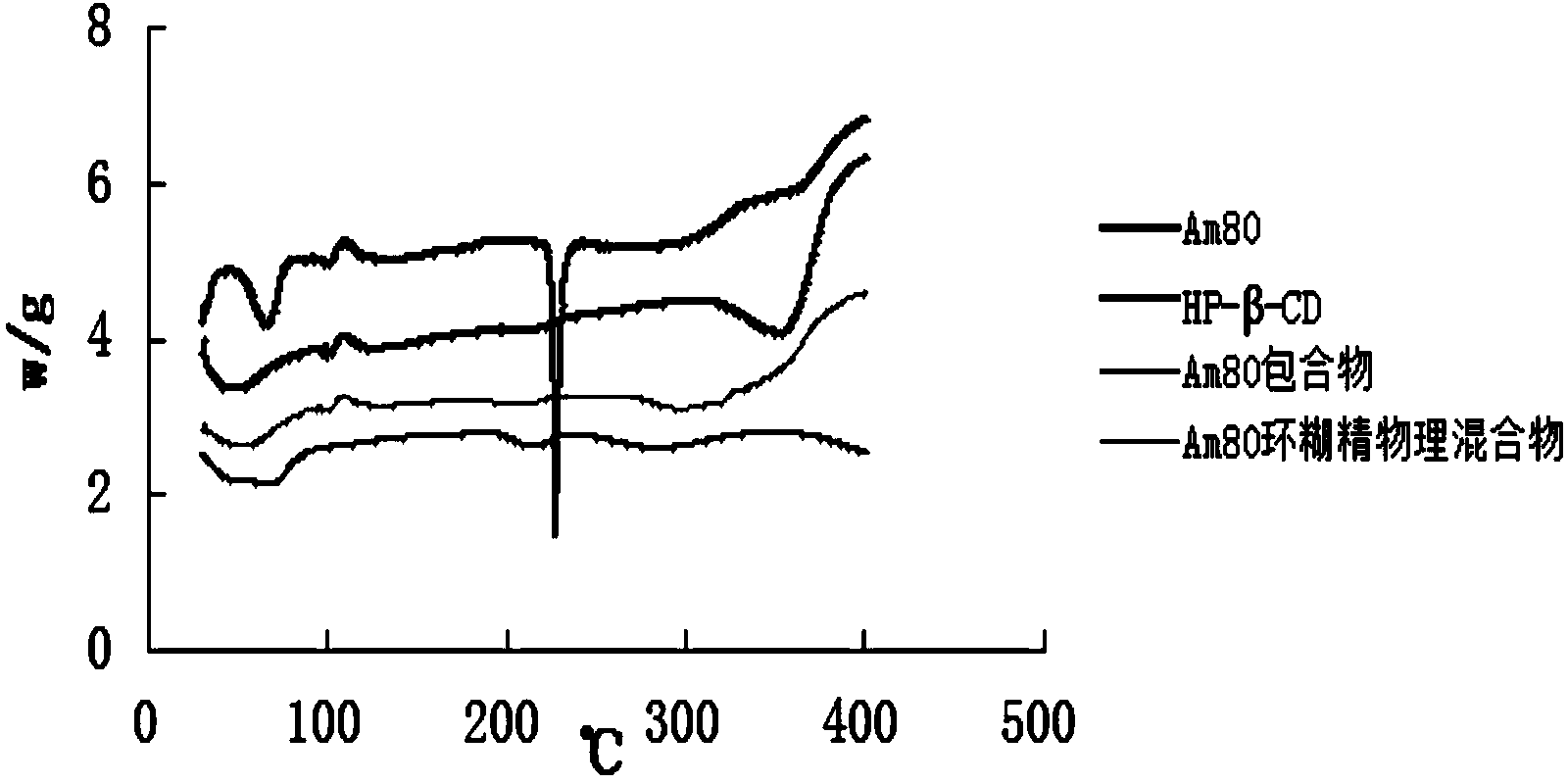
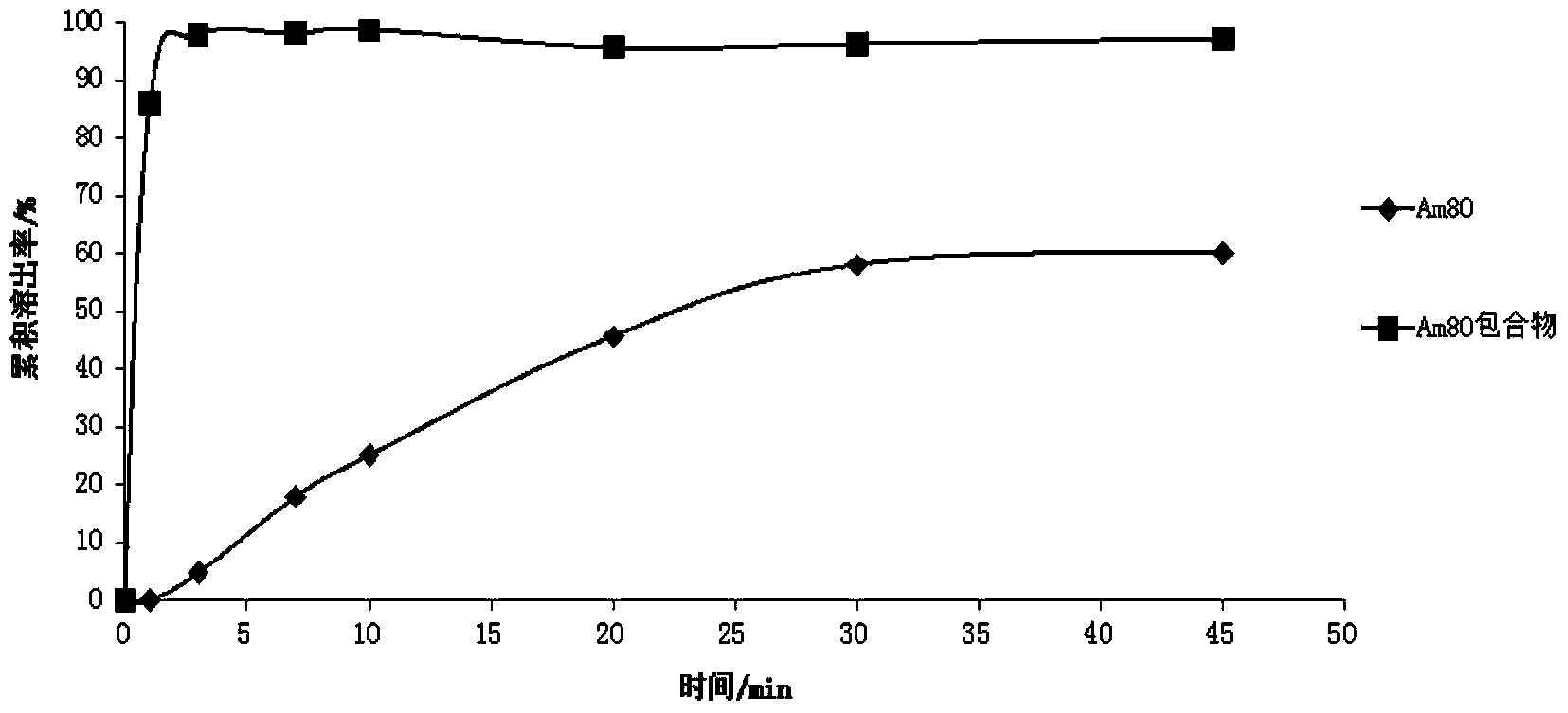
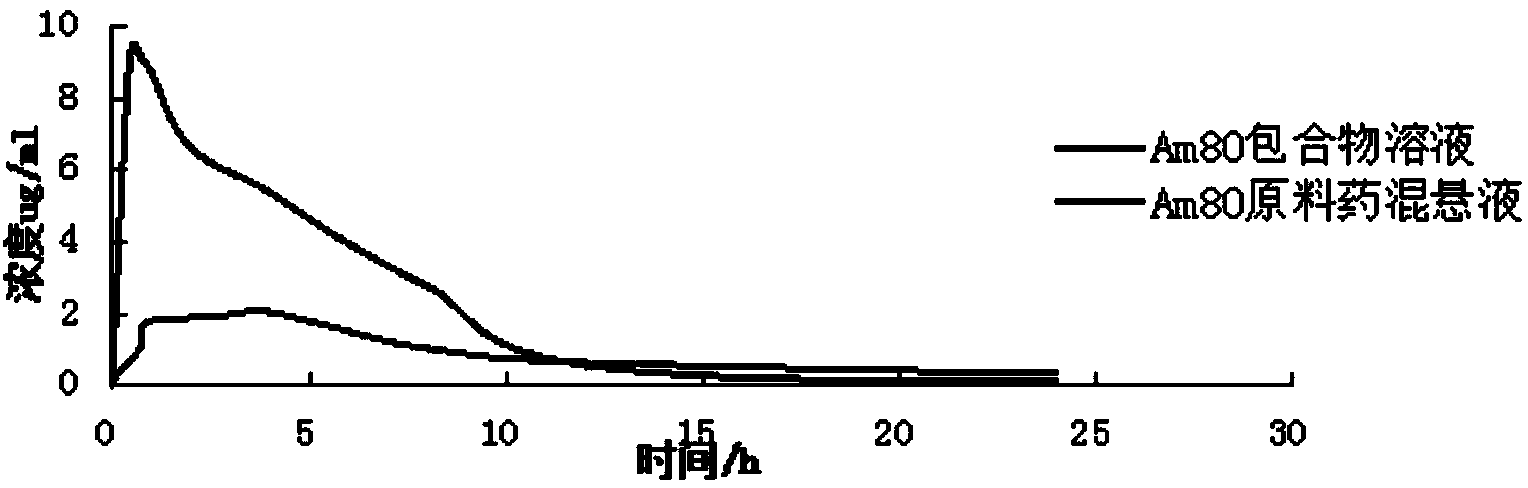
Tamibarotene cyclodextrin or cyclodextrin derivative clathrate and preparation method thereof
A technology of tamibarotene and cyclodextrin, applied in the field of medicine, can solve the problems of poor oral absorption, difficult oral or injection administration, etc., and achieves the effects of good dissolution, good clinical application and high bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1 Preparation of Liquid Tamibarotene Hydroxypropyl-β-Cyclodextrin Inclusion Complex Solution
[0029] Proceed as follows:
[0030] (1) Weigh 61 mg of hydroxypropyl-β-cyclodextrin, pour into 5 ml of distilled water, and stir to dissolve.
[0031] (2) Also known as Tamibarotene 5mg, add 5ml of absolute ethanol to dissolve, and pour this solution into the above-mentioned hydroxypropyl-β-cyclodextrin solution.
[0032] (3) The mixed solution was stirred at 45°C for 1 hour by magnetic stirring method, the ethanol was removed by rotary evaporation under reduced pressure, and the unincluded drug was removed by filtration to obtain the inclusion complex of tamibarotene hydroxypropyl-β-cyclodextrin derivative substance solution.
Embodiment 2
[0033] Example 2 Preparation of Solid Tamibarotene Hydroxypropyl-β-cyclodextrin Inclusion Compound
[0034] Proceed as follows:
[0035] (1), (2), (3) are the same as embodiment 1.
[0036] (4) Freeze-drying the inclusion compound solution of the tamibarotene hydroxypropyl-β-cyclodextrin derivative to obtain a solid tamibarotene hydroxypropyl-β-cyclodextrin inclusion compound.
Embodiment 3
[0037] Example 3 Preparation of Solid Tamibarotene Hydroxypropyl-β-cyclodextrin Inclusion Compound
[0038] Proceed as follows:
[0039] (1) Weigh 122mg of hydroxypropyl-β-cyclodextrin, put it into a mortar, add 2ml of distilled water, and grind to make a paste;
[0040] (2) Another 10 mg of tamibarotene was weighed, dissolved in 2 ml of absolute ethanol, and poured into the above-mentioned hydroxypropyl-β-cyclodextrin solution.
[0041] (3) The mixture was ground for 2 hours to obtain a uniform paste, which was filtered and evaporated to dryness under reduced pressure to obtain the inclusion compound of a solid tamibarotene cyclodextrin derivative.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com