Method for preparing dispersible tablets of tamibarotene
A technology for tamibarotene and dispersible tablets, which is applied in the field of preparation of tamibarotene dispersible tablets, can solve the problems of unqualified content uniformity, dry and itchy skin, and main drug dust floating, and achieves fluidity and reliability. Good pressure, avoid adverse reactions, improve the effect of bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Embodiment 1 Comparison of physical mixing method and solvent deposition method for preparing Tamibarotene Tablets
[0019] Process A: Preparation by physical mixing method
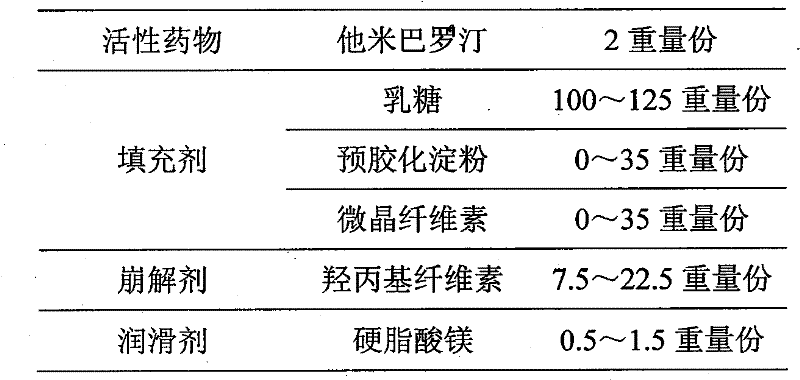
[0020]
[0021] Preparation process: all auxiliary materials (except magnesium stearate) pass through a 80-mesh sieve, and mix evenly. Magnesium stearate through a mesh sieve, further mixed evenly, and directly compressed into tablets.
[0022] Process B: Preparation by solvent deposition
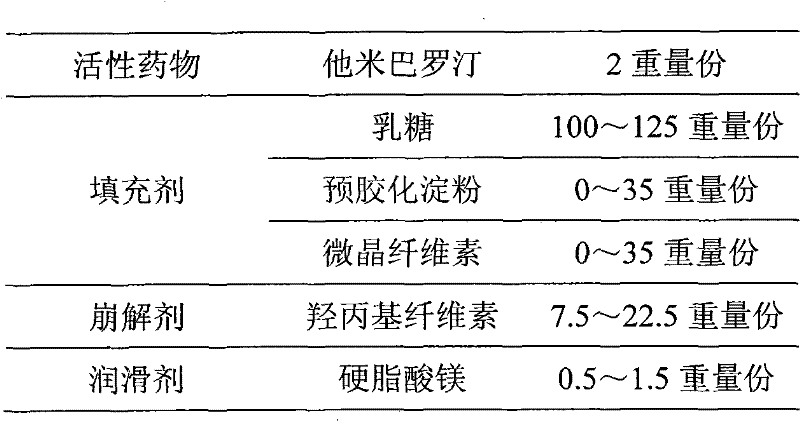
[0023]
[0024] Preparation process: pass all excipients (except magnesium stearate) through 80-mesh sieve, mix evenly, dissolve tamibarotene raw material in ethanol, spray evenly on the above-mentioned excipients mixed uniformly, at 30-40°C Dry to dryness, pass through an 80-mesh sieve and mix evenly, then add magnesium stearate that has passed through an 80-mesh sieve, further mix evenly, and directly compress into tablets.
[0025] Comparing the two preparation processes, the properties of Tamibarote...
Embodiment 2
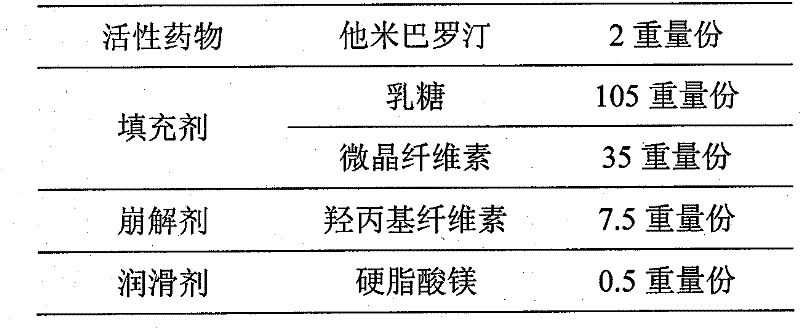
[0029]
[0030] Preparation process: pass all excipients (except magnesium stearate) through 80-mesh sieve, mix evenly, dissolve tamibarotene raw material in medicinal absolute ethanol to make tamibarotene ethanol solution, and then spray evenly In the uniformly mixed auxiliary materials, dry to dry at 30-50°C, pass through an 80-mesh sieve and mix evenly, then add magnesium stearate that has passed through an 80-mesh sieve, further mix evenly, and directly compress into tablets.
Embodiment 3
[0032]
[0033] Preparation process: Dissolve the tamibarotene raw material in medicinal absolute ethanol to make a tamibarotene / ethanol solution, and then evenly spray it on the pregelatinized starch that has passed through a 80-mesh sieve, mix well, Dry to dry at 30-50°C, pass through 80-mesh sieve for later use, pass lactose and hydroxypropyl cellulose through 80-mesh sieve, mix evenly with the mixed powder treated by solvent deposition method by equal volume addition method, and then add Magnesium stearate with 80-mesh sieve, further mixed evenly, and directly compressed into tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com