Method for preparing polypeptide used for treating osteoporosis
A peptide fragment and peptide resin technology, applied in the field of medicinal chemistry, can solve the problems of difficulty, low product yield, difficult product purification, etc., and achieve the effects of less by-products, high product yield and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] Example 1: Preparation of Teriparatide according to Strategy 1
[0083] 1. Peptide resin fragment 1 (amino acids 34-26 of the teriparatide sequence)
[0084] 1. Preparation of Fmoc-Phe-Wang Resin
[0085] Weigh 1.2g of Wang Resin with a degree of substitution of 0.8mmol / g, add it to a solid-phase reaction column, wash it twice with DMF, and after swelling the resin with DMF for 30 minutes, weigh 0.51g of Fmoc-Phe-OH, 0.13g of HOBT and 0.01g DMAP was dissolved in DMF, activated by adding 0.16mL DIC in an ice-water bath, and then added to the above-mentioned reaction column equipped with resin. After 2 hours of reaction, 5mL pyridine and 5.5ml acetic anhydride were added to block for 12 hours. Washed 6 times with DMF to obtain Fmoc-Phe-Wang Resin, the detection degree of substitution was 0.20mmol / g.
[0086] 2. Preparation of peptide resin fragments
[0087] Weigh 1.5g of Fmoc-Phe-Wang Resin with a substitution degree of 0.20mmol / g, add it to a solid-phase reaction co...
Embodiment 2
[0107] Example 2: Preparation of Teriparatide according to Strategy 2
[0108] 1. Preparation of side chain protection peptide fragment b (25-11 amino acids of teriparatide sequence)
[0109] 1. Preparation of side chain protected peptide fragment b-peptide resin
[0110] Weigh 5.35g of 2-CTC resin with a degree of substitution of 0.47mmol / g, add it to a solid-phase reaction column, wash it twice with DMF, and swell the resin with DMF for 30 minutes, then take 3.24g of Fmoc-Arg(pbf)-OH Dissolve in DMF, activate by adding 1.7mL DIPEA in an ice-water bath, add to the above-mentioned reaction column equipped with resin, react for 2 hours, add 10mL of anhydrous methanol to seal for 1 hour, and wash with DMF 6 times. Fmoc protection was removed with DBLK, followed by 6 washes with DMF.
[0111]Dissolve 1.77g Fmoc-Leu-OH, 0.74g HOBt, and 0.86ml DIC in a mixed solution of DCM and DMF with a volume ratio of 1:1, add it to a solid-phase reaction column, and react at room temperature ...
Embodiment 3
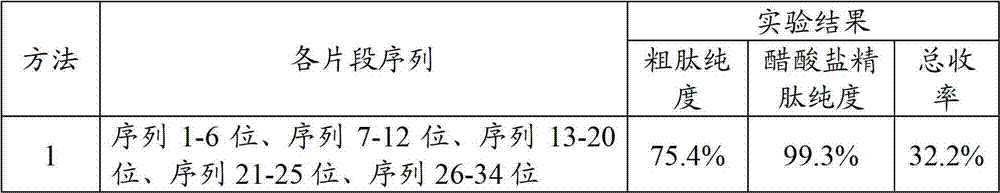
[0129] Teriparatide was prepared according to strategies 3, 4 and 5, respectively. The purity and total yield of the teriparatide crude peptide and the teriparatide acetate refined peptide prepared by each method were counted, and the results are shown in Table 1.
[0130] The teriparatide result that each method makes in table 1 embodiment 1~3
[0131]
[0132]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com