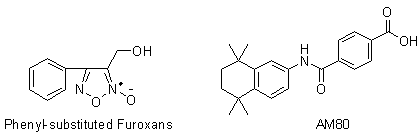Nitric oxide donor tamibarotene derivatives and their preparation method and use
A technology of Tamibarotene and Nitric Oxide, applied in organic chemistry, drug combination, pharmaceutical formulation, etc., can solve the problem of few research reports
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
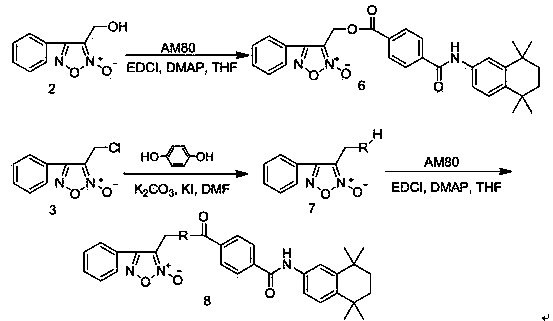
[0045] Example 1: 3-Hydroxymethyl-4-phenyl-1,2,5-oxadiazole-2-oxide ( 2 ) preparation
[0046] Add cinnamyl alcohol (53.7g, 0.40mol) and sodium nitrite (55.2g, 0.80mol) into 500mL of chloroform, stir at room temperature until the cinnamyl alcohol is completely dissolved, then slowly add glacial acetic acid (48.0g, 0.80mol), after the dropwise addition, continue to stir and react at room temperature for 1.0h, filter with suction, wash the filtrate twice with distilled water, dry over anhydrous sodium sulfate, filter with suction, concentrate under reduced pressure to remove the solvent, and obtain a brownish-yellow oily substance, which is subjected to silica gel column chromatography Separation (ethyl acetate:petroleum ether=1:5), the eluate was concentrated under reduced pressure to give a light yellow solid ( 2 ) (48.0g, 62.4%), mp 64~66℃.
Embodiment 2
[0047] Example 2: 3-Chloromethyl-4-phenyl-1,2,5-oxadiazole-2-oxide ( 3 ) preparation
[0048] 3-Hydroxymethyl-4-phenyl-1,2,5-oxadiazole-2-oxide ( 2 ) (38.4g, 0.2mol) and anhydrous pyridine (32.2mL, 0.4mol) were added to 600mL of anhydrous dichloromethane, stirred at room temperature to dissolve completely, cooled to 0~5°C in an ice-water bath, and then slowly dropped in 1.0h Add thionyl chloride (28.4mL, 0.4mol), remove the ice water bath after the dropwise addition, stir the reaction at room temperature for 12.0h, slowly pour the reaction solution into 500mL ice water under vigorous stirring, separate the organic layer, and successively use saturated chloride Sodium solution, saturated sodium bicarbonate solution, saturated sodium chloride solution and distilled water washed, dried over anhydrous sodium sulfate, suction filtered, the filtrate was concentrated to remove solvent, separated by silica gel column chromatography (ethyl acetate:petroleum ether=1:20), washed Dehy...
Embodiment 3
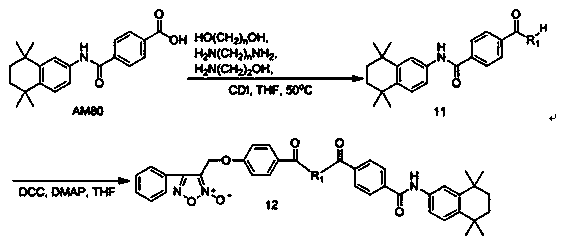
[0049] Example 3: 3-((4-carboxyphenoxy)methyl)-4-phenyl-1,2,5-oxadiazole-2-oxide ( 5 ) preparation
[0050] 3-Chloromethyl-4-phenyl-1,2,5-oxadiazole-2-oxide ( 3 ) (10.5g, 0.05mol) and methyl p-hydroxybenzoate (7.6g, 0.05mol) were dissolved in 250mL DMF, then anhydrous potassium carbonate (27.6g, 0.2mol) and potassium iodide (8.3g, 0.05mol) were added , stirred at room temperature for 12 hours, then poured the reaction solution into 1000mL water to precipitate solids under vigorous stirring, filtered the filter cake in 500mL ether, washed it with saturated sodium bicarbonate solution, saturated sodium chloride solution and distilled water successively, and no dried over sodium sulfate, suction filtered, the filtrate was concentrated to remove the solvent, the residue was subjected to silica gel column chromatography (ethyl acetate:petroleum ether=1:5), and the eluent was concentrated under reduced pressure to obtain a white crystalline solid ( 4 ) (13.6g, 83.4%), mp 97.0~98...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com