Sodium fusidate freeze-drying powder injection preparation with double auxiliary materials for injection
A technology of sodium fusidate and freeze-dried powder injection, which is applied in the field of freeze-dried sodium fusidate injection for injection with double excipients and its preparation field, and can solve the problems of low product clarity and solution color pass rate, and high drying temperature , Long freeze-drying time and other problems, to achieve the effect of reducing production freeze-drying time, lowering drying temperature, and excellent product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1, sodium fusidate freeze-dried powder for injection, in 1000 bottles
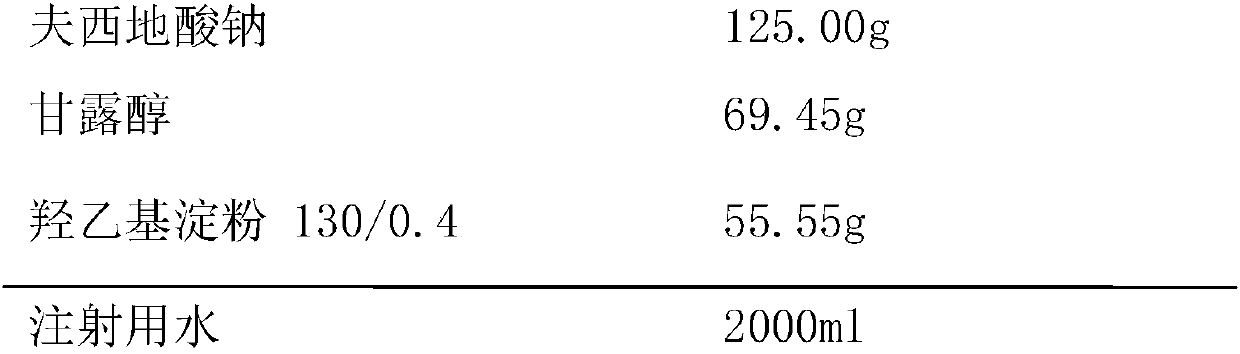
[0025] Prescription (specification: 125mg / bottle):
[0026]
[0027] Preparation Process:
[0028] a) Dissolve the prescribed amount of mannitol in water for injection, place it in a constant temperature water bath magnetic stirrer and stir at 80°C until it is completely dissolved, then add the prescribed amount of hydroxyethyl starch 130 / 0.4 and the prescribed amount respectively The sodium fusidate was kept at 80°C and continued to stir until it was completely dissolved, and the volume was adjusted to 2000ml with water for injection, and the pH value of the solution was adjusted to 7.5.
[0029] b) The above solution is circulated and filtered successively through 0.45 μm and 0.22 μm filter membranes for 30 minutes;
[0030] c) Sampling inspection, put the vial containing the liquid medicine into the vacuum freeze dryer and turn on the machine, quickly cool down to minus 45°C withi...
Embodiment 2
[0031] Embodiment two, sodium fusidate freeze-dried powder for injection, in 1000 bottles
[0032] Prescription (specification: 125mg / bottle):
[0033]
[0034] Preparation Process:
[0035] a) Dissolve the prescribed amount of mannitol in water for injection, place it in a constant temperature water bath magnetic stirrer and stir at 80°C until it is completely dissolved, then add the prescribed amount of hydroxyethyl starch 130 / 0.4 and the prescribed amount respectively The sodium fusidate was kept at 80°C and continued to stir until it was completely dissolved, and the volume was adjusted to 2000ml with water for injection, and the pH value of the solution was adjusted to 7.5.
[0036] b) The above solution is circulated and filtered successively through 0.45 μm and 0.22 μm membranes for 30 minutes.
[0037] c) Sampling inspection, put the vial containing the liquid medicine into the vacuum freeze dryer and turn on the machine, quickly cool down to minus 45°C within 55-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com