Method for synthesizing 5,6,7,8-tetrahydro-5,5,8,8-tetramethyl-2-naphthylamine
A synthesis method, tetramethyl technology, applied in the field of synthesis of 5,6,7,8-tetrahydro-5,5,8,8-tetramethyl-2-naphthylamine, can solve the problem of high pollution and safety Poor performance, high cost and other problems, to achieve the effect of high reaction yield, high safety, simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
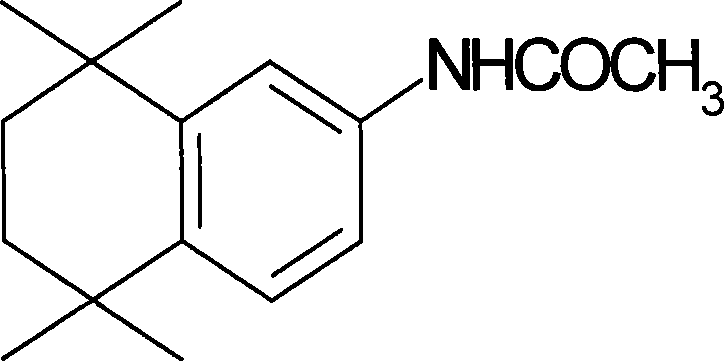
[0014] 5,6,7,8-tetrahydro-5,5,8,8-tetramethyl-2-naphthamide, its structural formula is as follows:
[0015]
[0016] Put 20g into a three-necked flask, add 200ml ethylene glycol and 5.8g potassium hydroxide, heat to 100°C, stir for 2 hours, cool, pour the reaction solution into a large amount of water, let it stand overnight, filter, and recrystallize the solid with methanol / water , Get yellow solid 14.2g, yield 85.7%, mp: 71-74, 1H-NMR (300MZ, CHCl3) 1.24 (6H) 1.27 (6H) 1.69 (4H) 3.5-3.9 (2H) 6.56 (1H) 6.68 ( 1H) 7.13 (1H).
[0017] Among them, the preparation method of 5,6,7,8-tetrahydro-5,5,8,8-tetramethyl-2-naphthamide can be referred to the description in EP0478787.
Embodiment 2
[0019] Put 20g of 5,6,7,8-tetrahydro-5,5,8,8-tetramethyl-2-naphthamide into a three-necked flask, add 200ml glycerol and 7.0g sodium hydroxide, and heat to 150℃ After stirring for 2 hours, cooling, the reaction solution was poured into a large amount of water, placed overnight, filtered, and the solid was recrystallized with methanol / water to obtain 14.5 g of a yellow solid with a yield of 87.5%.
Embodiment 3
[0021] Put 18g of 5,6,7,8-tetrahydro-5,5,8,8-tetramethyl-2-naphthamide into a three-necked flask, add 100ml of 1,2-propanediol and 6.5g of sodium hydroxide, and heat up After stirring at 130°C for 2 hours, cooling, the reaction solution was poured into a large amount of water, placed overnight, filtered, and the solid was recrystallized with methanol / water to obtain 12.1 g of a yellow solid with a yield of 83.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com