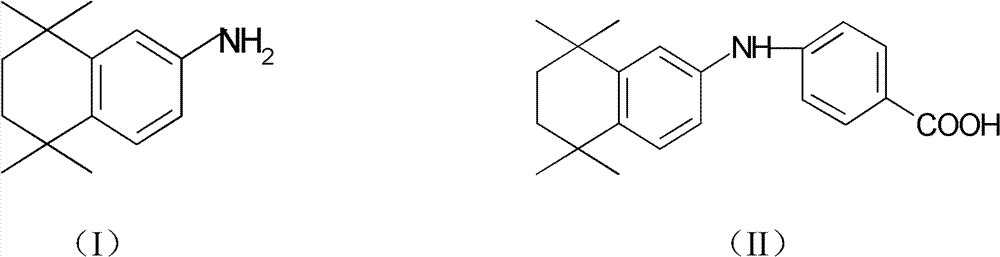
Method for synthesizing 5,6,7,8-tetrahydro-5,5,8,8-tetramethyl-2-naphthylamine
A synthesis method and tetramethyl technology are applied in the synthesis field of 5,6,7,8-tetrahydro-5,5,8,8-tetramethyl-2-naphthylamine, which can solve the problem of high pollution and safety It has the problems of poor performance and high cost, and achieves the effects of high reaction yield, high safety and simple operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
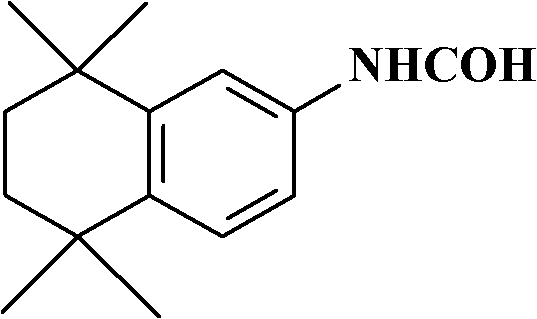
[0014] 5,6,7,8-tetrahydro-5,5,8,8-tetramethyl-2-naphthalenecarboxamide, its structural formula is as follows:
[0015]
[0016] Take 20g into a three-necked flask, add 200ml of ethylene glycol and 5.8g of potassium hydroxide, raise the temperature to 100°C, stir for 2 hours, cool down, pour the reaction solution into a large amount of water, leave it overnight, filter, and recrystallize the solid with methanol / water , to obtain yellow solid 14.2g, yield 85.7%, mp: 71-74, 1H-NMR (300MZ, CHCl3) 1.24 (6H) 1.27 (6H) 1.69 (4H) 3.5-3.9 (2H) 6.56 (1H) 6.68 ( 1H) 7.13(1H).
[0017] Wherein, the preparation method of 5,6,7,8-tetrahydro-5,5,8,8-tetramethyl-2-naphthylcarboxamide can refer to the description in EP0478787.
Embodiment 2
[0019] Put 20g of 5,6,7,8-tetrahydro-5,5,8,8-tetramethyl-2-naphthylcarboxamide into a three-necked flask, add 200ml glycerol and 7.0g sodium hydroxide, and heat up to 150°C , stirred for 2 hours, cooled, the reaction solution was poured into a large amount of water, left overnight, filtered, and the solid was recrystallized from methanol / water to obtain 14.5 g of a yellow solid, with a yield of 87.5%.
Embodiment 3
[0021] Put 18g of 5,6,7,8-tetrahydro-5,5,8,8-tetramethyl-2-naphthylcarboxamide into a three-necked flask, add 100ml of 1,2-propanediol and 6.5g of sodium hydroxide, and heat up to 130°C, stirred for 2 hours, cooled, poured the reaction solution into a large amount of water, left overnight, filtered, and recrystallized the solid with methanol / water to obtain 12.1 g of a yellow solid, with a yield of 83.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com