Phospholipase A2 sensitive glycerin skeleton anti-tumor prodrug and high-dispersing preparation thereof
An anti-tumor drug and anti-tumor technology, applied in the field of biomedicine, can solve the problems of low encapsulation rate, leakage of drugs into external water, etc., and achieve high-efficiency anti-tumor effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 11- 18
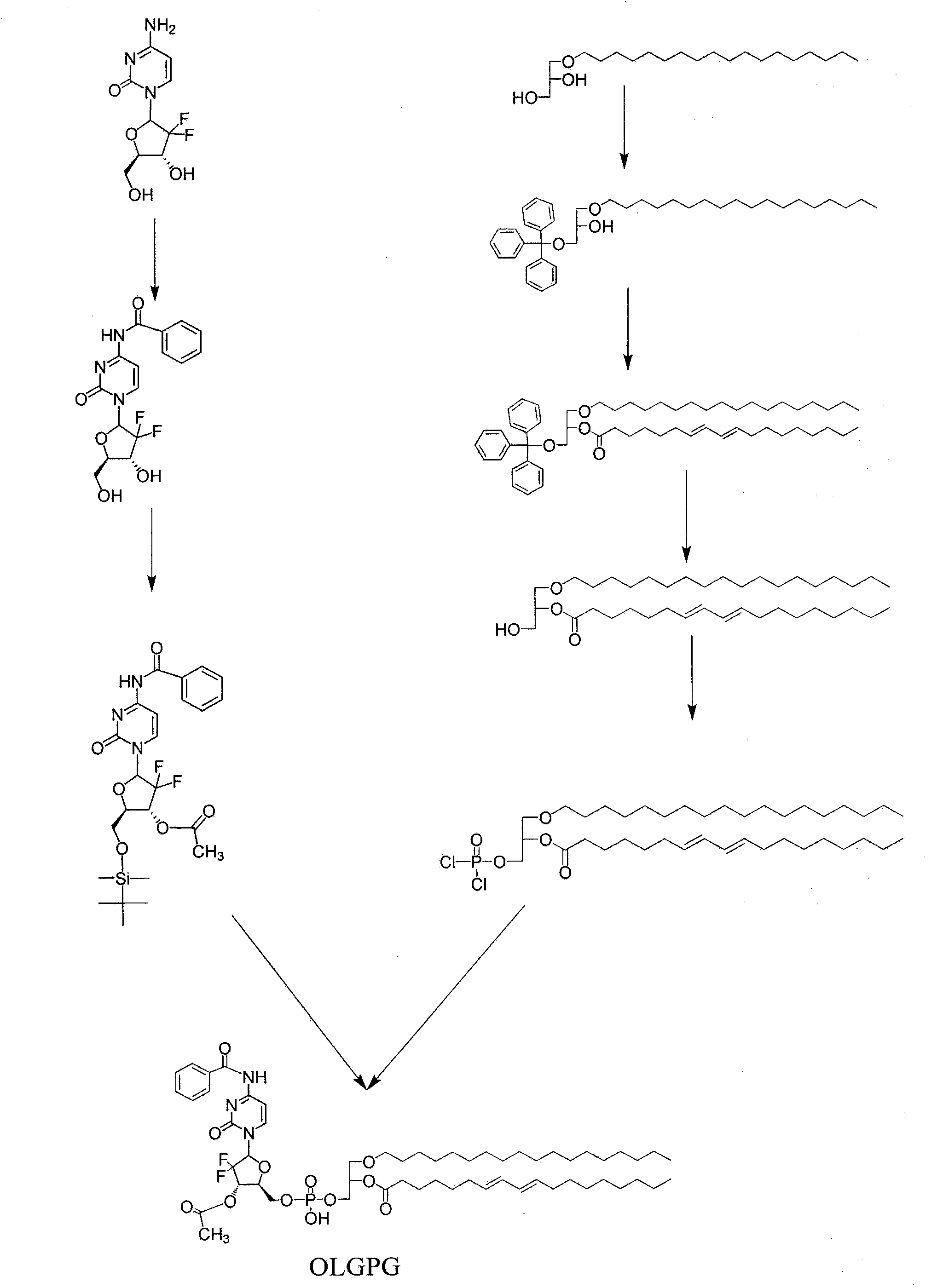
[0035] Example 1.1-octadecyl-2-conjugated linoleoyl-3-phosphoryl gemcitabine glyceride (OLGPG)
[0036] 1. Synthesis of N-benzoyl-3'-O-acetyl gemcitabine (BAG)
[0037] Dissolve gemcitabine (0.2638g, 1mmol) in 50ml of ethanol, reflux at 80°C, add benzoic anhydride (0.2728g, 1.2mmol) to continue the reaction for 1 hour, then add 1 part of benzoic anhydride (1.2mmol) per hour, and continue to add 3 time, a total of 3 hours, after the last 1 portion of benzoic anhydride was added, the reflux reaction was continued for 1 hour, and the solvent was removed under reduced pressure to obtain a colorless transparent viscous liquid. Dissolved in chloroform containing 4% methanol, separated on a silica gel column, collected dichloromethane: methanol = 15: 1 the corresponding components washed out from the eluent, and removed the solvent under reduced pressure to obtain white powdery N-benzoyl gemcitabine (BG).
[0038] Dissolve BG (0.3683g, 1.00mmol) and imidazole (0.2238g, 3.29mmol) in...
Embodiment 21- 18
[0053] Example 2. Preparation of sodium salt of 1-octadecyl-2-conjugated linoleoyl-3-phosphoryl gemcitabine glyceride
[0054] Molecular formula C 57 h 89 f 2 N 3 o 12 PNa. Take 1.078 g of 1-octadecyl-2-conjugated linoleoyl-3-phosphoryl gemcitabine glyceride, dissolve it in 10 ml of chloroform relative to 0.001 mol, add methanol solution containing 0.001 mol of NaOH, shake and sonicate, reduce The solvent was evaporated under pressure, and recrystallized from methanol to obtain white crystals of sodium salt of 1-octadecyl-2-conjugated linoleoyl-3-phosphoryl gemcitabine glyceride. Thin layer chromatography showed a spot. Other alkali salts such as potassium salts, calcium salts, magnesium salts, ammonia salts, and organic amine salts have similar preparation methods.
Embodiment 3
[0055] Example 3. Preparation of 1-stearyl ether-2-conjugated linoleoyl-3-phosphoryl fluoride darabine glyceride liposomes
[0056] Take 25 mg of 1-octadecyl ether-2-conjugated linoleoyl-3-phosphoryl fluoride darabine glyceride and 0.1 g of soybean lecithin in a 250 ml flask, dissolve in 20 ml of dichloromethane, and evaporate under reduced pressure. To obtain a layer of organic fat-soluble film, add 10ml of phosphate buffer solution with pH 7.4, oscillate, most of the film falls off, and sonicate at 50°C until a uniform suspension is obtained. Observe under a microscope, most of the particles are less than 1 micron in diameter. It is 1-stearyl ether-2-conjugated linoleoyl-3-phosphoryl fluoride darabine glyceride liposome.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com