparation of the synergistic combination and the shelf life observed to be more than one year. The cream based preparation is a potent anti-dermatophytic as described and illustrated by in vitro and in vivo evaluations.
Owner:COUNCIL OF SCI & IND RES
Pharmaceutical preparations containing highly volatile silicones
PendingUS20140079791A1Improve stability penetration propertyImprove stabilityAntibacterial agentsBiocidePharmaceutical formulationHexamethyldisiloxane
The subject of the present invention is a transdermal preparation containing pharmaceutically active ingredient, wherein the particles of the active ingredient are coated with highly volatile silicones or a mixture thereof, and these coated particles are dispersed in a gel or cream base. The volatile silicone component is hexamethyldisiloxane and / or octamethyltrisiloxane and / or decamethylpentacyclo-siloxane. A further subject of the present invention is a method for the preparation of such pharmaceutical compositions.
Owner:EGIS PHARM PLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com