Preparation of tamibarotene-cytarabine conjugate and nano pharmacosomes and anti-tumor application of tamibarotene-cytarabine conjugate and nano pharmacosomes
A technology of cytarabine conjugates and tamibarotene, which is applied in the field of amphiphilic tamibarotene-cytarabine conjugates and their nano-pharmaceuticals, in vivo and in vitro evaluation, and can solve the problem of Drug-resistant bone marrow suppression and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
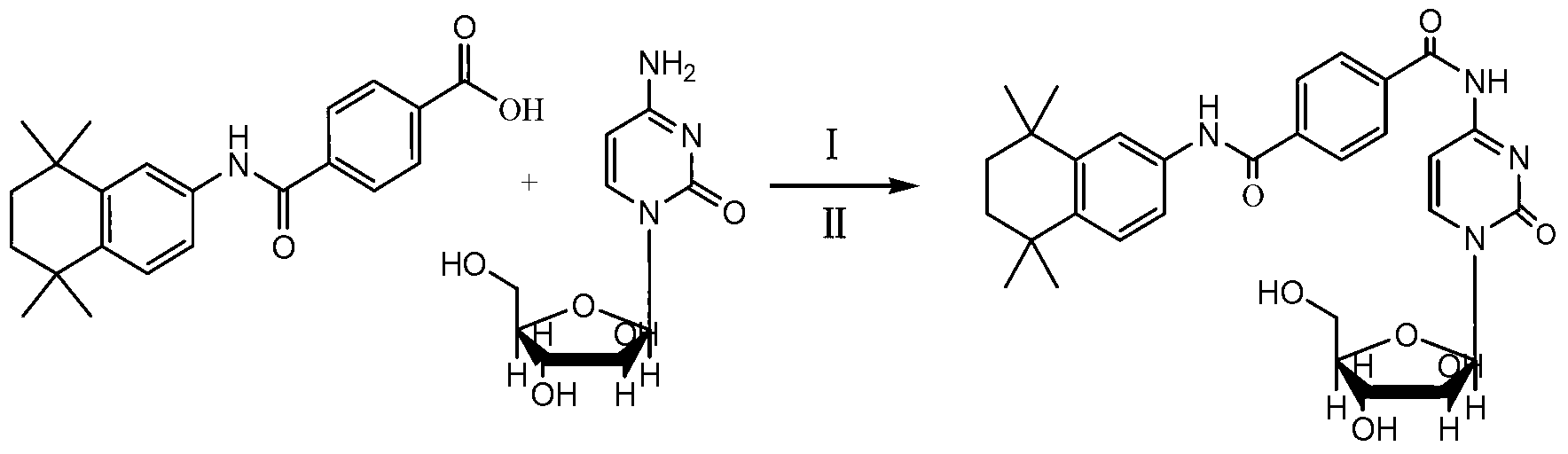
[0028] Example 1 Preparation of Tamibarotene-Cytarabine Conjugate
[0029] Dissolve 0.702 g (2 mmol) Am80 (tamibarotene), 0.454 g (2.2 mmol) DCC (dicyclohexylcarbodiimide) and 0.270 g (2 mmol) HOBT (1-hydroxybenzotriazole) in Add appropriate amount of anhydrous pyridine solution, stir in ice bath for 60 minutes, add 0.558g (2mmol) Ara-C·HCl (cytarabine hydrochloride), stir in oil bath at 40°C for 2 days, TLC (chloroform / methanol, 6: 1) The points showing Am80 and Ara-C become shallower, spin the reaction solution to dryness under reduced pressure, and remove pyridine; add an appropriate amount of chloroform to dissolve, filter out DCU (dicyclohexyl urea), and spin the filtrate to dryness under reduced pressure. Grind and wash with ether overnight, drain the ether, dissolve the residue in chloroform, wash with 0.01N hydrochloric acid, saturated NaCl and saturated KHSO 4 Wash several times, concentrate the chloroform layer, wash with anhydrous Na 2 SO 4 Dry overnight, filter,...
Embodiment 2
[0032] Example 2 Preparation of Tamibarotene-Cytarabine Nano-pharmaceutical Body
[0033] Accurately weigh the tamibarotene-cytarabine conjugate and lecithin prepared in Example 1 with an appropriate mass ratio, dissolve in an appropriate amount of tetrahydrofuran; and take a certain volume of PBS (PH=7.4) For the buffer solution, slowly and uniformly inject the tetrahydrofuran solution (the needle is located under the liquid surface) into the PBS solution at an appropriate speed under constant stirring, after the addition is completed, continue to stir for a certain period of time, remove the tetrahydrofuran by rotary evaporation under reduced pressure at 37°C, and obtain the drug plastid. No precipitation by visual inspection, uniform dispersion system with light blue opalescence; spherical vesicle structure can be seen under optical microscope. As measured by a laser nanometer particle size analyzer, the particle size of the drug plasmid is 170-250nm, and the Zeta potentia...
Embodiment 3
[0034] Example 3 Inhibitory Effect of Tamibarotene-Cytarabine Conjugate on Proliferation of Human Leukemia Cell HL60
[0035] Test compound: Tamibarotene-cytarabine conjugate (Am80-Ara-C) prepared in Example 1;
[0036] Human leukemia cell line HL-60 was regularly subcultured with RPMI1640 medium (containing 15% extinguished fetal bovine serum, penicillin 100u / ml, streptomycin 100u / ml).
[0037] Drug preparation:
[0038]Positive control group: Weigh an appropriate amount of tamibarotene (Am80), cytarabine (Ara-C) and the physical mixture of tamibarotene and cytarabine (Am80+Ara-C), add DMSO medium, ultrasonically dissolved fully, and the final concentration was 10 -1 mol / L. Dissolve the test drug in DMSO to prepare a solution with the same amount and concentration as the positive control group. Before use, it was diluted step by step to make the final concentration 2×10 -3 mol / L, 4×10 -4 mol / L, 8×10 -5 mol / L, 1.6×10 -5 mol / L, 3.2×10 -6 mol / L, 6.4×10 -7 mol / L, 1.28×1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com