Preparation method of melanotan-II
A technology of notan and amino resin, which is applied in the field of preparing menanotan, can solve the problems of unfavorable industrial production, complicated operation, and low application value, and achieve the effects of increasing yield, simple operation, and reducing production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
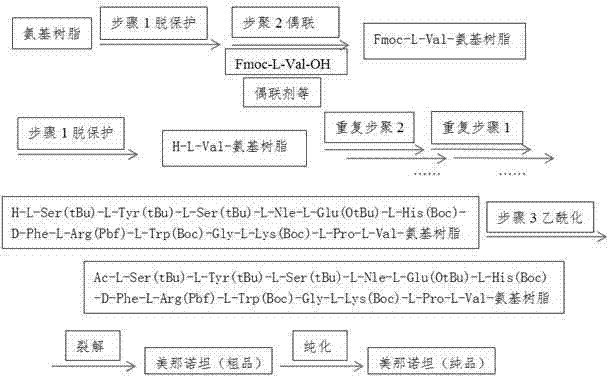
[0047] Such as figure 1 As shown, the preparation method claimed in the present invention comprises the following steps:
[0048] Step 1, Fmoc-L-Val-Rink Amide resin preparation
[0049] 1. Put 10 g of Rink Amide resin with a substitution degree of 0.6 mmol / g in the reactor, add NMP to wash twice, and then use NMP to swell for 1 hour.
[0050] 2. Add the piperidine NMP solution with a volume ratio of 1:4 to the swollen Rink Amide resin to remove the Fmoc protecting group on the Rink Amide resin. After the removal, wash with NMP 4 times, DCM 2 times, and methanol 2 times. Second-rate.
[0051] 3. Weigh 6.11g Fmoc-L-Val-OH, 2.43g HOBt, 6.83g HBTU, add 54ml NMP to dissolve, add 6.27ml DIPEA after completely dissolved, mix well and add to the reactor to start the reaction.
[0052] 4. React for 6 hours, finish the reaction, wash with NMP three times, measure 7.6ml of acetic anhydride and 6.5ml of pyridine, mix them in 40ml of NMP, add them to the reactor for 2 hours, wash with ...
Embodiment 2
[0070] Such as figure 1 As shown, the preparation method claimed in the present invention comprises the following steps:
[0071] Step 1, Fmoc-L-Val-Rink Amide resin preparation
[0072] 1. Put 30 g of Rink Amide resin with a substitution degree of 0.1 mmol / g in the reactor, add NMP to wash twice, and then use NMP to swell for 1 hour.
[0073] 2. Add the piperidine NMP solution with a volume ratio of 1:4 to the swollen Rink Amide resin to remove the Fmoc protecting group on the Rink Amide resin. After the removal, wash with NMP 4 times, DCM 2 times, and methanol 2 times. Second-rate.
[0074] 3. Weigh 10.2g Fmoc-L-Val-OH, 4.05g HOBt, 11.4g HBTU, add 80ml NMP to dissolve, add 10.5ml DIPEA after completely dissolved, mix well and add to the reactor to start the reaction.
[0075] 4. React for 1 hour, finish the reaction, wash with NMP three times, measure 3.8ml of acetic anhydride and 3.3ml of pyridine, mix them in 70ml of NMP, put them into the reactor for 2 hours, wash with...
Embodiment 3
[0093] Such as figure 1 As shown, the preparation method claimed in the present invention comprises the following steps:
[0094] Step 1, Fmoc-L-Val-Rink Amide resin preparation
[0095]1. Put 5 g of Rink Amide resin with a substitution degree of 1.6 mmol / g in the reactor, add NMP to wash twice, and then use NMP to swell for 1 hour.
[0096] 2. Add the piperidine NMP solution with a volume ratio of 1:4 to the swollen Rink Amide resin to remove the Fmoc protecting group on the Rink Amide resin. After the removal, wash with NMP 4 times, DCM 2 times, and methanol 2 times. Second-rate.
[0097] 3. Weigh 4.07g Fmoc-L-Val-OH, 1.62g HOBt, 4.55g HBTU, add 36ml NMP to dissolve, add 4.18ml DIPEA after completely dissolved, mix well and add to the reactor to start the reaction.
[0098] 4. React for 20 hours, finish the reaction, wash with NMP three times, measure 7.6ml of acetic anhydride and 6.5ml of pyridine, mix them in 40ml of NMP, add them to the reactor for 2 hours, wash with N...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of substitution | aaaaa | aaaaa |
| degree of substitution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com