Composition of sodium fusidafe for injection and preparing method thereof
A technology of sodium fusidate and its composition, applied in the field of pharmaceutical preparations, can solve the problems of high buffer salt consumption, high cost, irritation and pain at venipuncture site, achieve simple process, improve tolerance and reduce production cost Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Take 4 / 5 of the prescribed amount of water for injection, add the prescribed amount of disodium edetate, and stir to dissolve. Add the prescribed amount of mannitol, disodium hydrogen phosphate and citric acid, stir to dissolve completely, let cool to room temperature, add the prescribed amount of sodium fusidate, stir to dissolve, add water for injection to the full amount. Add special activated carbon for injection to the liquid medicine, stir for 30 minutes, decarbonize and filter. 0.22μm microporous membrane filter to sterilize. Filling liquid medicine, half plug. Freeze-dried, cork and cap.
[0020] The final dosage of buffering agent and metal ion complexing agent is selected according to the results of dissolution, reconstitution and compatibility. The research results are shown in Table 1
[0021] Table 1 Selection results of buffer dosage
[0022] prescription 1 2 3 4 5 6 7 8 fusidic acid
Embodiment 2
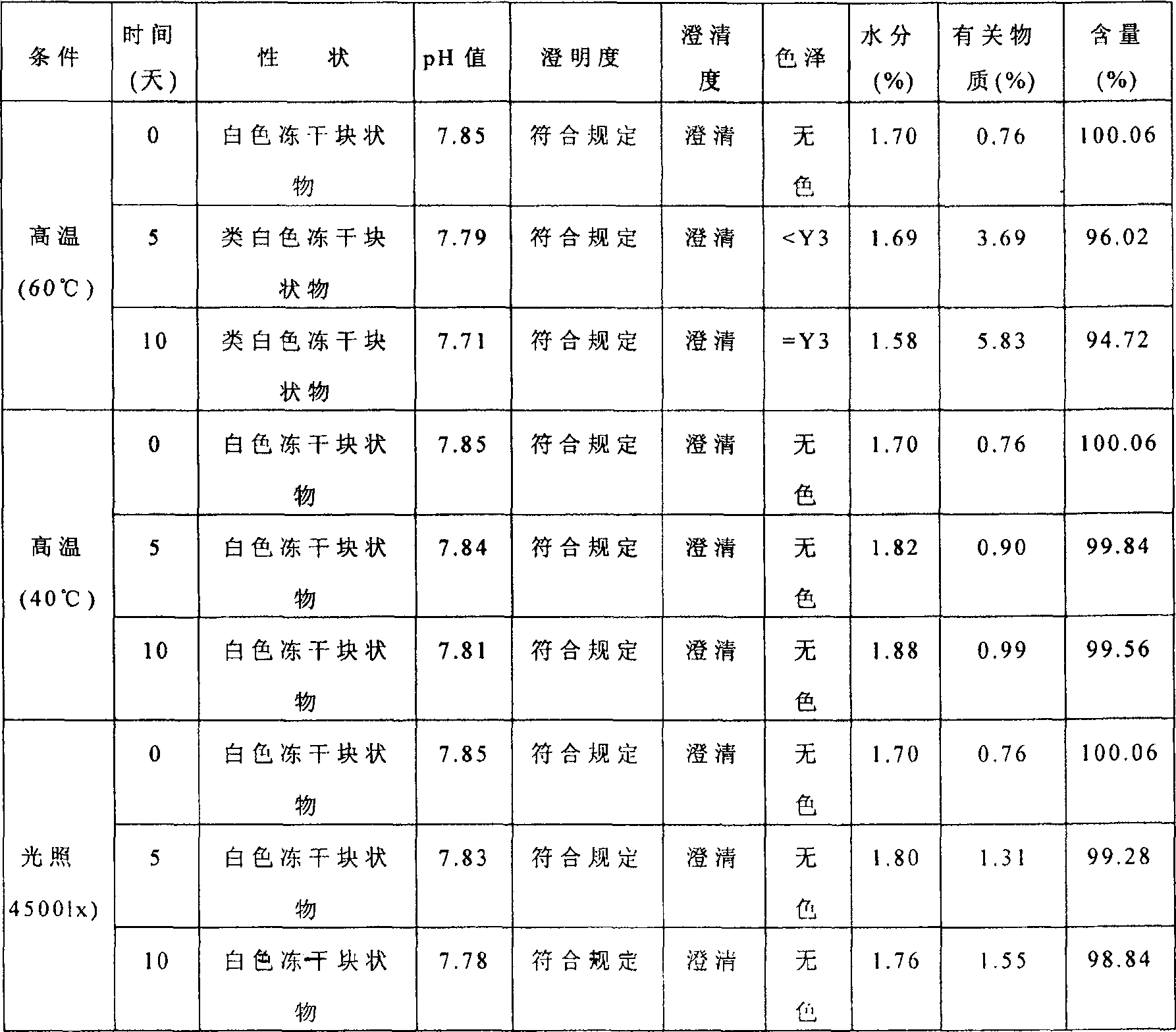
[0024] Sodium fusidate for injection prepared according to prescription 6 was placed under high temperature (60°C), high temperature (40°C) and light (45001x) conditions for 10 days respectively, and samples were taken at 5 and 10 days respectively to check the quality The index results are shown in Table 2.
[0025] The results showed that sodium fusidate for injection was placed in high temperature (60°C), light (45001x) and high temperature (40°C) environment for 10 days, and all quality indicators were checked, compared with 0 days, except for high temperature (60°C). The substance increases, the content decreases, and the color changes; except for the light (45001x) related substances increase slightly, other indicators have no obvious changes.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com