Tamibarotene solid preparation and preparation method thereof
A technology of Tamibarotene and solid preparations, which is applied in the field of medicine, can solve problems such as the composition and preparation methods of Tamibarotene solid preparations, and achieve excellent quality results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
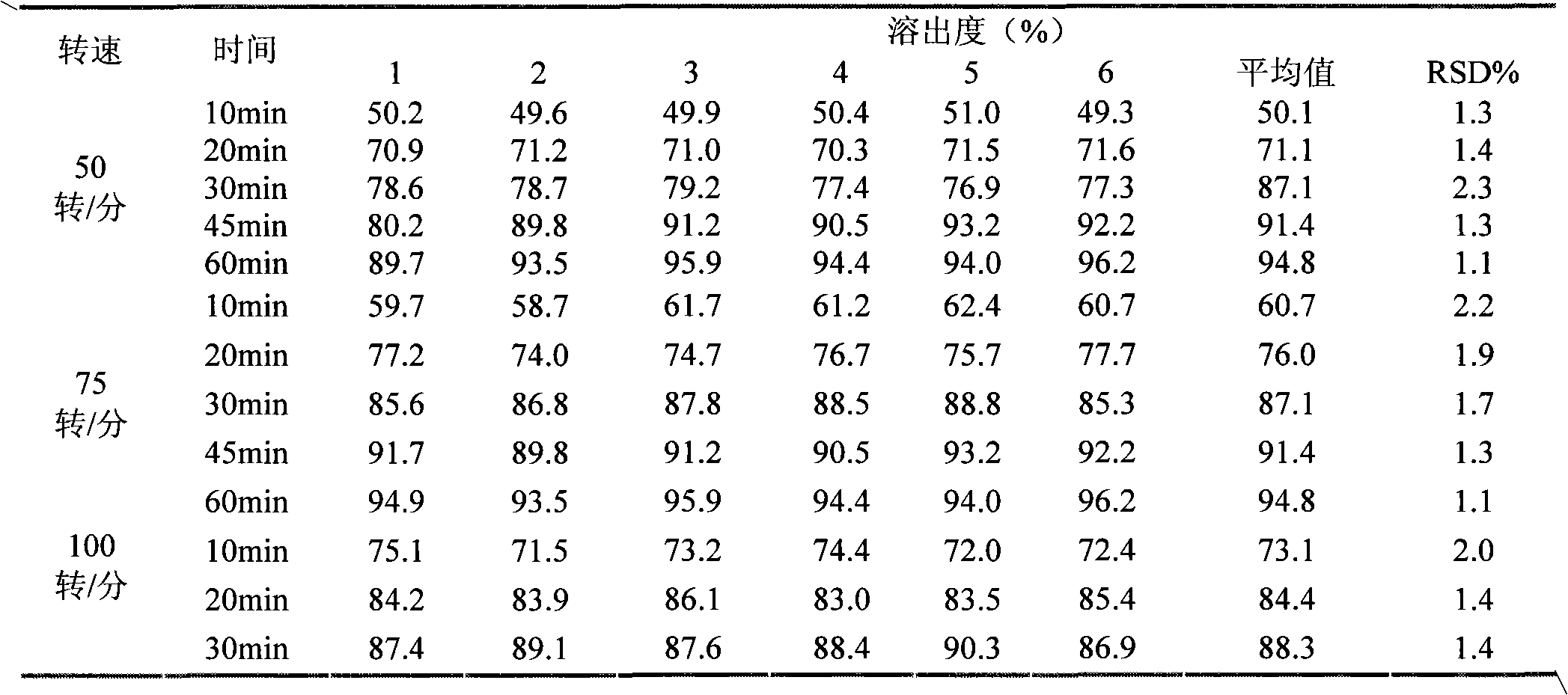
Image
Examples
Embodiment 1
[0097] Tamibarotene 1.71g, disintegrant 5.13g, filler 78.23g, lubricant 0.43g.
Embodiment 2
[0099] Tamibarotene accounts for 1.96g, disintegrant 4.36g, filler 78.66g, lubricant 0.52g.
Embodiment 3
[0101] Tamibarotene 2.56g, disintegrant 4.54g, filler 77.81g, lubricant 0.59g.
[0102] The disintegrant in the above-mentioned embodiment is a high-efficiency disintegrant.
[0103] The disintegrants of the above examples are preferably croscarmellose sodium, crospovidone, sodium carboxymethyl starch, microcrystalline cellulose, pregelatinized starch, low-substituted hydroxypropyl methylcellulose, amino acid One or several types of processing agar.
[0104] The fillers in the above examples include but are not limited to the fillers described in the prior art, especially the lubricants commonly used in solid preparations in "Pharmacy", such as talcum powder and magnesium stearate.
[0105] Lubricants in the above examples include but are not limited to lubricants described in the prior art, especially lubricants commonly used in solid preparations in Pharmaceutics, such as talcum powder and magnesium stearate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com