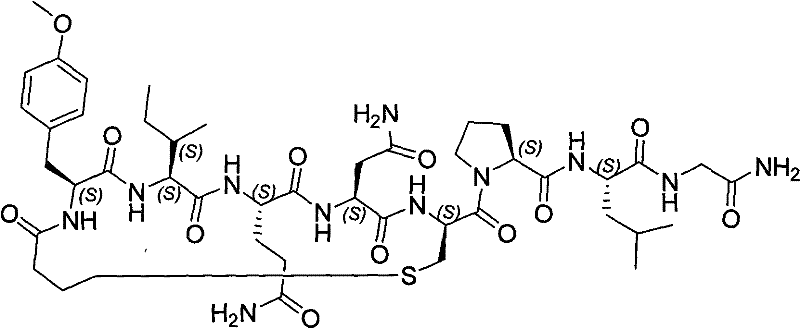
Solid phase preparation method of carbetocin
A technology of carbetocin and solid-phase synthesis, which is applied in the field of solid-phase synthesis of polypeptides, and achieves the effects of simple reaction operation, high yield, and easy post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Embodiment 1: the preparation of Fmoc-Gly-amino resin
[0045] Add 14.2g of amino resin, with a substitution degree of 0.7mmol / g, into the solid-phase reaction column, add DCM to swell the resin for 30 minutes, and place 5.95g of Fmoc-Gly-OH, 7.6g of HATU, and 2.97g of HOAt in an ice bath. Dissolve in DMF, add to the above resin and react for 10 minutes, then add 2.4ml TMP, and react at room temperature for 45 minutes. After washing with DMF for 3 times, washing with DCM for 3 times, shrinking with methanol for 3+5+8min to obtain Fmoc-Gly-amino resin, the detection degree of substitution is 0.6mmol / g. The so-called "3+5+8min" means shrinking with methanol three times for 3min, 5min and 8min respectively.
Embodiment 2
[0046] Embodiment 2: the preparation of Fmoc-Gly-amino resin
[0047] Add 14.2g of Rink Amide amino resin, with a substitution degree of 0.2mmol / g, into the solid-phase reaction column. After adding DCM to swell the resin for 30 minutes, mix 1.7g of Fmoc-Gly-OH, 2.2g of HATU, and 0.85g of HOAt on ice Dissolve in DMF in a bath, add to the above resin and react for 10 minutes, then add 5-15ml DIPEA to improve the reaction efficiency, and react at room temperature for 45 minutes. After washing with DMF for 3 times, washing with DCM for 3 times, shrinking with methanol for 3+5+8min to obtain Fmoc-Gly-amino resin, the detection degree of substitution is 0.13mmol / g. The so-called "3+5+8min" means shrinking with methanol three times for 3min, 5min and 8min respectively.
Embodiment 3
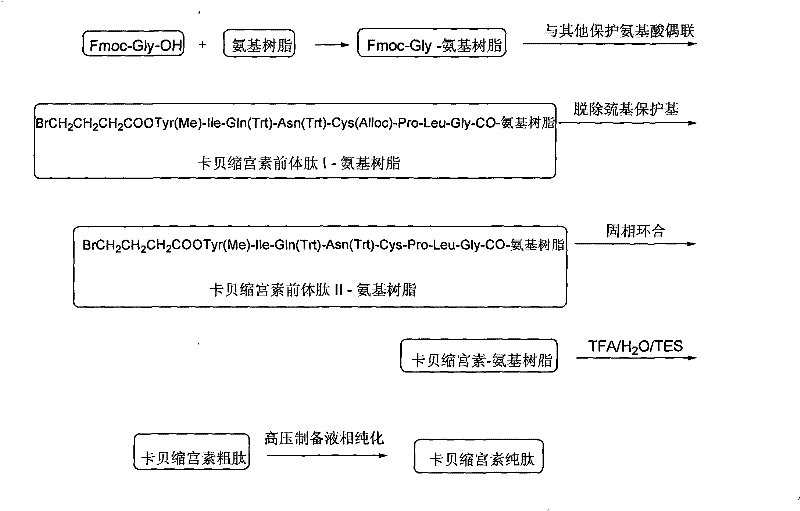
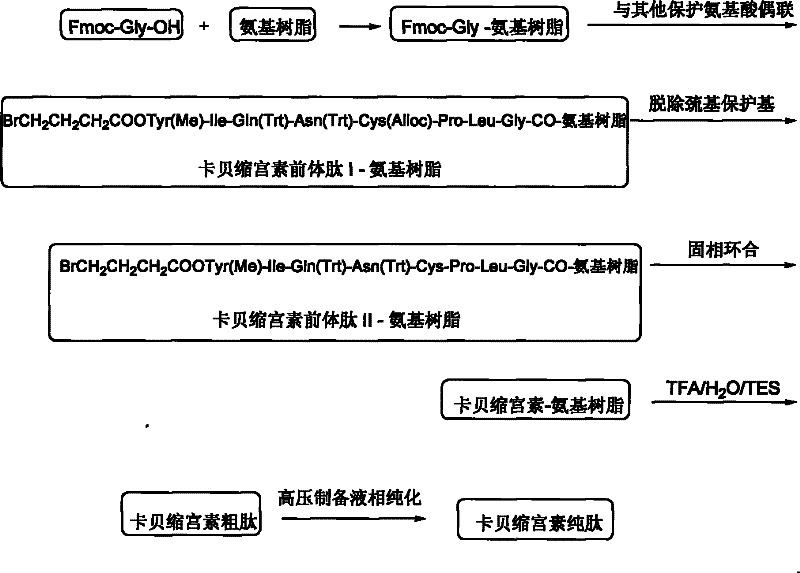
[0048] Embodiment 3: carbetocin precursor peptide I-amino resin (BrCH 2 CH 2 CH 2 Preparation of COOTyr(Me)-Ile-Gln(Trt)-Asn(Trt)-Cys(Alloc)-Pro-Leu-Gly-CO-aminoresin)
[0049] Weigh 1 mmol of Fmoc-Gly-amino resin into the reactor, swell with DCM for 0.5 h, then use 20% DBLK for 10 min to remove Fmoc protection, and connect Fmoc-Leu-OH after washing. Dissolve 1.78g Fmoc-Leu-OH, 0.49g HOBt, and 0.61ml DIC in DCM (a small amount of DMF can be added to aid dissolution), activate in an ice-water bath for 7 minutes, add to a solid-phase reactor, and react at room temperature for 1-2 hours. The end point of the reaction was determined by the ninhydrin method. Repeat the above steps, and use the amino acids of the Fmoc protecting group to sequentially complete the remaining connections to obtain the carbetocin precursor peptide I-amino resin. The structure is: BrCH 2 CH 2 CH 2 COOTyr(Me)-Ile-Gln(Trt)-Asn(Trt)-Cys(Alloc)-Pro-Leu-Gly-CO-aminoresin.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com