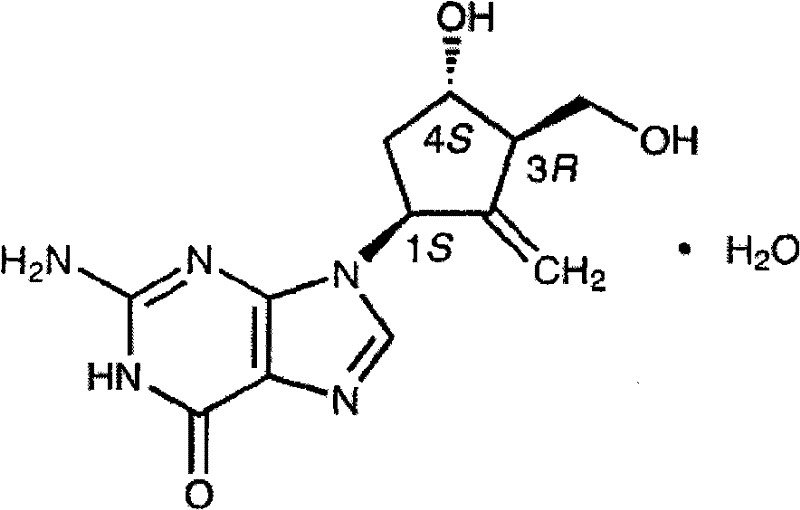
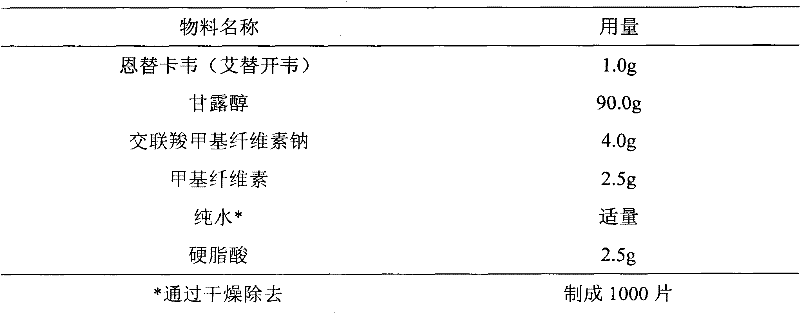
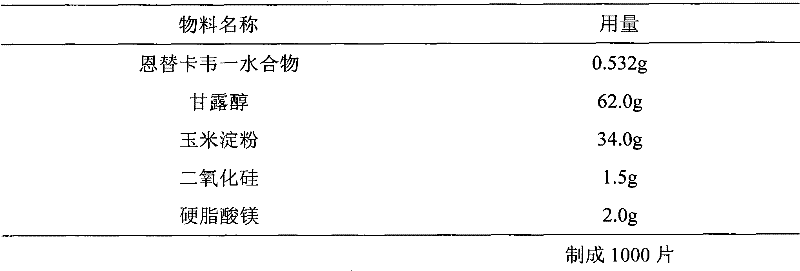
Entecavir tablet and preparation method thereof
A technology for entecavir and cavir tablets, applied in the field of entecavir tablets and their preparation, can solve the problems of complex technological process and difficulty in industrialized production, and achieve the effects of good product quality, rapid dissolution rate, and good content uniformity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033]
[0034] 0.532 g of entecavir monohydrate is equivalent to 0.5 g of entecavir.
[0035] Preparation method: mix entecavir monohydrate (d(0.9)=8.6μm) with mannitol and cornstarch evenly, add purified water to granulate, granulate, and dry in a fluidized bed to obtain dry granules, and add colloidal dioxide to the dry granules Silicone and magnesium stearate are mixed and compressed into tablets.
[0036] Stability test
[0037] The product of embodiment 1 and the product of comparative example 2 (0.1N hydrochloric acid is solvent) are placed on 60 ℃, under the condition of 75% relative humidity, in 5 days, 10 days sampling uses HPLC to detect content and related substance, and with 0 The results are shown in Table 1 below.
[0038] Table 1:
[0039]
[0040] It can be seen that the addition of hydrochloric acid can lead to a significant change in product stability.
Embodiment 2
[0042]
[0043] 1.064g of entecavir monohydrate is equivalent to 1.0g of entecavir.
[0044] Preparation method: Entecavir monohydrate is micronized to a particle size of d(0.9)=47.3 μm, mixed evenly with entecavir monohydrate, lactose monohydrate, pregelatinized starch, and cross-linked povidone, and then added into povidone aqueous solution to granulate , granulated, and dried in a fluidized bed to obtain dry granules, which are mixed with magnesium stearate and compressed into tablets.
Embodiment 3
[0046]
[0047] 0.532 g of entecavir monohydrate is equivalent to 0.5 g of entecavir.
[0048] Preparation method: Entecavir monohydrate is micronized to a particle size of d(0.9)=11.8 μm, 0.532 g of entecavir monohydrate and about 6 g of lactose monohydrate are mixed uniformly in equal increments, and then mixed with the remaining lactose monohydrate , microcrystalline cellulose, and half of the amount of crospovidone are mixed evenly, and the povidone aqueous solution is added to granulate, granulated, and dried in a fluidized bed to obtain dry granules, and the remaining crospovidone and stearin are added to the dry granules Magnesium acid mixed, compressed into tablets.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com