Flurbiprofen cataplasm and preparation method thereof
A flurbiprofen and solvent technology, which is applied in the field of pharmaceutical preparations, can solve the problems of low solubility of poorly soluble drugs, poor transdermal absorption performance, affecting treatment effects, etc., and achieves improved storage stability, improved transdermal absorption performance, penetration good skin absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
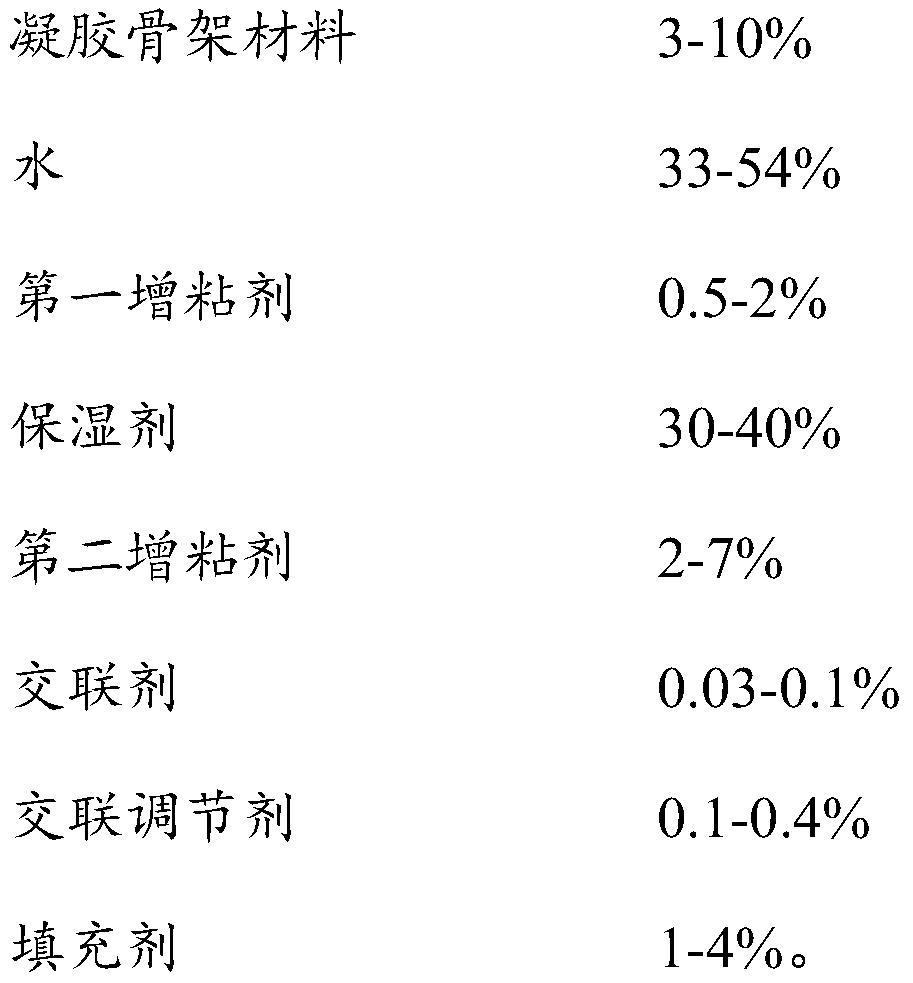
[0051]The cataplasm of insoluble drugs provided in this embodiment is composed of a backing, a paste and an anti-adhesive film, and the composition of the paste is as follows:
[0052]
[0053]
[0054] The preparation method comprises the following steps:
[0055] Add flurbiprofen to a solution composed of polyethylene glycol-12-hydroxystearate (KolliphorHS 15), Span 83 and crotamiton, mix and stir evenly to obtain component A;
[0056] Dissolve tartaric acid and polyvinyl alcohol in purified water, heat to 85°C, stir evenly until it becomes a transparent colorless liquid, cool down to 35°C, add gelatin and stir evenly until a clear and translucent colloid is obtained to obtain component B;
[0057] Dissolve sodium carboxymethylcellulose in glycerin, add polyethylene glycol, NP-700, micropowder silica gel, disodium edetate and aluminum hydroxide, and stir evenly to obtain component C.
[0058] In the reaction kettle, first add component B, then add component A, and sti...
Embodiment 2
[0061] The insoluble drug cataplasm provided in this embodiment is composed of a backing, a paste and an anti-adhesive film, and the composition of the paste is as follows:
[0062]
[0063] The preparation method is as follows:
[0064] Dissolve flurbiprofen in a solution composed of Span 83, Kolliphor HS 15, N-methylpyrrolidone (NMP) and crotamiton, mix and stir evenly to obtain component A;
[0065] Dissolve tartaric acid and polyvinyl alcohol in purified water, heat to 85°C, stir evenly until it becomes a transparent colorless liquid, cool down to 35°C, add gelatin and stir evenly until a clear and translucent colloid is obtained to obtain component B;
[0066] Dissolve sodium carboxymethylcellulose in glycerin first, then add polyethylene glycol, NP-700, micropowder silica gel, titanium dioxide, disodium edetate and aluminum glycolate, and stir evenly to obtain component C.
[0067] In the reaction kettle, first add component B, then add component A, and stir to make ...
Embodiment 3
[0070] The insoluble drug cataplasm provided in this embodiment is composed of a backing, a paste and an anti-adhesive film, and the composition of the paste is as follows:
[0071]
[0072]
[0073] The preparation method is as follows:
[0074] Dissolve flurbiprofen in a solution composed of KolliphorHS 15, Span 83 and N-methylpyrrolidone (NMP), mix and stir evenly to obtain component A;
[0075] Dissolve tartaric acid and polyvinyl alcohol in purified water, heat to 85°C, stir evenly until it becomes a transparent colorless liquid, cool down to 35°C, add gelatin and stir evenly until a clear and translucent colloid is obtained to obtain component B;
[0076] Dissolve sodium carboxymethylcellulose in glycerin first, then add polyethylene glycol, NP-800, micropowder silica gel, titanium dioxide, disodium edetate and aluminum hydroxide, and stir evenly to obtain component C.
[0077] In the reaction kettle, first add component B, then add component A, and stir to make a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com