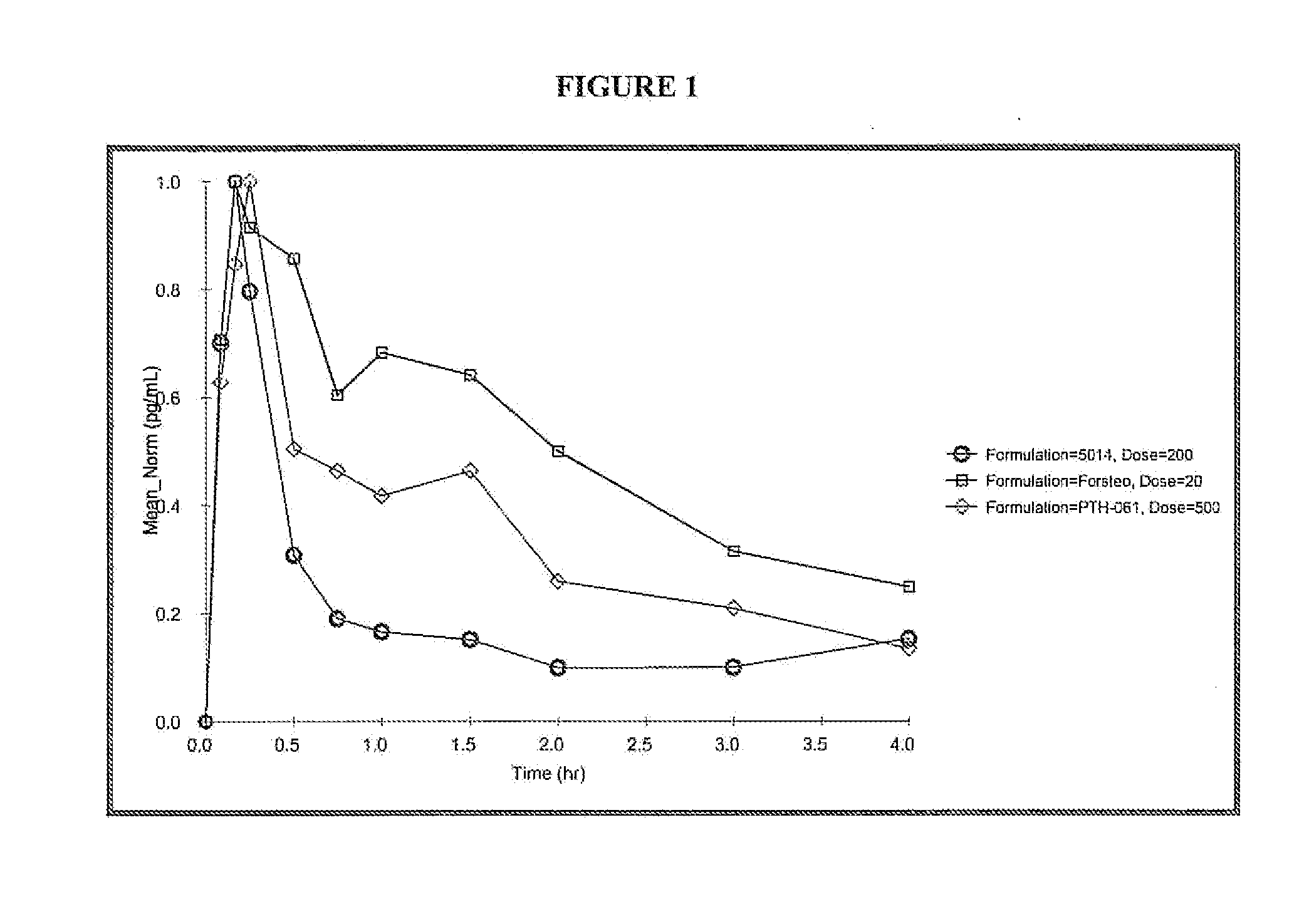
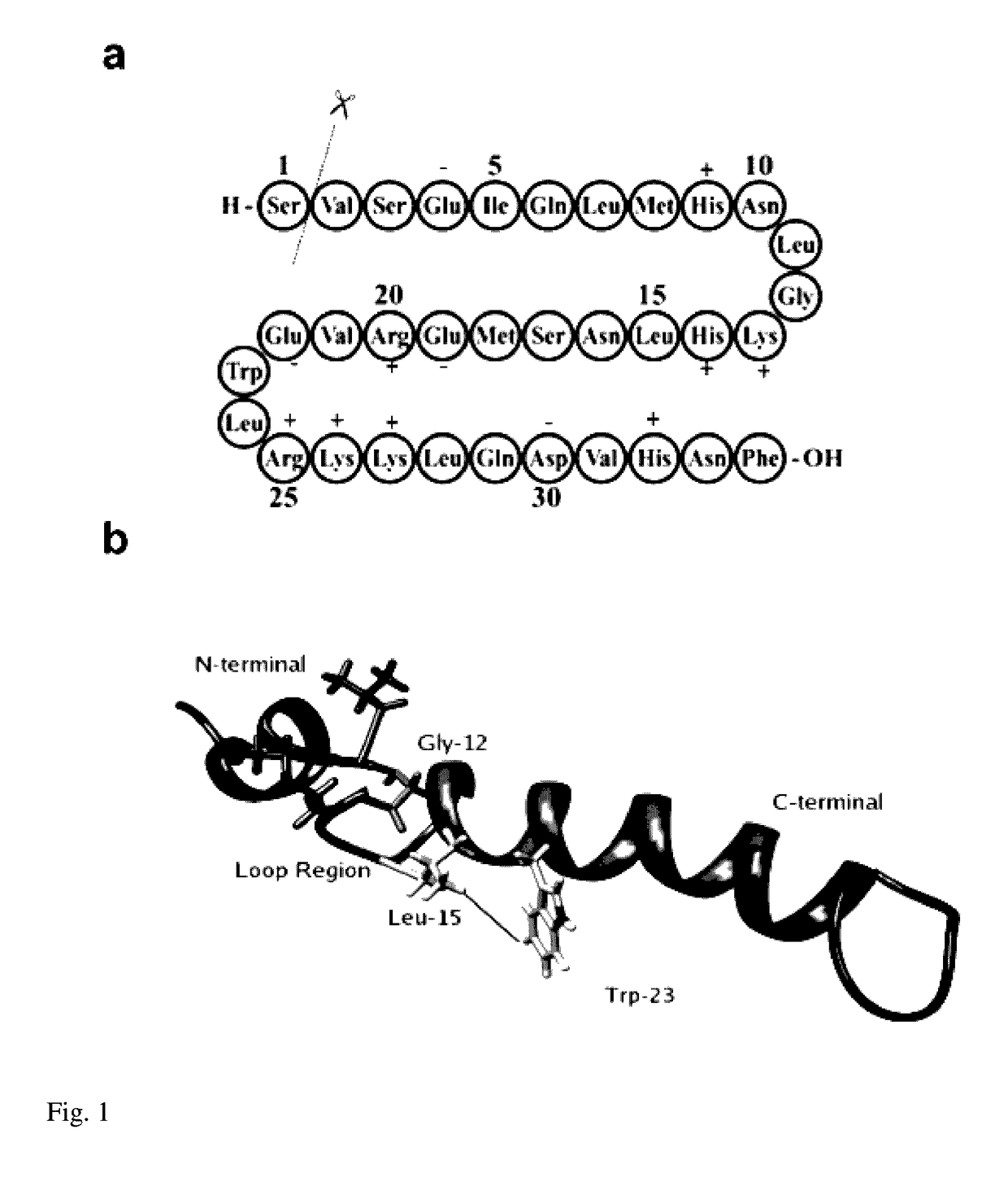
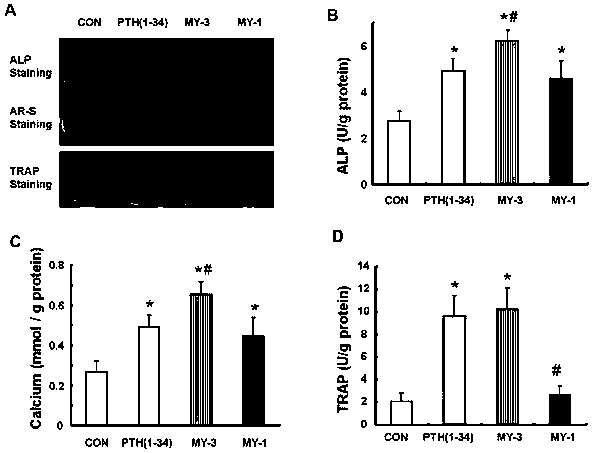
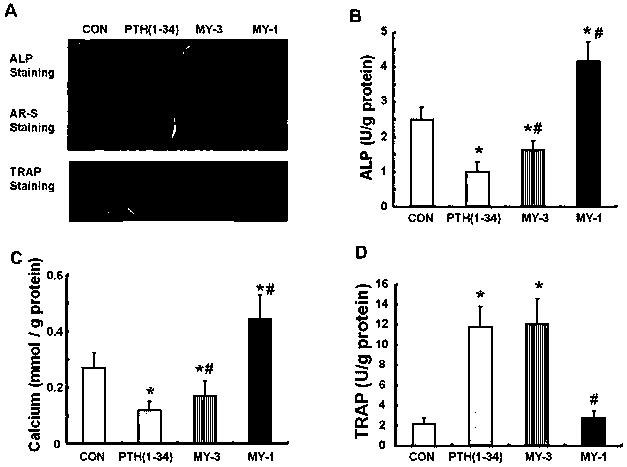
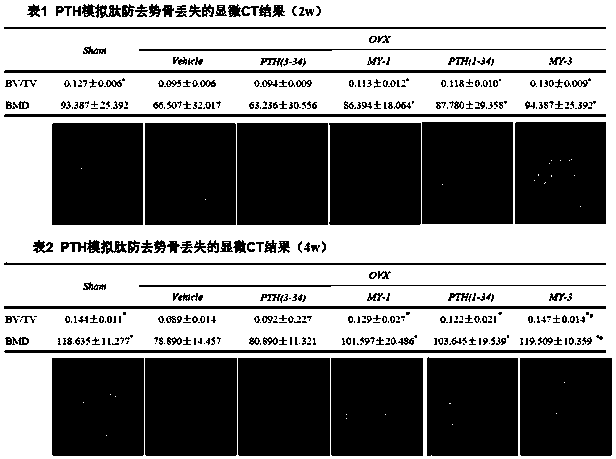
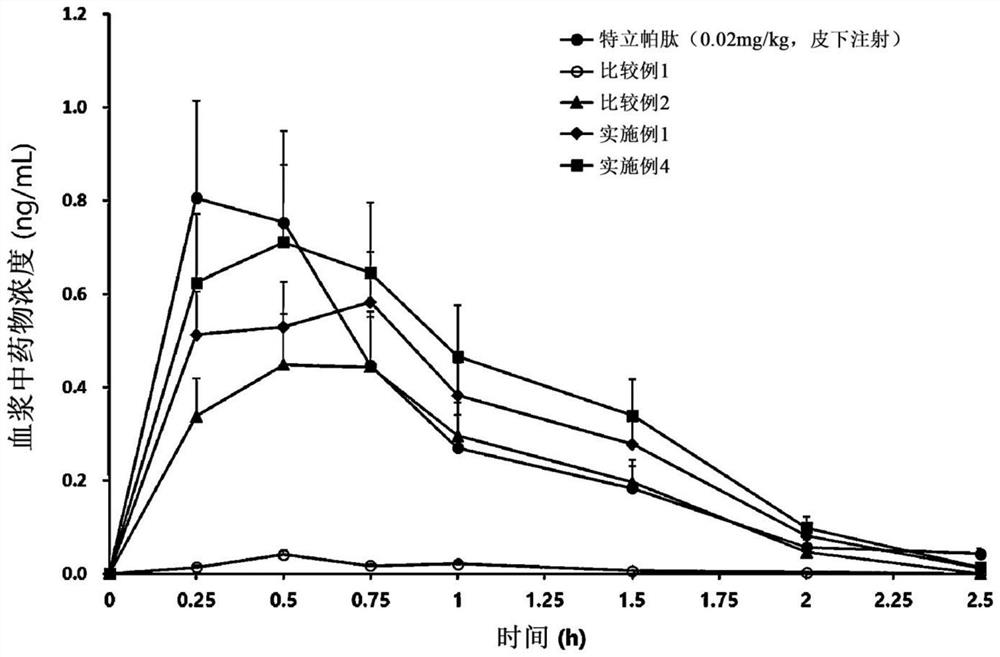
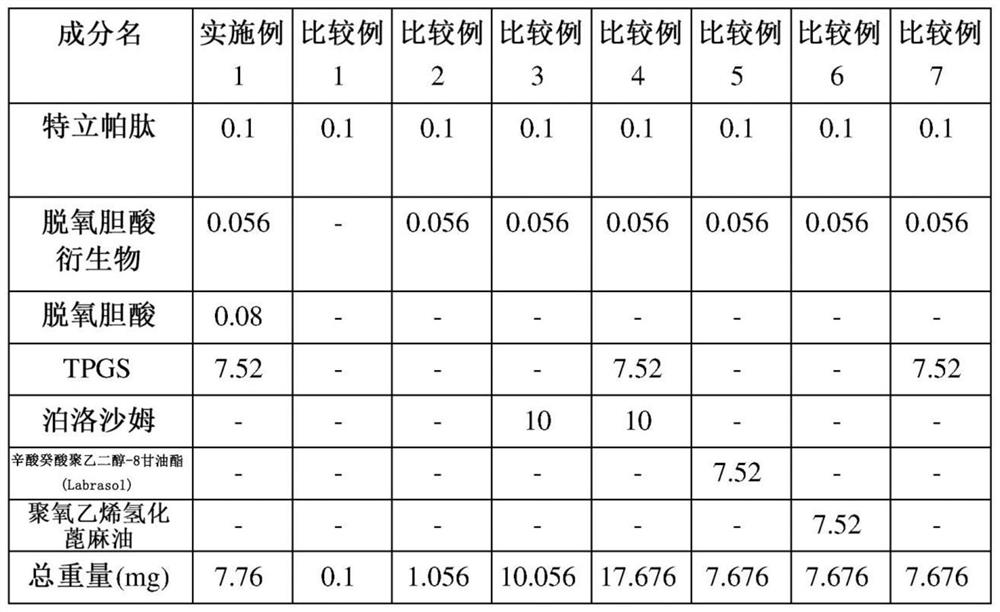
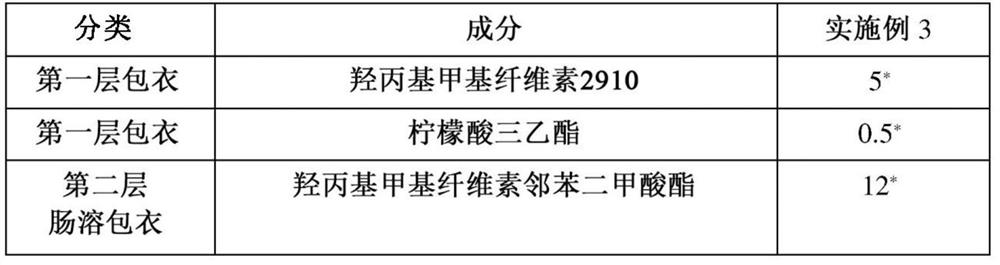
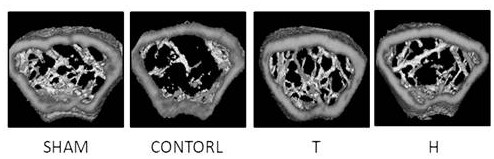
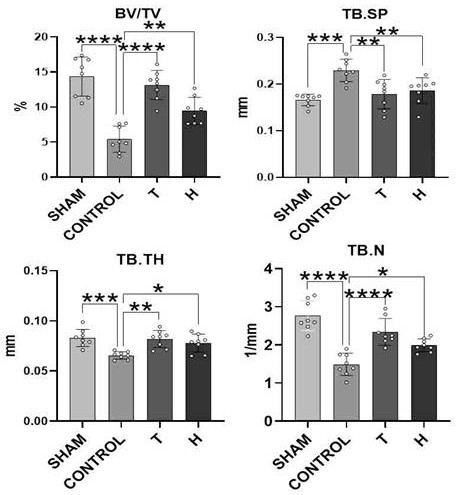
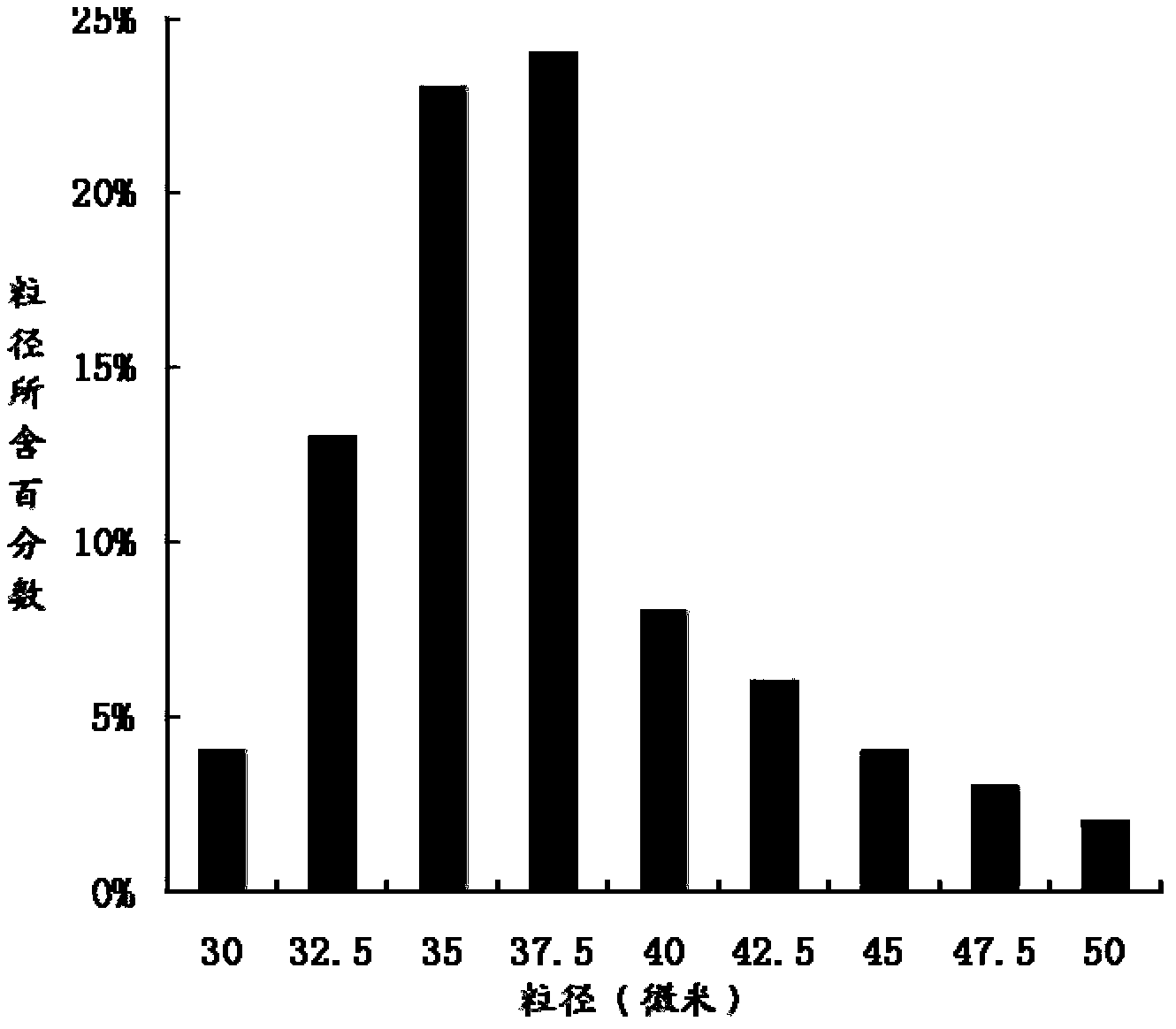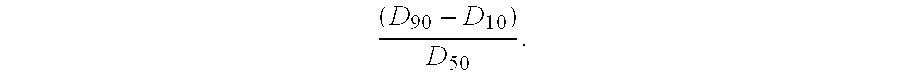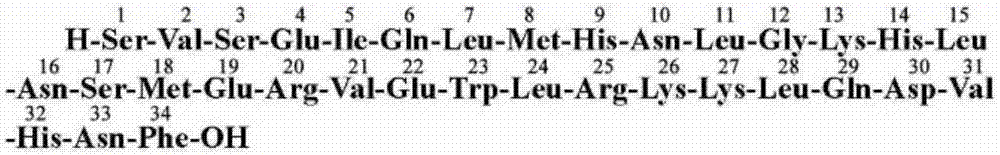Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
42 results about "Teriparatide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Teriparatide is used to treat bone loss (osteoporosis) in people who have a high risk of getting fractures.
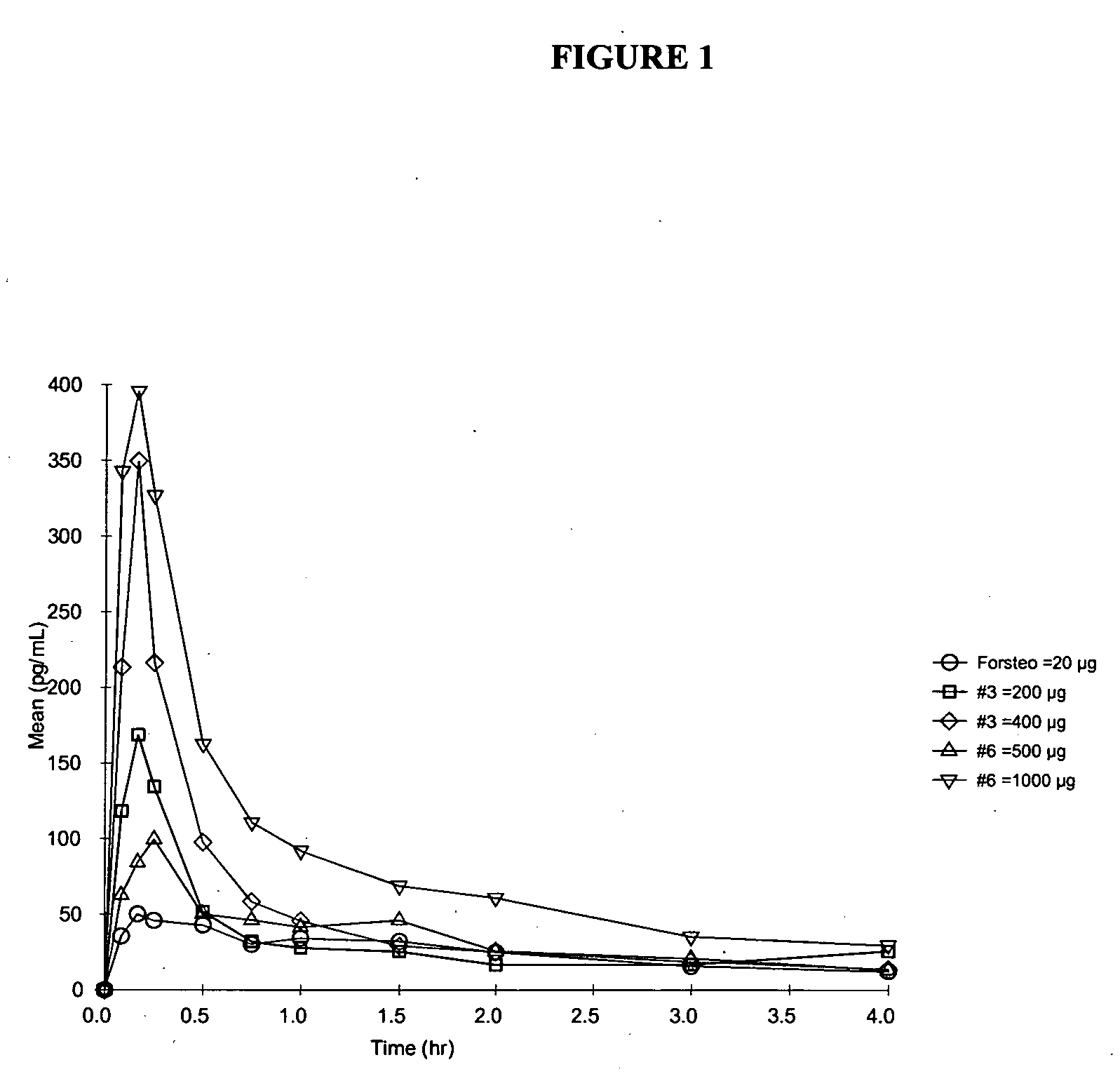
Stable pharmaceutical dosage forms of teriparatide
InactiveUS20060189533A1Low viscosityReduce adhesionPeptide/protein ingredientsPharmaceutical delivery mechanismTeriparatideBioavailability
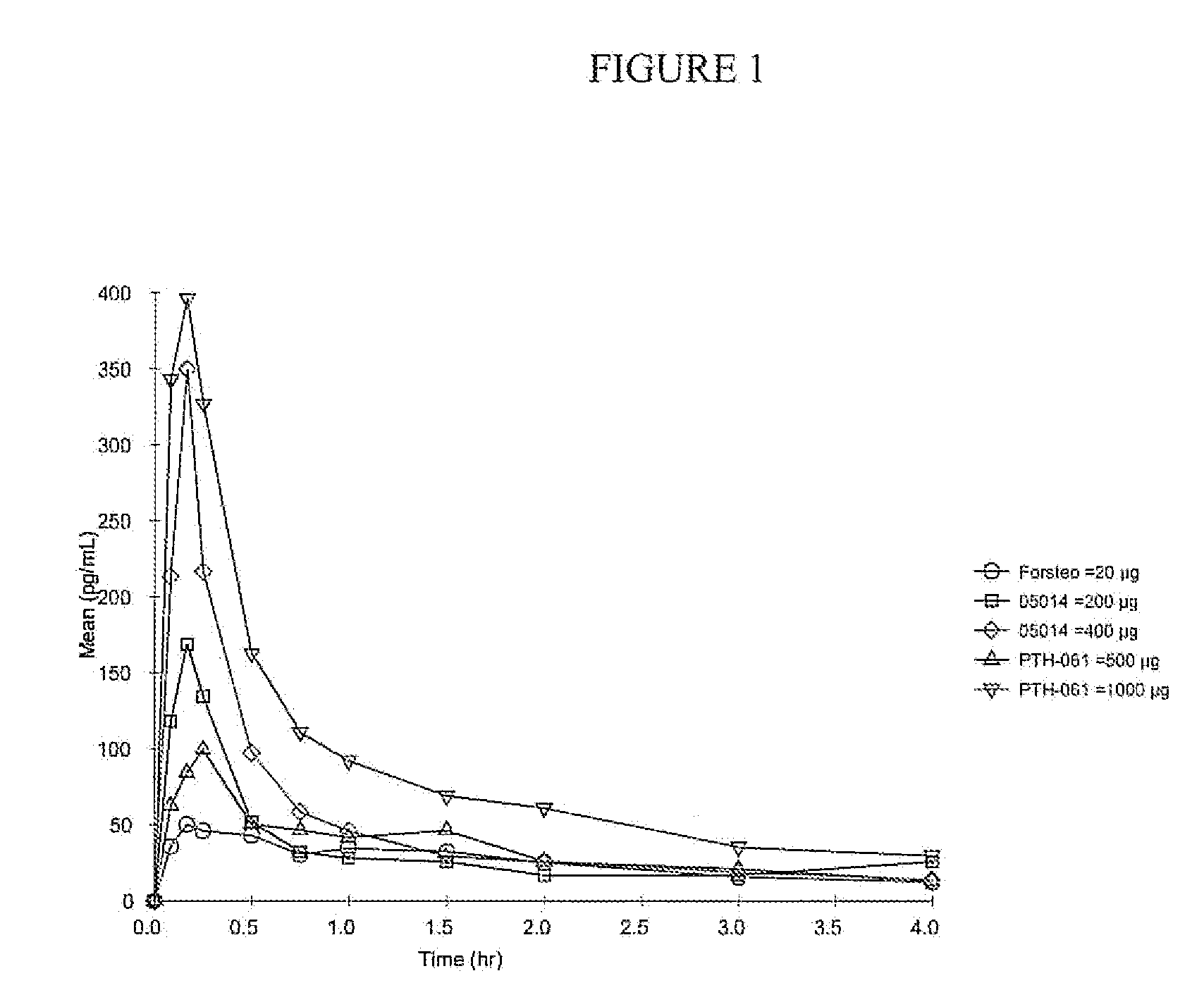
A parathyroid hormone (1-34) (PTH) dosage form is described that is suitable for multi-use administration. A dosage form of parathyroid hormone (1-34) (PTH) comprising an aqueous pharmaceutical formulation for aerosolized intranasal delivery of PTH having a bioavailability of about 5% or greater, wherein the formulation comprises a therapeutically effective amount of PTH and polysorbate, and wherein least 90% of the PTH can be recovered after storage for 24 weeks at 5° C.
Owner:NASTECH PHARMA
Method for preparing teriparatide
InactiveCN103467595AStable in natureNot easy to fall offPeptide preparation methodsParathyroid hormonesCombinatorial chemistryTeriparatide
The invention belongs to the technical field of pharmaceutical chemistry, relates to a peptide preparation method and particularly relates to a method for preparing teriparatide. The method comprises the steps of (1) sequentially coupling amino acids on a solid-phase carrier, starting from a carbon terminal, according to the amino acid sequence of teriparatide by adopting a solid-phase synthesis method, adopting Boc-Ser-OH as a raw material when 17-th serine is coupled, enabling free hydroxyl of the 17-th serine and carboxyl of 16-th asparagine to be subjected to ester condensation, and then, sequentially coupling the other amino acids; (2) carrying out pyrolysis to obtain crude peptide, and then, converting an ester bond between the 17-th serine and the 16-th asparagine into an amide bond, thereby obtaining a crude peptide solution of teriparatide. The method for preparing teriparatide, provided by the invention, is novel, the purity of teriparatide is improved, the operation is simple, convenient and practical, the total yield is high, the purity is high, the cost is low, and the industrial production is facilitated.
Owner:HYBIO PHARMA
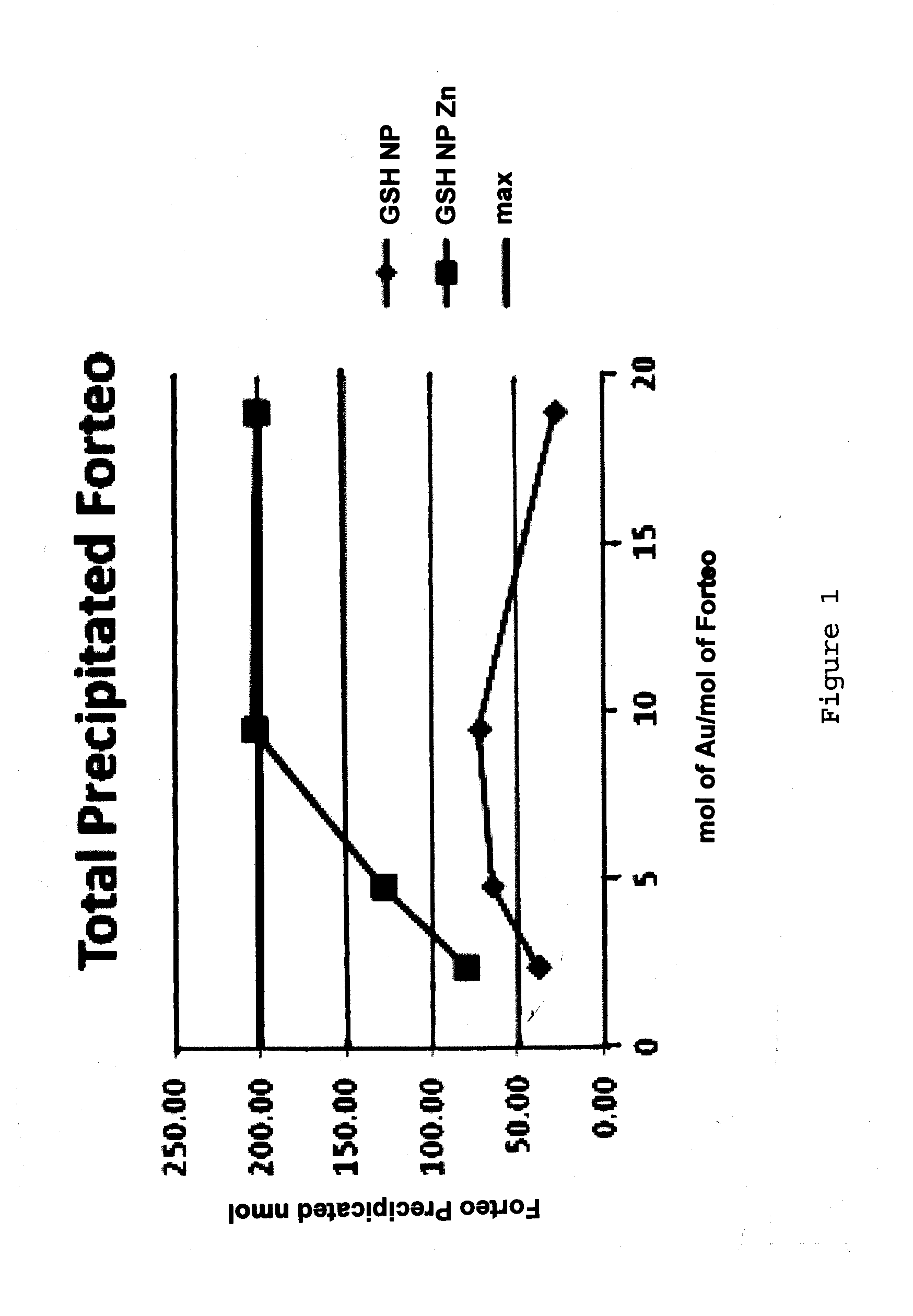
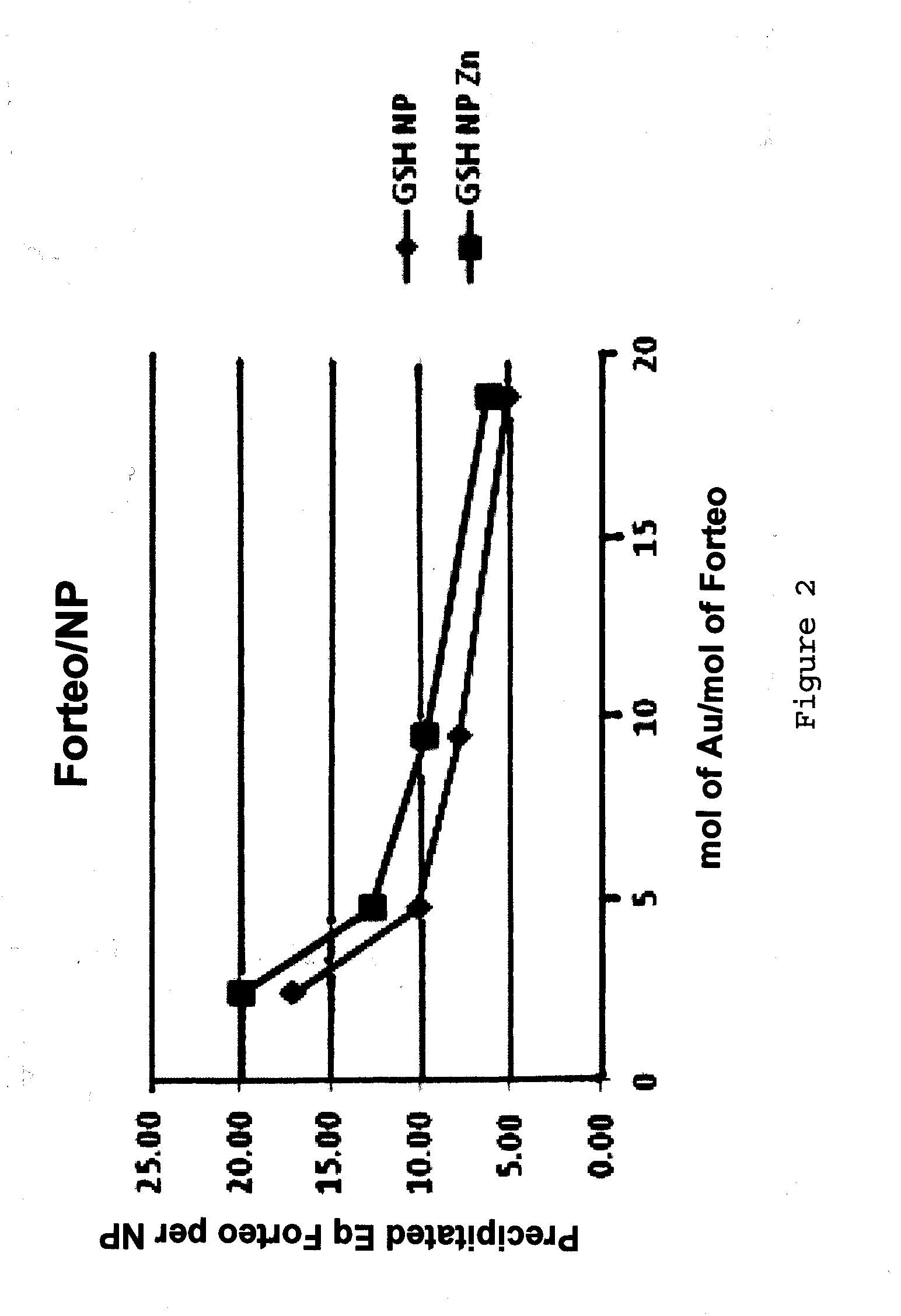
Nanoparticle peptide compositions
InactiveUS20140248365A1Increase contentHigh densityPowder deliveryPeptide/protein ingredientsCombinatorial chemistryTeriparatide
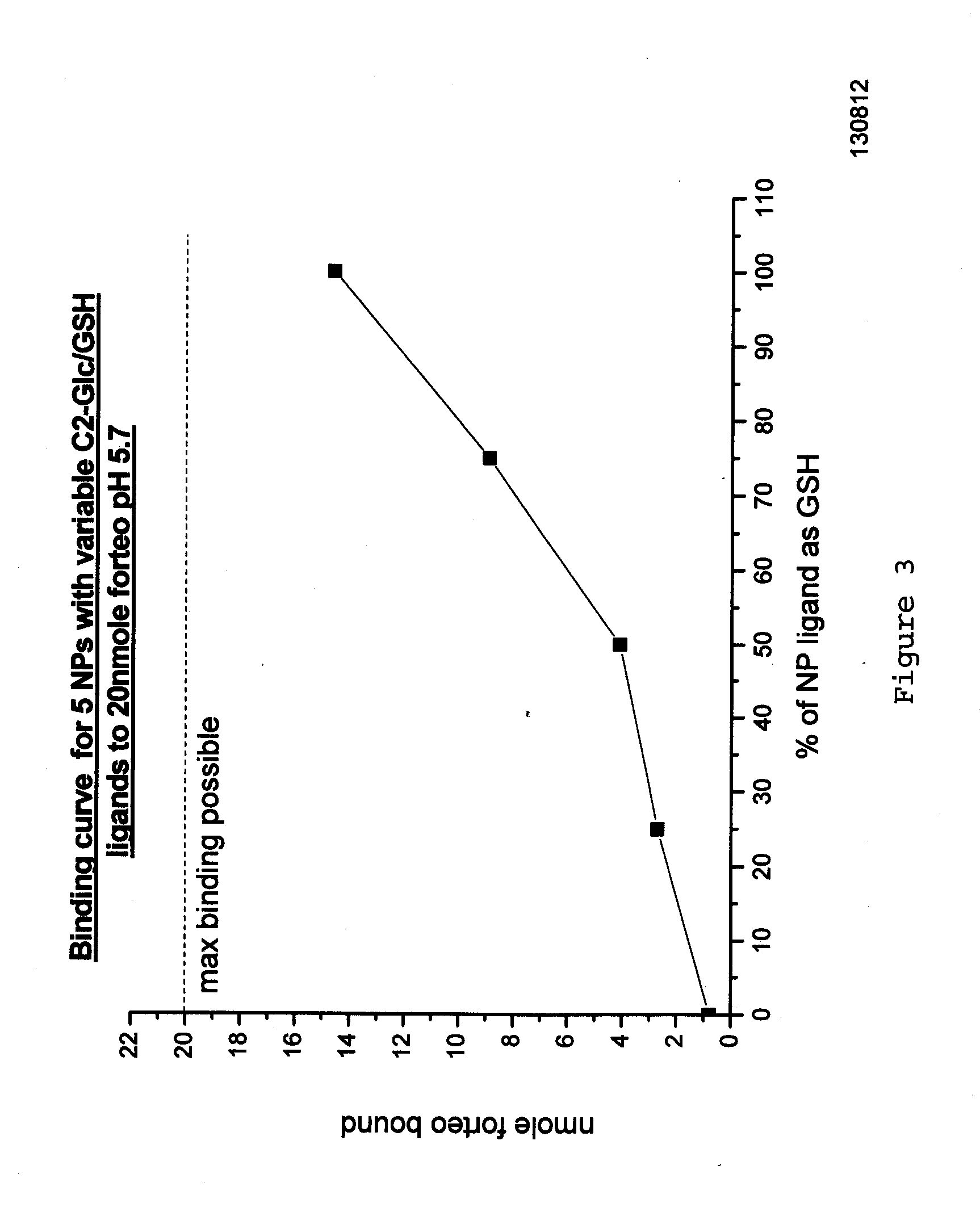
The present invention relates to teriparatide peptide-carrying nanoparticles, particularly for use in medicine, and includes methods for treatment of disorders, e.g., of bone density. Nanoparticle composition comprise a nanoparticle comprising a core comprising a metal and / or a semiconductor; and a corona comprising a plurality of ligands covalently linked to the core, wherein said plurality of ligands comprise at least one glutathione; and at least one teriparatide peptide that is non-covalently bound to the corona.
Owner:MIDATECH LTD
Stabilized teriparatide solutions
A stabilized pharmaceutical composition in the form of a solution for parenteral administration of a parathyroid hormone is described wherein the therapeutically active ingredient is stabilized with a buffer and a polyol. Preferred preparations contain in an aqueous solution human PTH(1-34), mannitol, an acetate or tartrate buffering agent and m-cresol or benzyl alcohol as a preservative.
Owner:ELI LILLY & CO
Composition for teriparatide injection, and preparation method and preparation thereof
InactiveCN103301058ANo degradationGuaranteed stabilityPowder deliveryPeptide/protein ingredientsPen InjectorTeriparatide
The invention relates to the technical field of medicaments, and particularly relates to a composition for teriparatide injection, and a preparation method and preparation thereof. The composition for teriparatide injection comprises teriparatide, a freezing and drying protecting agent and a pH regulator, wherein the mass ratio of the teriparatide to the freezing and drying protecting agent is 1:(10-100000). The composition for teriparatide injection is simple in formula; the freezing and drying protecting agent ensures that the teriparatide is not degraded in preparation and preservation processes; and the pH regulator is used for regulating the pH value in a preparation process and regulating the pH value after the composition for teriparatide injection is redissolved, so as to maintain the stability of the teriparatide. The used auxiliary materials are safe, and do not cause toxic reaction. Therefore, the composition for teriparatide injection provided by the invention is good in stability, simple and reasonable in formula, and good in redissolving performance, and can be prepared into powder-injection or injection and is needless to be prepared into a pen-type injector. Thus, the production technology is simple.
Owner:HYBIO PHARMA
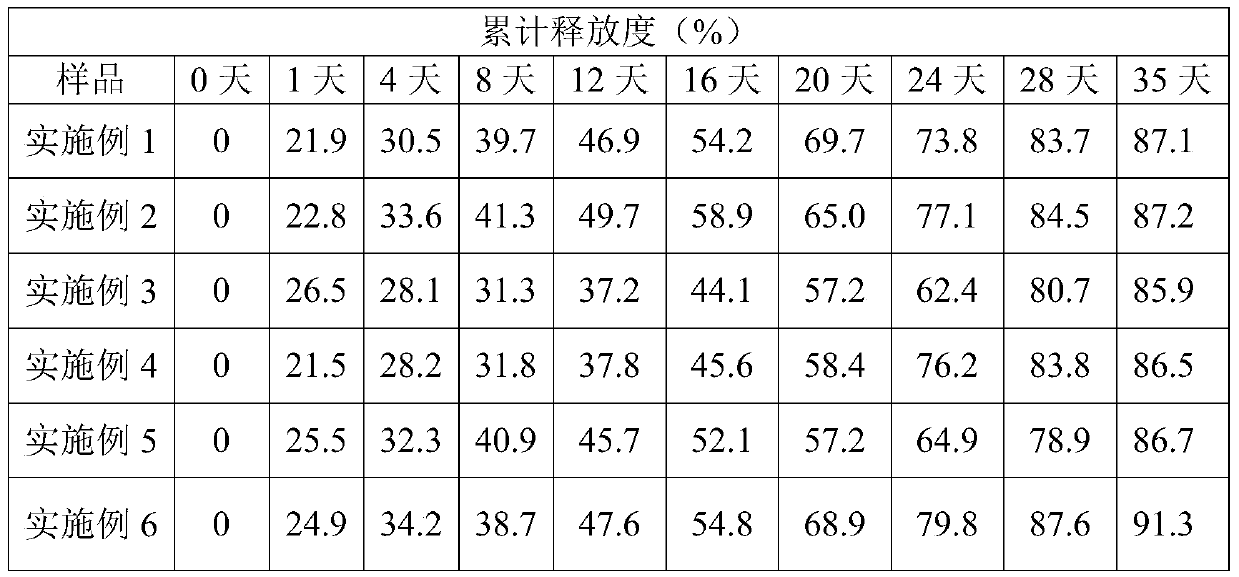
Teriparatide sustained-release microsphere and preparation method thereof
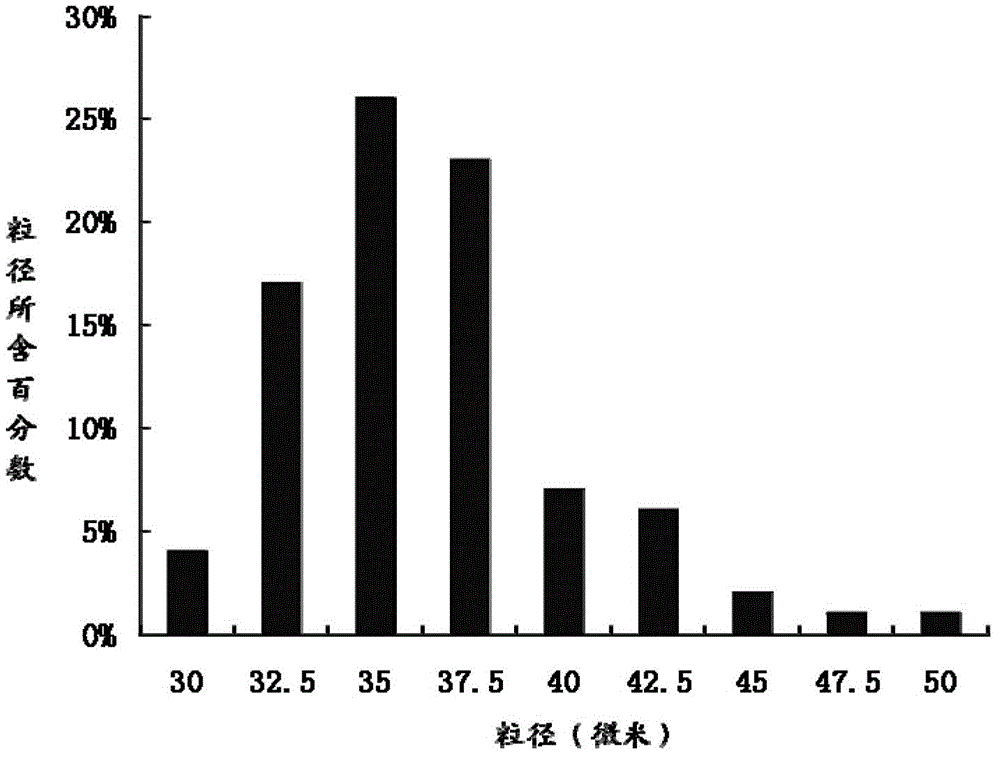
ActiveCN103157096AEasy to useImprove compliancePeptide/protein ingredientsSolution deliveryPolyesterMicrosphere
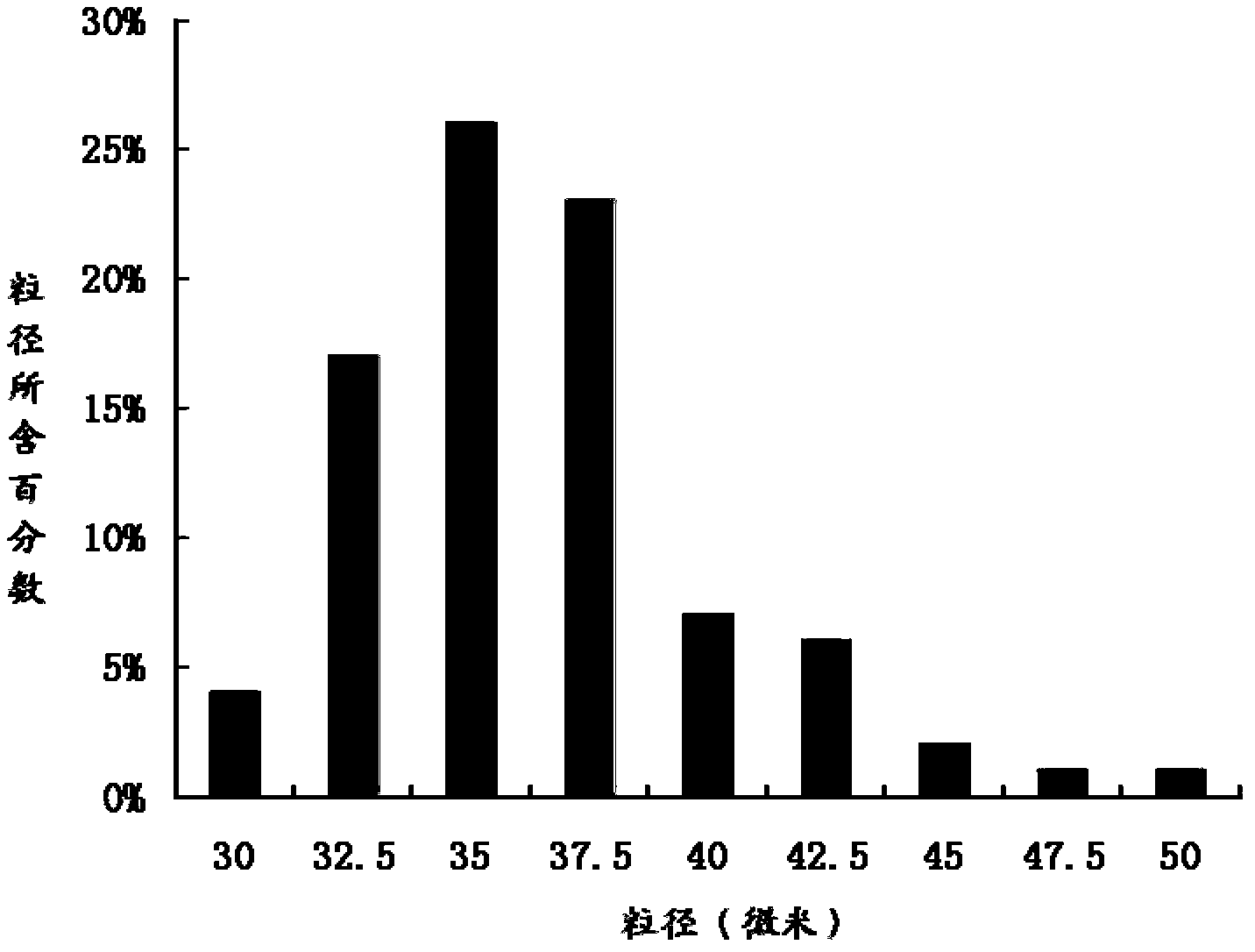
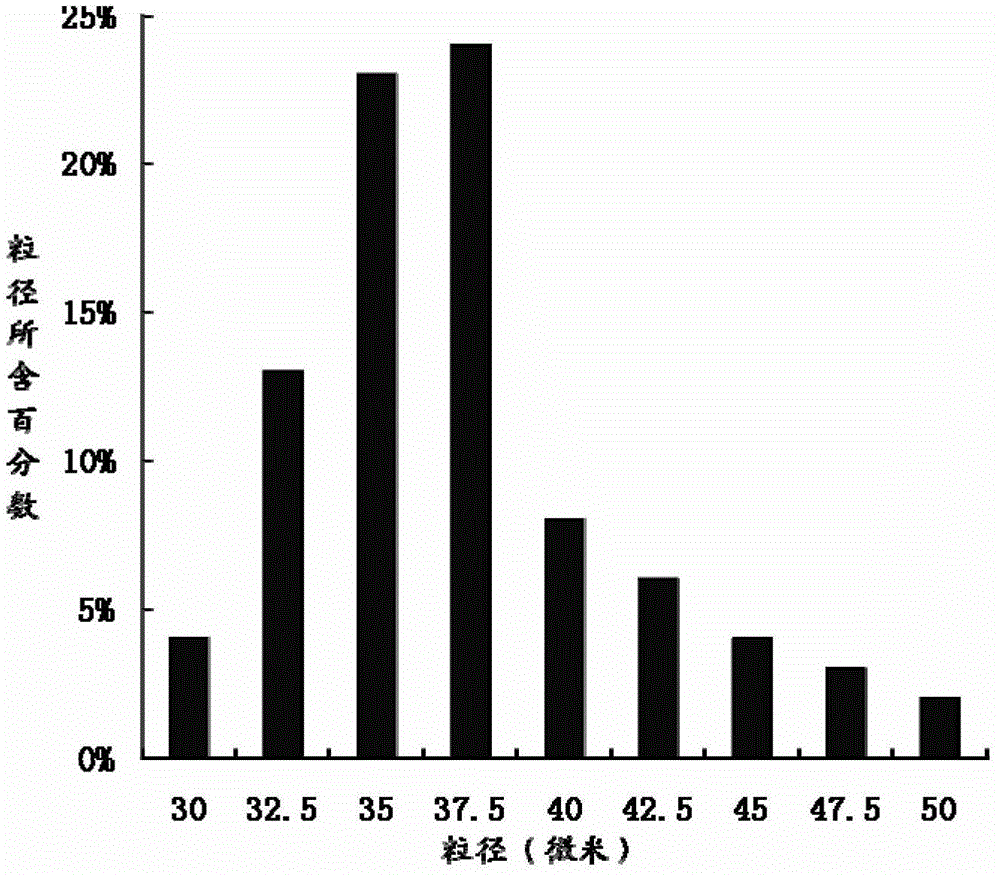
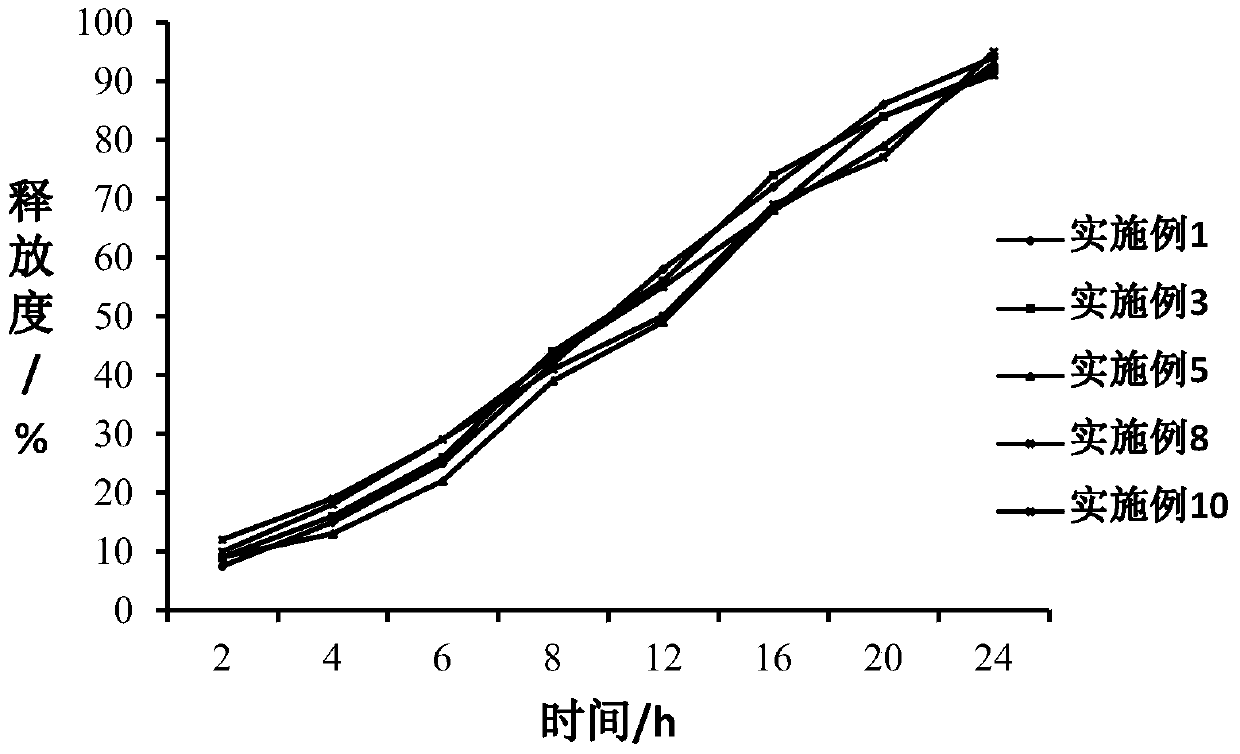
The invention relates to the technical field of medicines, and in particular relates to a teriparatide sustained-release microsphere and a preparation method thereof. The teriparatide sustained-release microsphere comprises a teriparatide and a carrier, wherein the particle size of the teriparatide is 30 micrometers-50 micrometers; and the carrier is a mixture of one or two of polyester, starch, gelatin, Arabic gum, albumin, alginate and chitosan. The teriparatide microsphere is prepared by adjusting the constitution and molecular weight of the carrier and the medicine-loading rate and particle size of the microsphere according to the properties of the teriparatide. The prepared microsphere is stable in releasing and small in sudden releasing, the dose administration times can be reduced, the probability of infection is decreased, patients can conveniently take the microsphere, and the compliance of the patients is improved. Experiments show that the teriparatide has uniform particle size distribution, high encapsulation efficiency and large medicine-loading capacity. The sustained-release microsphere can be externally released for 10 days.
Owner:HYBIO PHARMA
Method for synthesizing teriparatide
ActiveCN104910269AHigh yieldGuaranteed purityPeptide preparation methodsParathyroid hormonesCouplingAcid hydrolysis
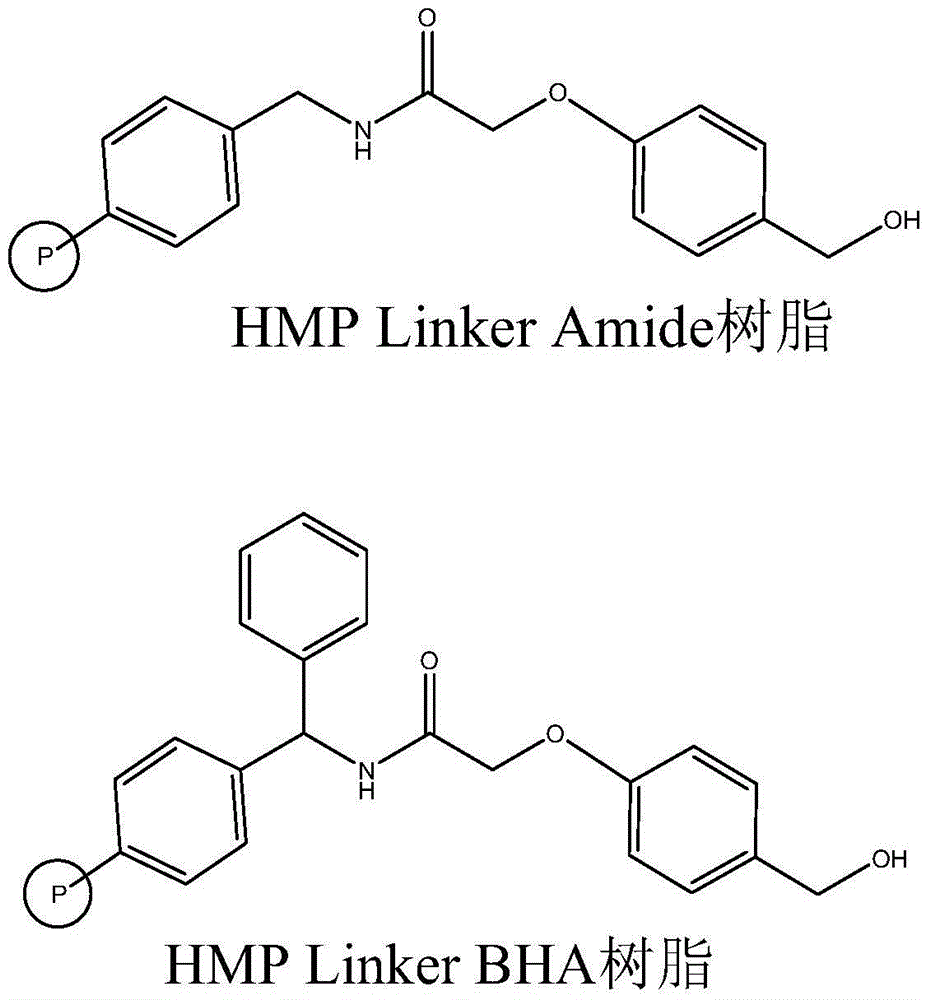
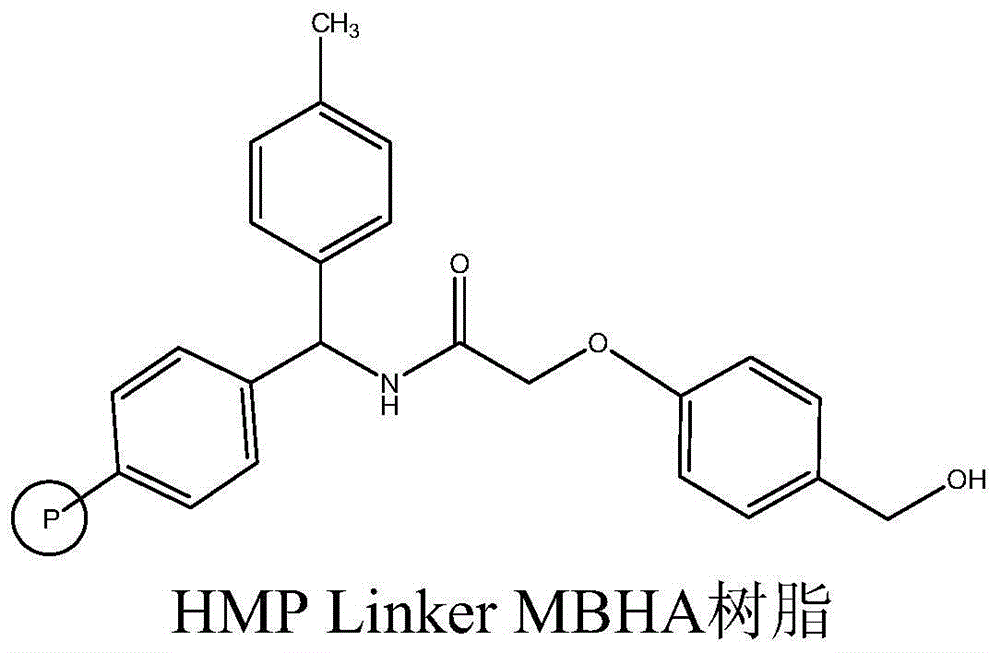
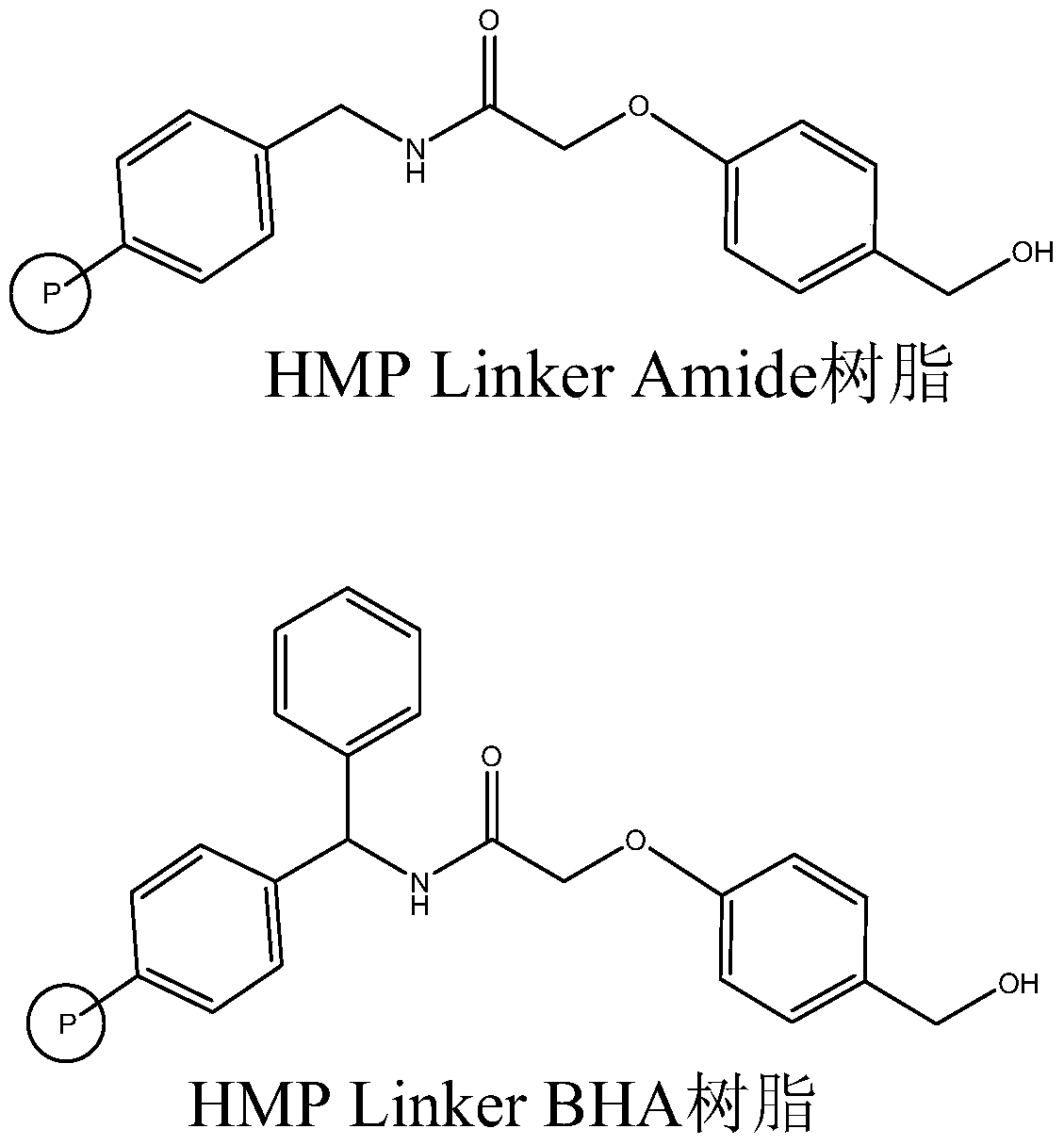
The invention relates to the field of medicine synthesis, and discloses a method for synthesizing teriparatide. The method comprises the following steps: performing esterification reaction between Phe coupled with a protecting group at an N end and HMP Linker resin under the action of a coupling reagent and an activating reagent to obtain peptide resin 1; and performing one-by-one extension coupling on the rest protective amino acids under the reaction of a condensation reagent and an activating reagent according to a sequence from a C end to the N end of a teriparatide amino acid sequence to obtain corresponding peptide resin after each extension coupling and finally obtain teriparatide resin, performing acid hydrolysis to obtain a teriparatide crude product, and purifying the teriparatide crude product into acetate so as to obtain a teriparatide pure product. According to the method disclosed by the invention, a suitable synthesis scheme is selected, the HMP Linker resin is used as carrier resin, the whole synthesis process is optimized, a high-purity product is obtained, the total yield of the teriparatide is improved, and the method is harmless to any environment.
Owner:CHENGDU SHENGNUO BIOTEC CO LTD
Method of modulating hematopoietic stem cells and treating hematologic diseases using intranasal parathyroid hormone
A method for modulating hematopoietic stem cells and treating a hematologic disease in a mammal comprising administering intranasally a therapeutically effective amount of a PTH formulation. The PTH formulation may contain teriparatide.
Owner:NASTECH PHARMA
Large-scale separation and purification method of teriparatide
InactiveCN106167522AHigh purityHigh product yieldPeptide preparation methodsParathyroid hormonesPurification methodsFreeze-drying
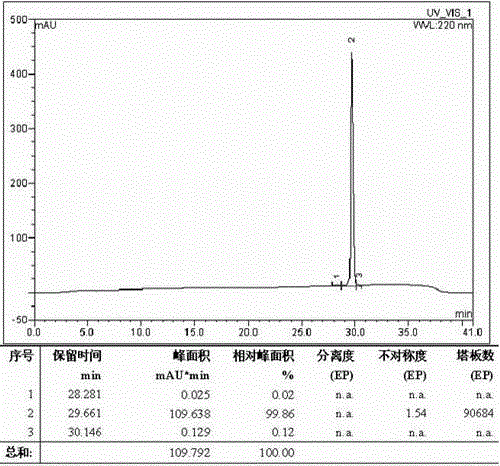
The invention relates to a large-scale separation and purification method of teriparatide by reversed high performance liquid chromatography. The method comprises the following steps: dissolving a teriparatide crude product in 30% acetonitrile aqueous solution, carrying out ultrasonic treatment, filtering with a filter membrane, and fetching a filtrate for later use; separating and purifying the filtered crude product by a high performance liquid chromatography instrument; letting a concentrated solution pass through a reversed-phase column for conversion to acetate; carrying out vacuum decompression rotary-evaporation concentration on a product with 99% purity after conversion to acetate, freeze-drying to obtain a powdery product, and detecting qualified so as to obtain the final product. The method is convenient to operate and excessive generating equipment is not required. Product purity is high and yield is high. Product purity reaches 99% and above, purification recovery rate reaches 60% and above. The technology can meet requirements of large-scale industrial production.
Owner:ZHEJIANG PEPTITES BIOTECH CO LTD
Method for preparing teriparatide
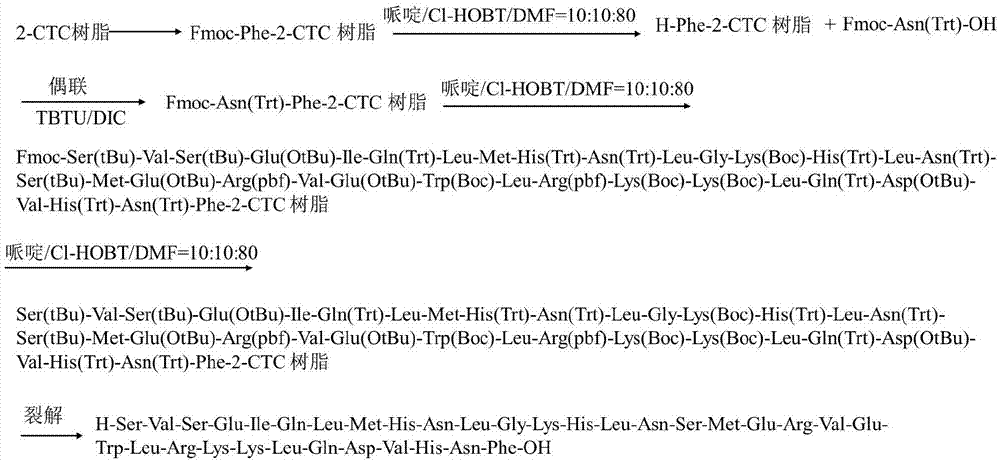
The invention discloses a method for preparing teriparatide. The method comprises synthesis and purification of the teriparatide, and belongs to the field of preparation of medicaments. The method disclosed by the invention comprises the following steps: 1) in the presence of an activator system, coupling a resin solid carrier with Fmoc-Phe-OH to obtain Fmoc-Phe-resin; 2) by solid-phase synthesis, sequentially coupling amino acids having N-terminal Fmoc protection and side chain protection according to a teriparatide backbone peptide sequence to obtain whole peptide resin; 3) when the whole peptide resin is condensed at amino acid No. 32, using COMU as a condensing agent and adopting a DMF solution of piperidine / Cl-HOBT as a deprotection agent during a synthesis process in each step; and 4) cracking the peptide resin to obtain a crude product, and subjecting the crude product to processes such as preliminary purification, purification and desalination to obtain the teriparatide, with a content of racemization impurity D-His32-teriparatide in process impurities being less than 0.1%. The invention provides a process for preparing the teriparatide with high purity, low cost and suitability for large-scale production. The process not only effectively controls technological impurities, but also obviously increases the total yield of the teriparatide.
Owner:YANGTZE RIVER PHARM GRP SICHUAN HAIRONG PHARM CO LTD +1
Crystalline teriparatide
InactiveCN1281467AImprove solubilitySkeletal disorderPeptide preparation methodsPurification methodsTeriparatide
Pure, stable crystalline forms of parathyroid hormone, particularly teriparatide, are described as well as methods of preparation and purification.
Owner:ELI LILLY & CO
Method for treating osteoporosis by intranasal delivery of teriparatide with an Anti-resorptive agent
A method for treating osteoporosis in a mammal by administering intranasally a therapeutically effective amount of a PTH formulation to the mammal in combination with administration of an anti-resorptive agent. The PTH formulation can contain teriparatide.
Owner:NASTECH PHARMA
Method for purifying teriparatide
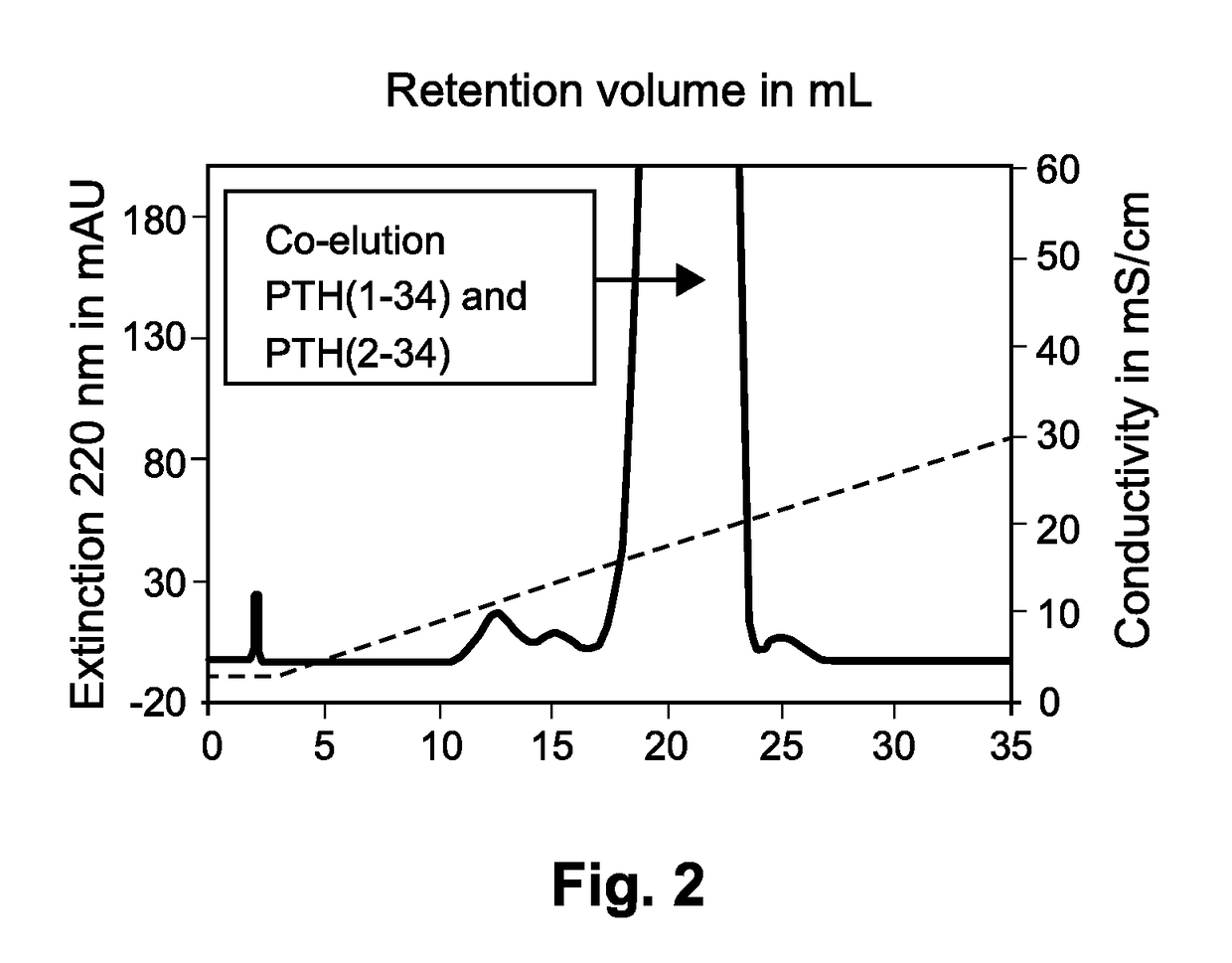
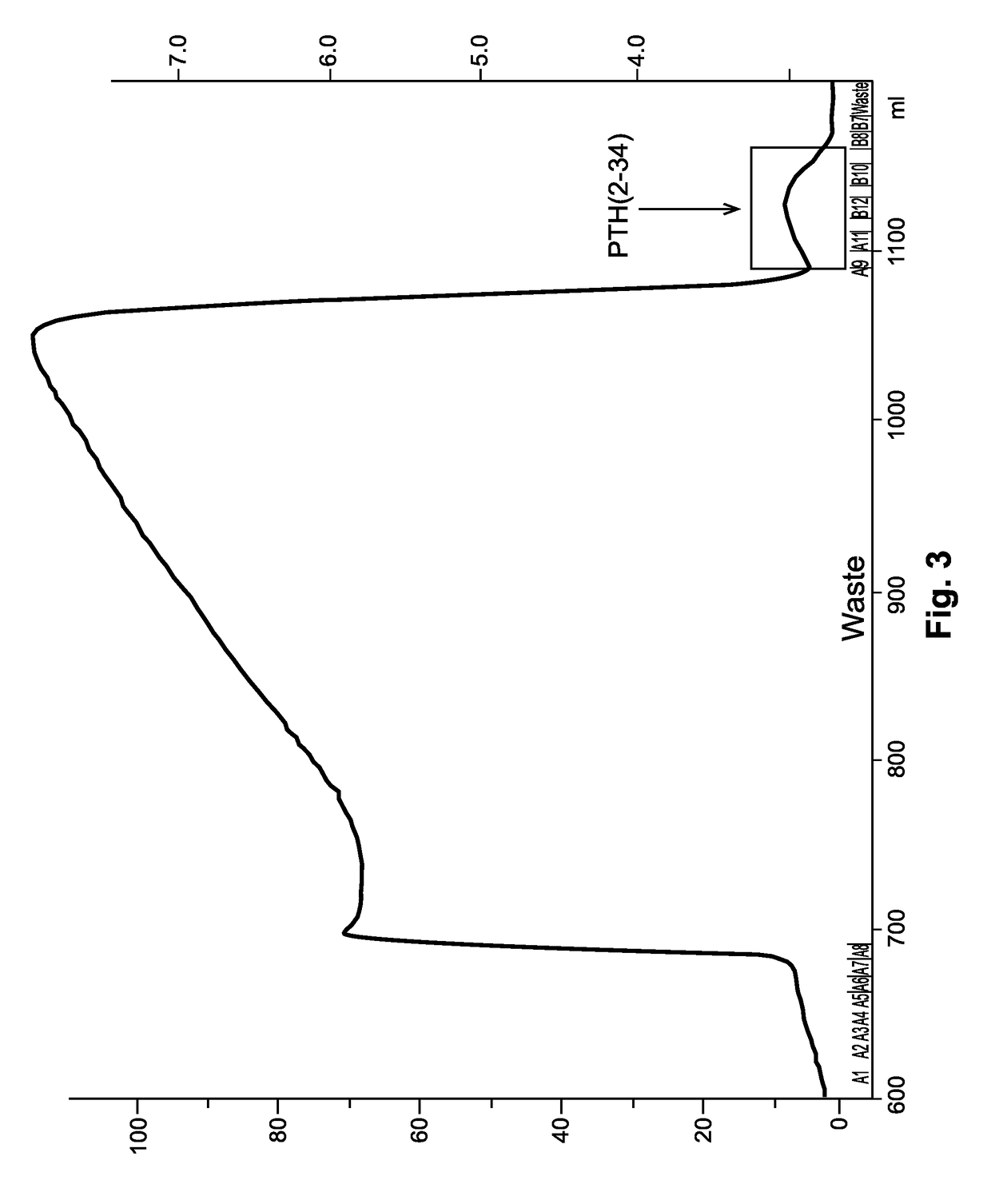
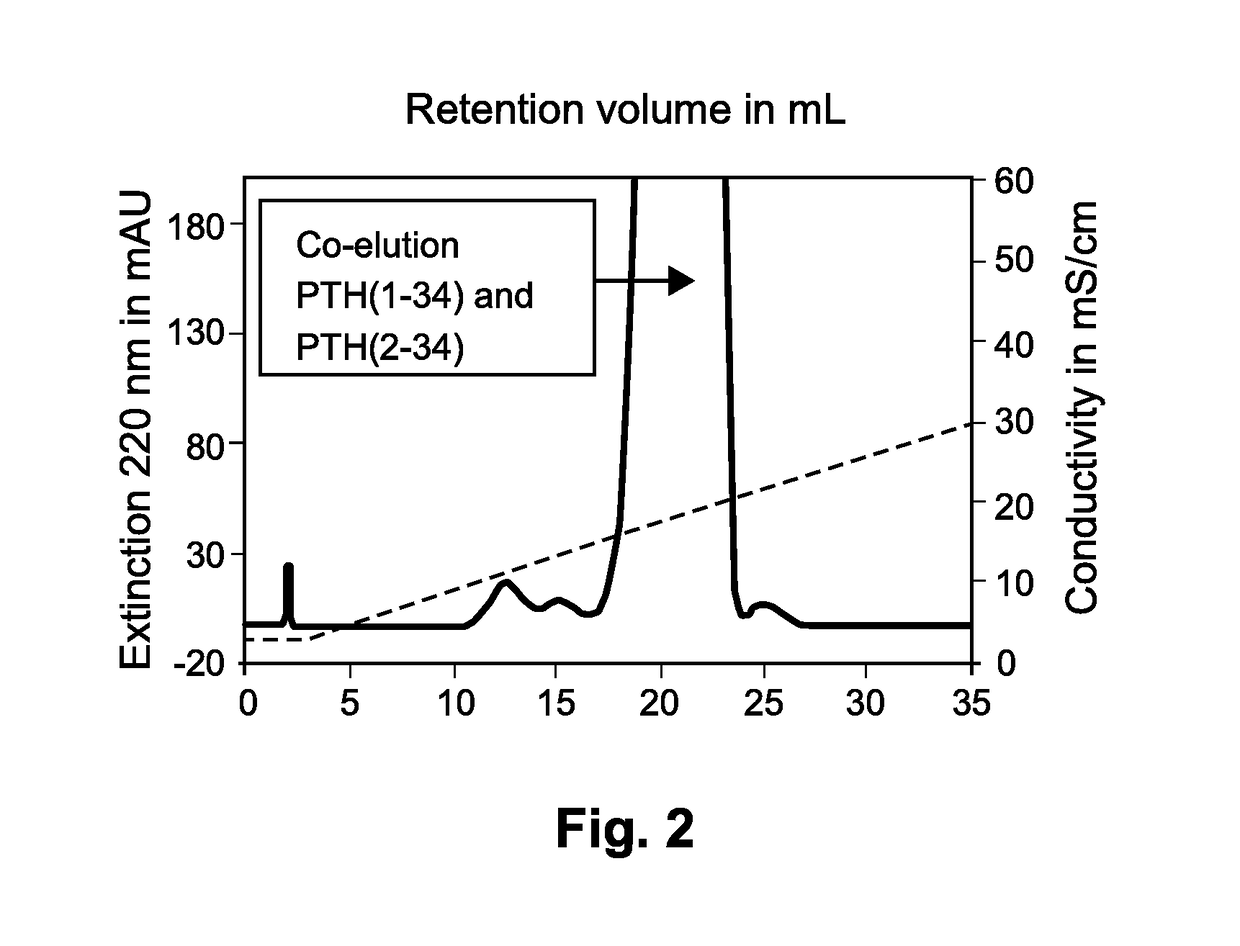
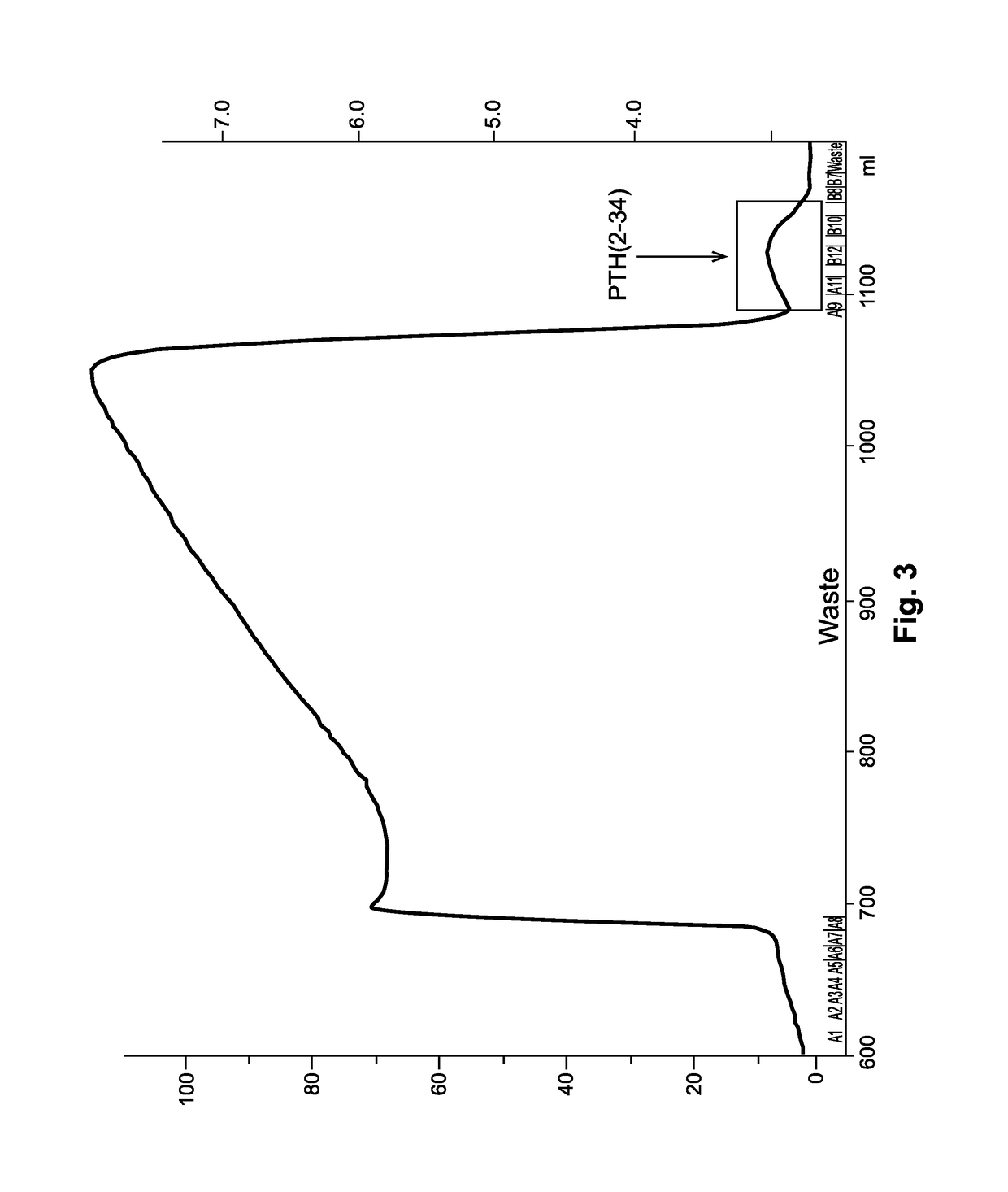
The present invention relates to a novel method for purifying teriparatide, a therapeutically active polypeptide fragment of full-length human parathyroid hormone. The method is based on ion exchange chromatography and is effective in separating the therapeutically active polypeptide from undesirable variants, such as truncated polypeptides. The method of the invention can be used at a preparative scale which allows it to be implemented into the production process for teriparatide. Accordingly, the invention also provides a method for the production of teriparatide which includes a step in which teriparatide is purified by the novel ion exchange chromatography method of the invention.
Owner:RICHTER HELM BIO TEC
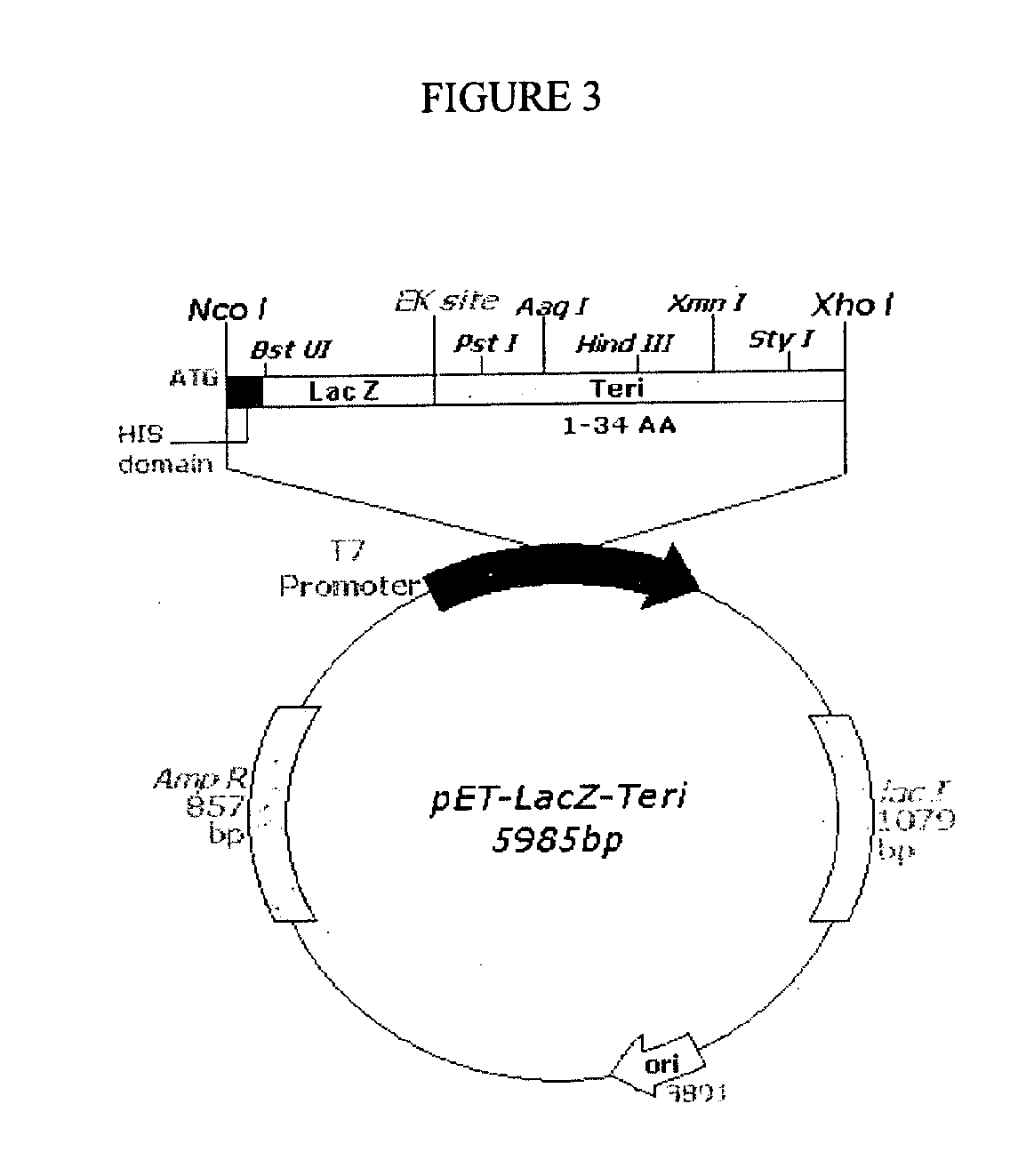
Novel orthogonal process for purification of recombinant human parathyroid hormone (RHPTH) (1-34)
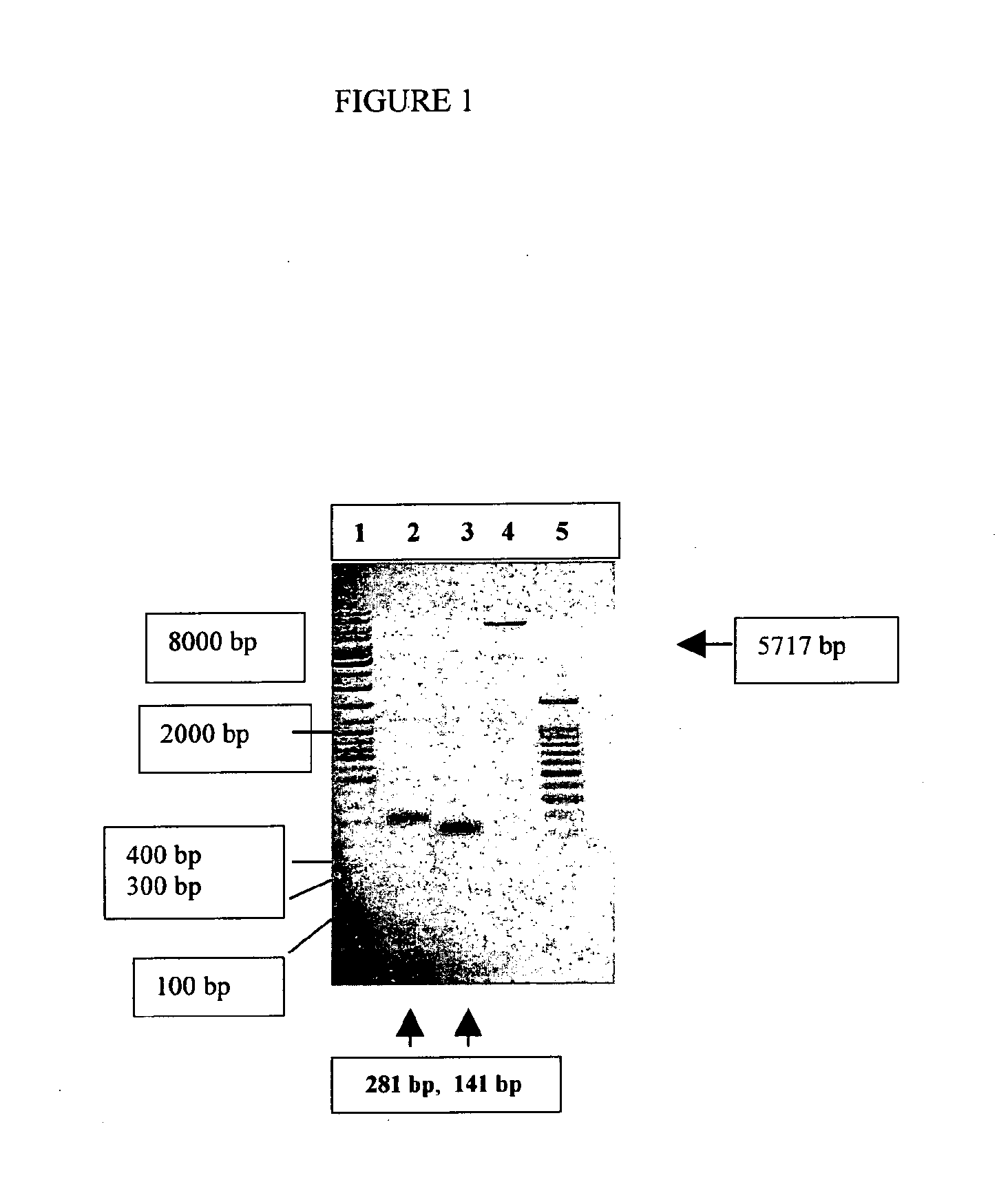
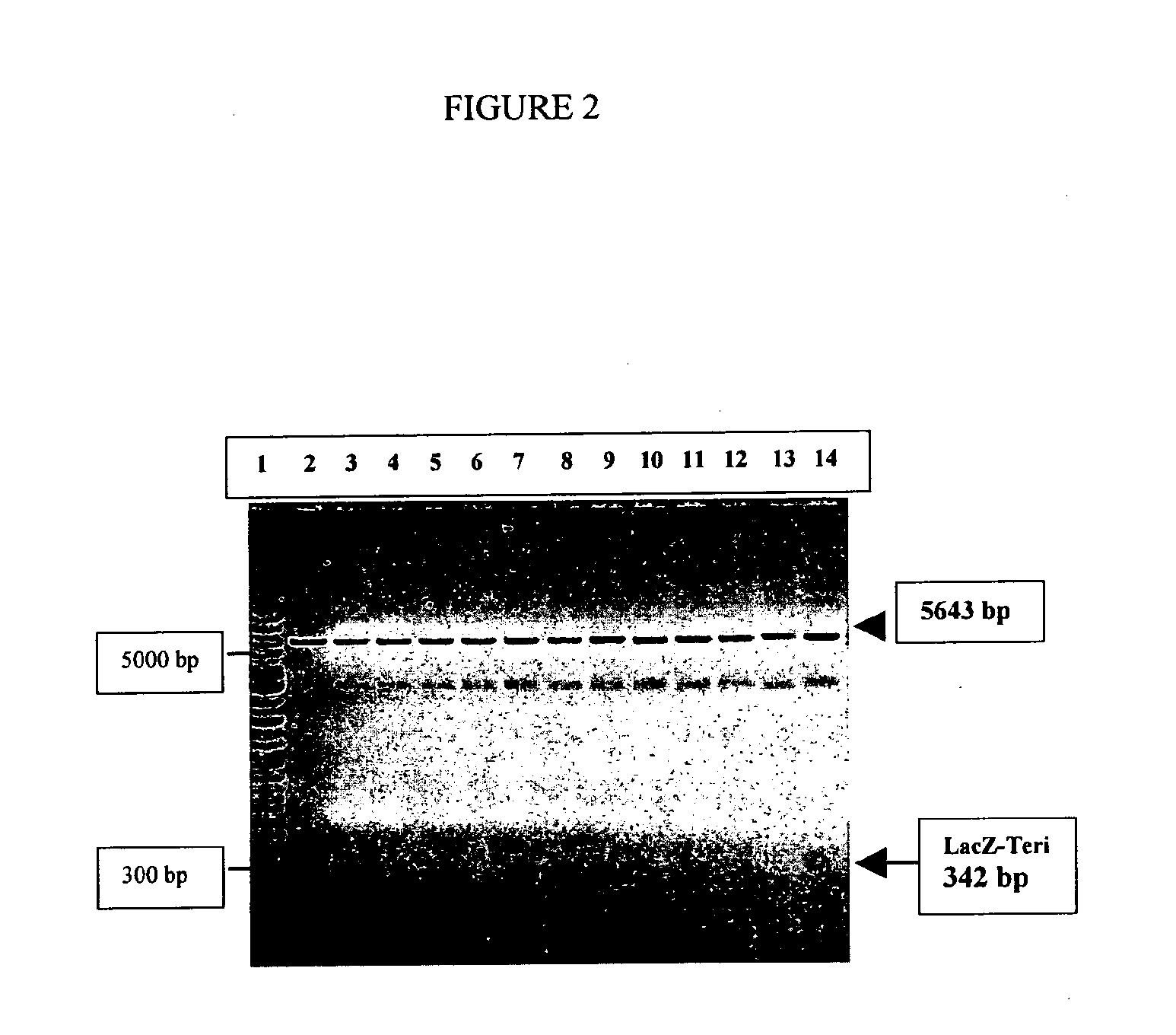
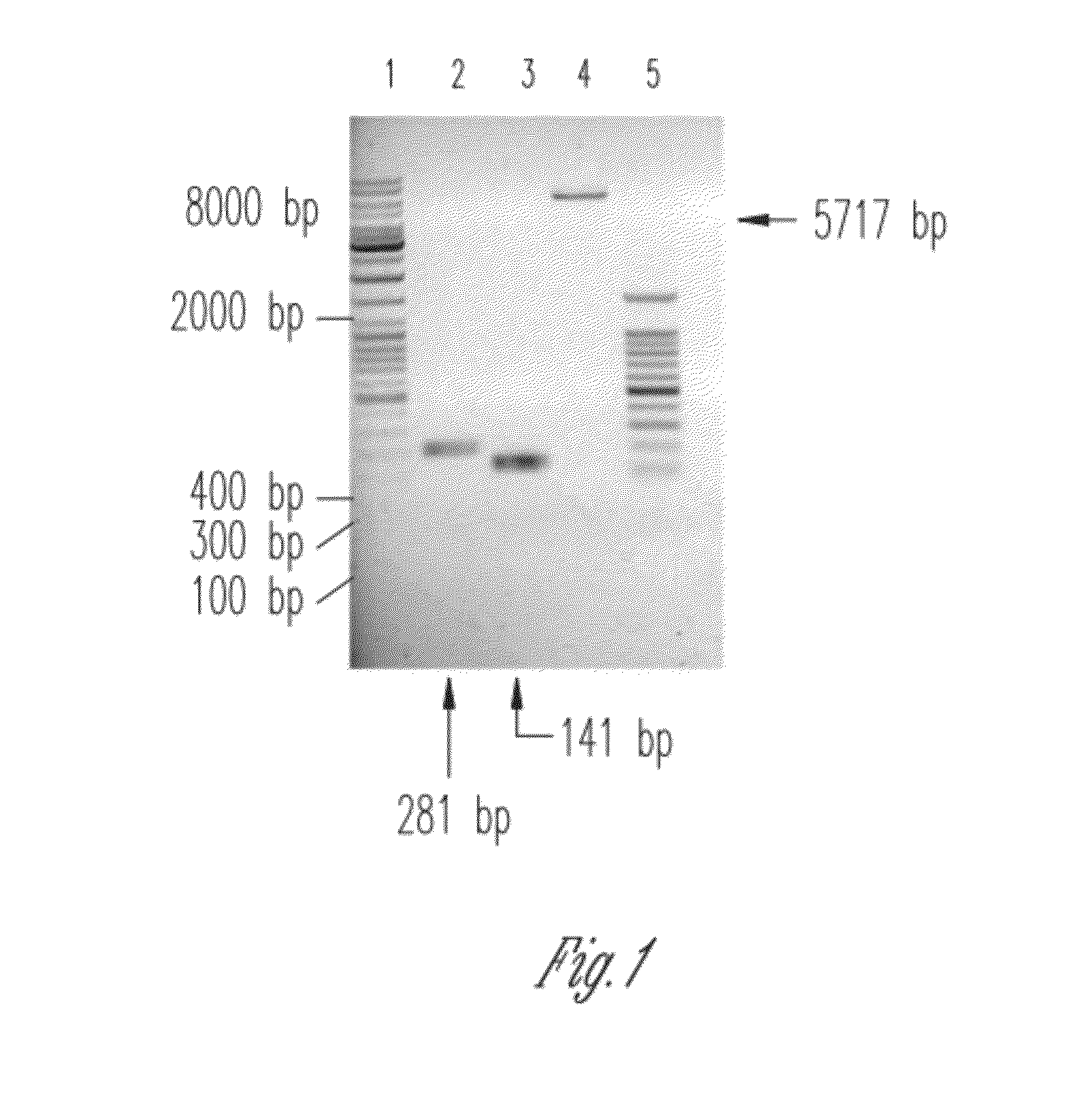
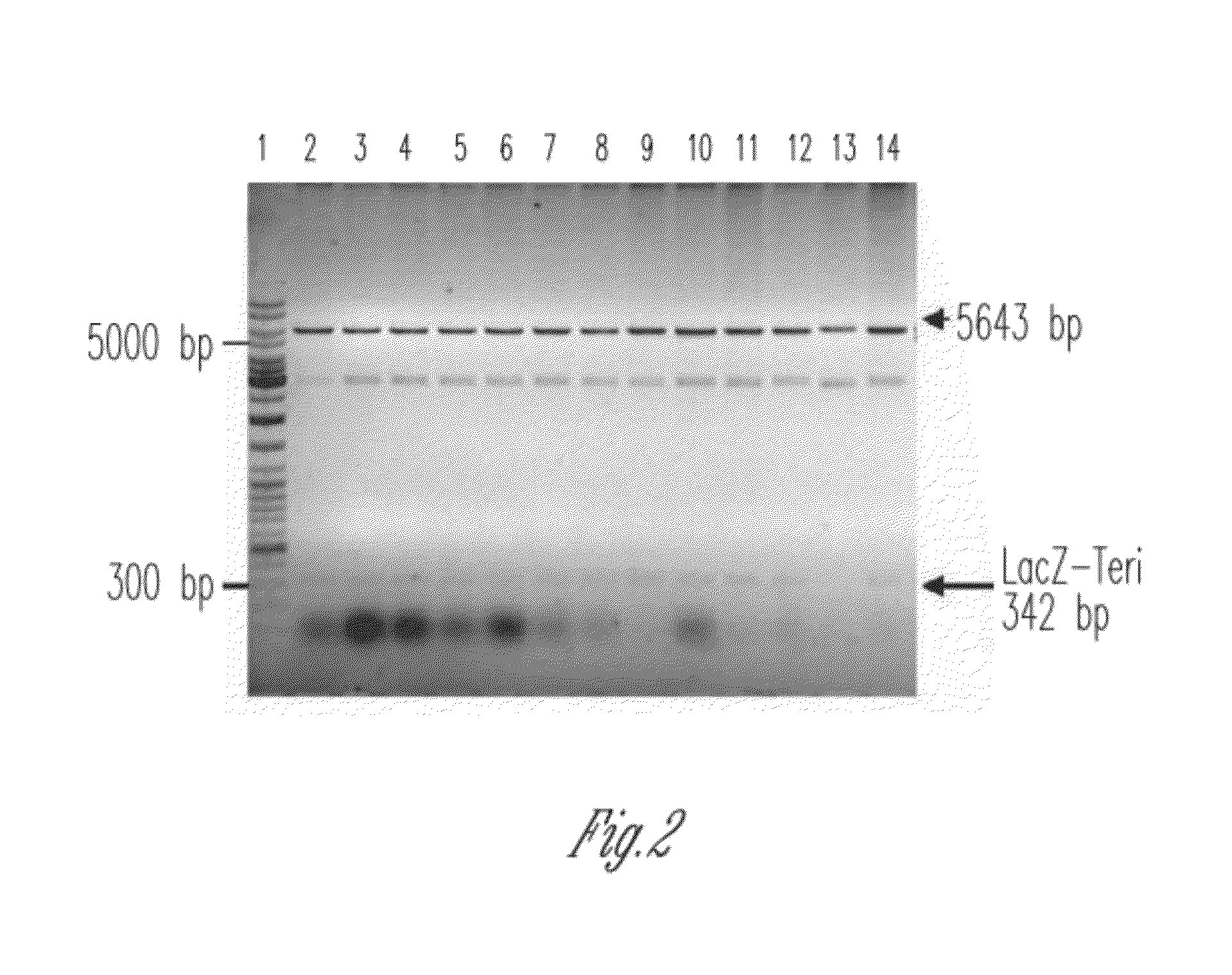
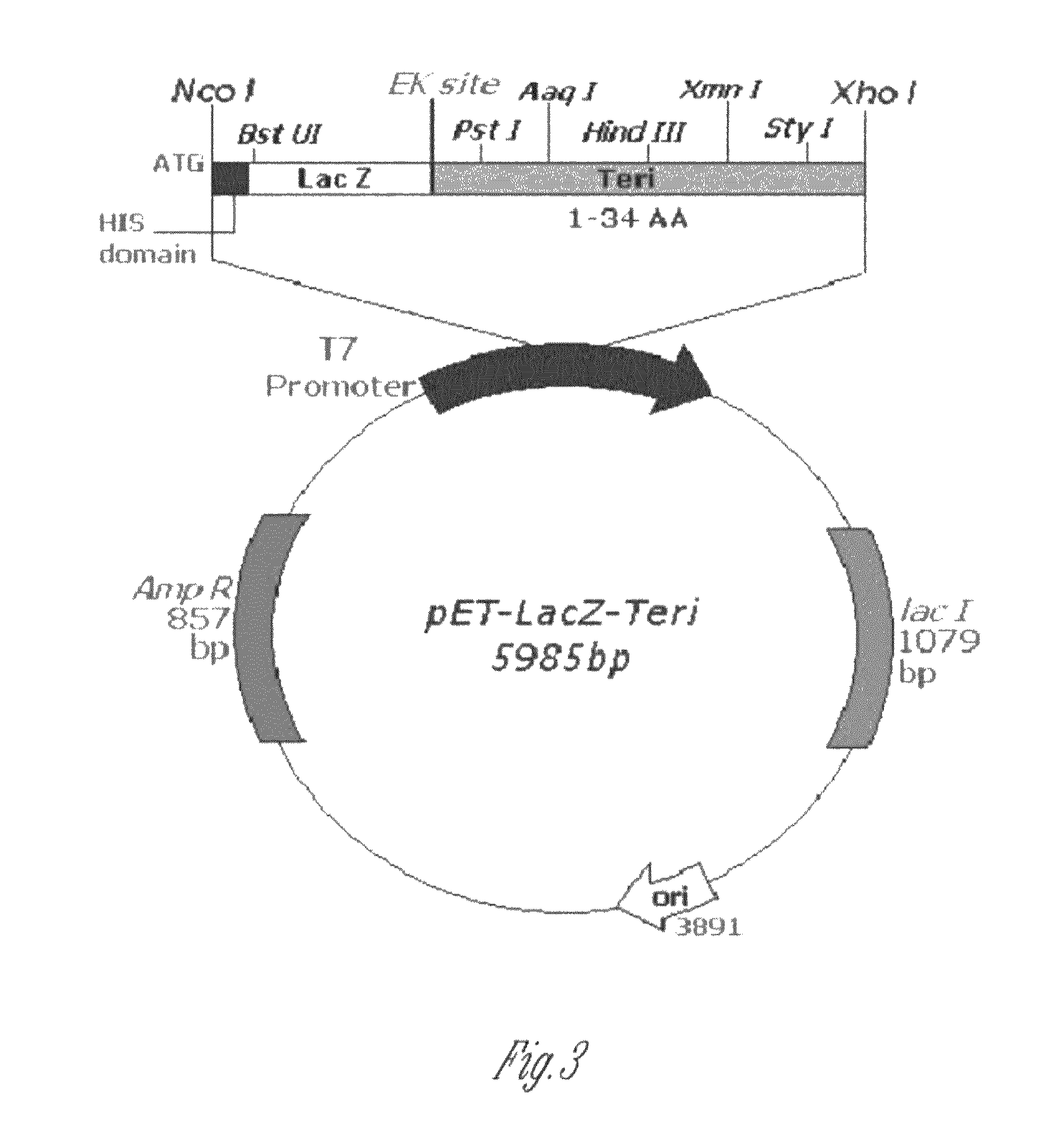
The present invention discloses a process for the preparation of rhPTH (1-34) also known as teriparatide by con-struction of a novel nucleotide, as an NcoI.IXhoI fragemt as set forth in SEQ. ID. No.:1 encoding a chimeric fusion protein as set forth in SEQ.ID. No.:2 comprising of a fusion partner consisting of 41 amino acids belonging to Escherichia coli β-galactosidase (LacZ) gene, an endopeptidase cleavage site, rhPTH (1-34) gene fragment, cloning the said nucleotide in an expression vector under the control of T7 promoter, transforming Escherichia coli with the said vector and expressing the chimeric fusion protein in fed batch fermentation. The present invention further discloses a low feed rate lactose induction for optimized expression of rhPTH (1-34) in Escherichia coli. The present invention also discloses an unique, novel two step orthogonal purification process for rhPTH (1-34) comprising of cation exchange chromatography optionally followed by preparative chromatography selected from HIC or RP-HPLC to yield a target protein of ≧99% purity. The present invention discloses a simple, cost-effective, environmentally benign method of producing high purity rhPTH (1-34).
Owner:USV LTD
Medicine composition sustained release micro-sphere preparation for treating osteoporosis and preparing method thereof
InactiveCN105497880AProlong the action timeGood treatment effectPowder deliveryOrganic active ingredientsMechanism of actionCalcitonin
The invention relates to a medicine composition sustained release micro-sphere preparation for treating osteoporosis and a preparing method thereof. The preparation is characterized in that one or more of teriparatide and bisphosphonate, or calcitonin or estrogen are wrapped by a degradable macro-molecule material carrier composed according to a certain proportion, and a sustained release micro-sphere is prepared and used for injection, so that the long-acting sustained release effect is achieved. The sustained release period of the sustained release micro-sphere injection is as long as a few days or a few months, while the number of times for drug use is reduced obviously, complementation of action mechanisms can be achieved by means of the drug combination, adverse drug reaction is reduced, and the preparation is beneficial for clinical treatment.
Owner:SHENZHEN JYMED TECH
Orthogonal process for purification of recombinant human parathyroid hormone (rhPTH) (1-34)
The present invention discloses a process for the preparation of rhPTH (1-34) also known as teriparatide by construction of a novel nucleotide, as an NcoI.IXhoI fragment as set forth in SEQ. ID. No.:1 encoding a chimeric fusion protein as set forth in SEQ.ID. No.:2 comprising of a fusion partner consisting of 41 amino acids belonging to Escherichia coli β-galactosidase (LacZ) gene, an endopeptidase cleavage site, rhPTH (1-34) gene fragment, cloning the said nucleotide in an expression vector under the control of T7 promoter, transforming Escherichia coli with the said vector and expressing the chimeric fusion protein in fed batch fermentation. The present invention further discloses a low feed rate lactose induction for optimized expression of rhPTH (1-34) in Escherichia coli. The present invention also discloses an unique, novel two step orthogonal purification process for rhPTH (1-34) comprising of cation exchange chromatography optionally followed by preparative chromatography selected from HIC or RP-HPLC to yield a target protein of ≧99% purity. The present invention discloses a simple, cost-effective, environmentally benign method of producing high purity rhPTH (1-34).
Owner:USV LTD
Method for purifying teriparatide
The present invention relates to a novel method for purifying teriparatide, a therapeutically active polypeptide fragment of full-length human parathyroid hormone. The method is based on ion exchange chromatography and is effective in separating the therapeutically active polypeptide from undesirable variants, such as truncated polypeptides. The method of the invention can be used at a preparative scale which allows it to be implemented into the production process for teriparatide. Accordingly, the invention also provides a method for the production of teriparatide which includes a step in which teriparatide is purified by the novel ion exchange chromatography method of the invention.
Owner:RICHTER HELM BIO TEC
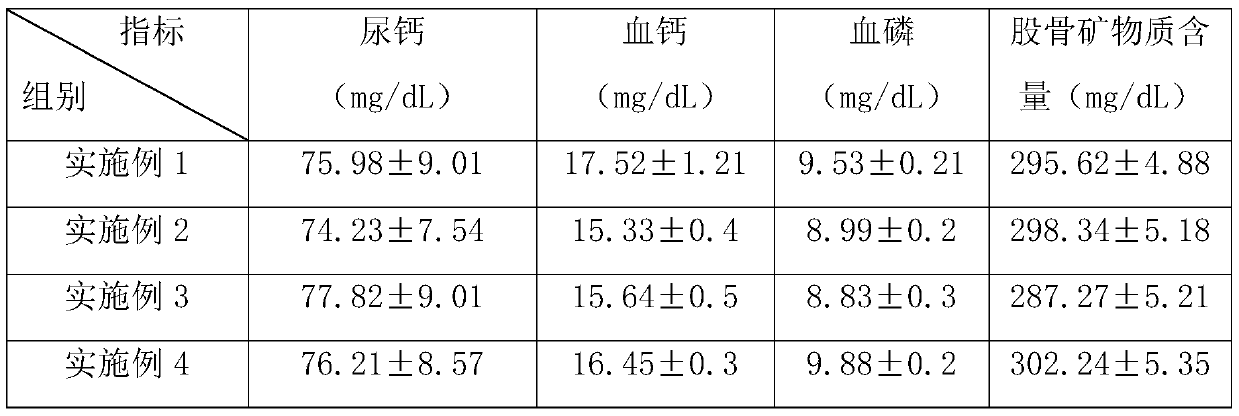
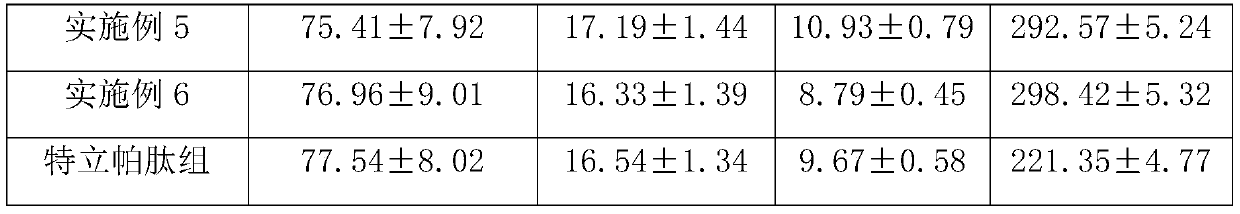
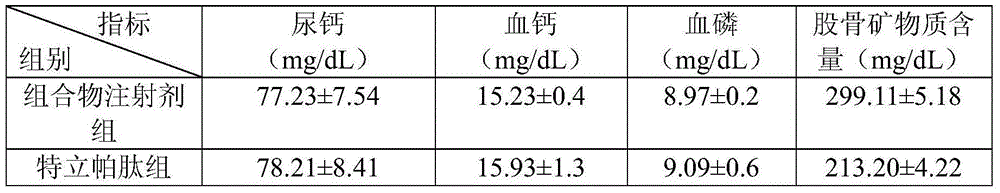
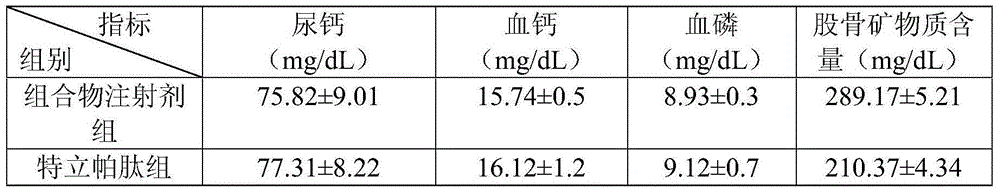
Medicine composite for treating osteoporosis and preparation method thereof
InactiveCN105169394AGood treatment effectReduced responseOrganic active ingredientsPeptide/protein ingredientsSerum phosphateTreatment effect
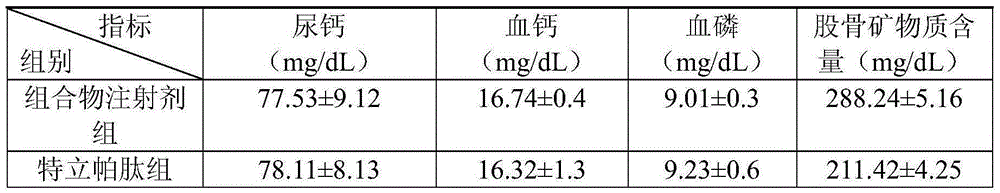
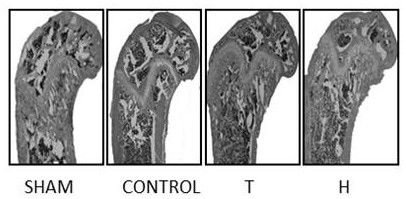
The invention relates to a medicine composite injection for treating osteoporosis and a preparation method thereof. In particular, active constituents of the injection comprise one or more constituents out of teriparatide combined with bisphosphonate series, calcitonin series and estrogen, and besides, pH regulating agents and excipient with the amount acceptable to pharmacy are added. According to the preparation method, the preparation process is investigated, the rat urinary calcium content, the blood calcium conent, the serum phosphate content and the thighbone mineral matter content further serve as indexes, the treatment effect of the injection on osteoporosis model rats is further investigated, the prepared composite injection is remarkable in effect, and the practical significance is achieved.
Owner:SHENZHEN JYMED TECH
Preparation method of teriparatide
PendingCN109897099AEfficient removalLess prone to side effectsPeptide preparation methodsParathyroid hormonesFreeze-dryingTeriparatide
The invention discloses a preparation method of teriparatide. The preparation method comprises steps as follows: 2-CTC resin is taken as a resin carrier and is coupled with Fmoc-Phe-OH under the action of an activator and a coupling reagent, and Fmoc-Phe-resin is obtained, an Fmoc deprotection reagent is added to the Fmoc-Phe-resin, after a reaction ends, washing is performed with N,N-DMF, Phe-resin is obtained, one-by-one extension coupling is performed on amino acid under the action of a condensation reagent and an activating reagent, and teriparatide-resin is obtained; a lysate containing PhSMe / PhOH / EDT system is added to the teriparatide-resin, and a crude product of teriparatide is obtained; the crude product of teriparatide is separated, purified and freeze-dried by use of a C18 column, and high-purity teriparatide is obtained. The preparation method of teriparatide is low in cost and adopts a simple process. The total yield of teriparatide is increased, and no harm is caused toany environment. The preparation method is suitable for large-scale production.
Owner:哈尔滨吉象隆生物技术有限公司
New process for preparing teriparatide
PendingCN111057139AHigh purityShort synthesis timePeptide preparation methodsParathyroid hormonesBiochemical engineeringPeptide sequence
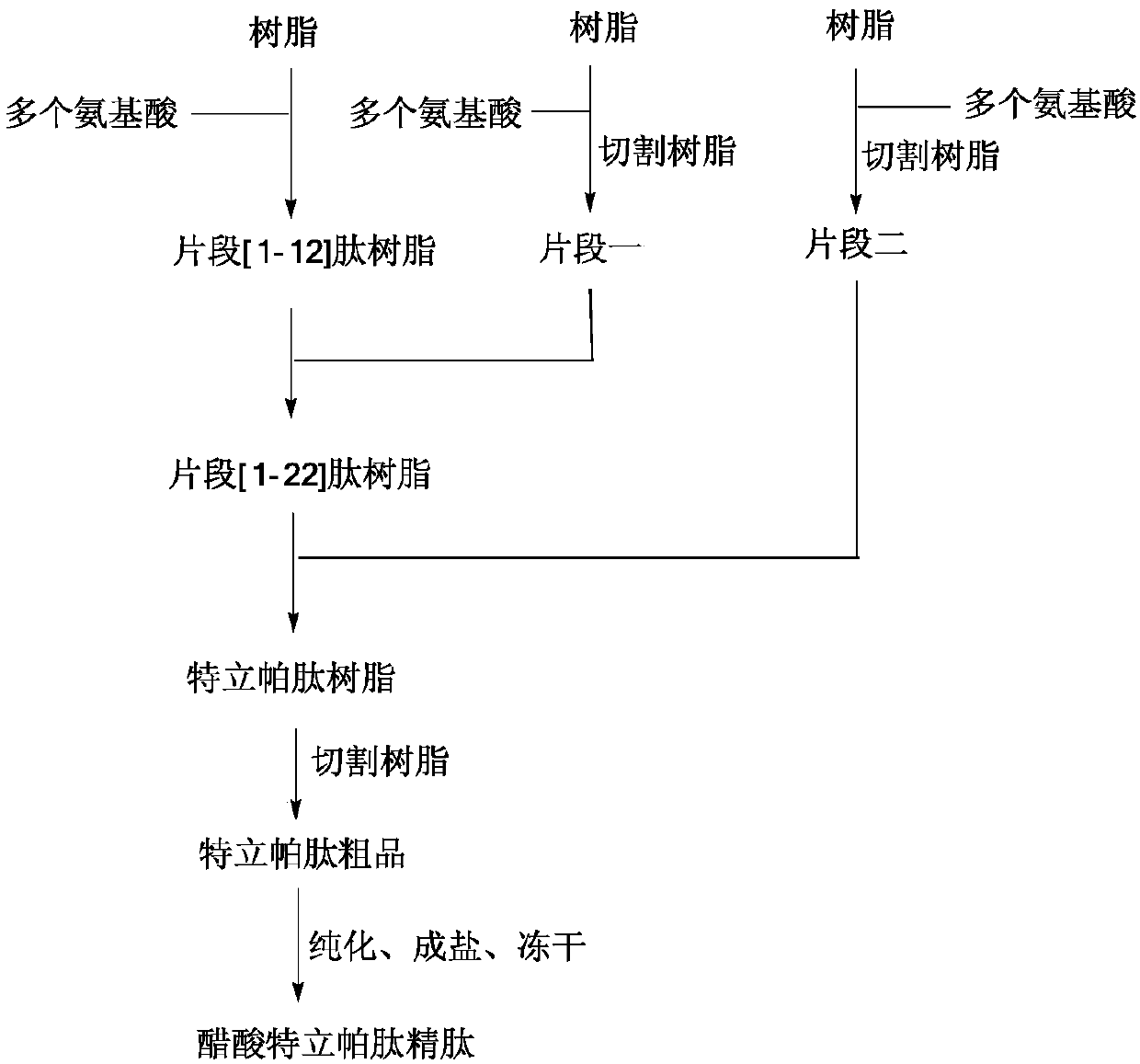
The invention provides a teriparatide synthesis method. According to the method, according to the main chain peptide sequence of teriparatide, the teriparatide is divided into a fragment [1-12] peptide resin, a fragment I and a fragment II, the three short peptides can be respectively synthesized, the fragment [1-12] peptide resin, the fragment I and the fragment II are linked one by one, a terminal Fmoc protecting group is removed to obtain a teriparatide resin, and finally cracking, purifying and salifying are performed to finally obtain the target product, wherein the structures of the fragments and the fragment resin are defined in the specification. The method is simple to operate, the obtained product is high in purity and low in cost, and industrial production is facilitated.
Owner:NANJING HUAWE MEDICINE TECH DEV
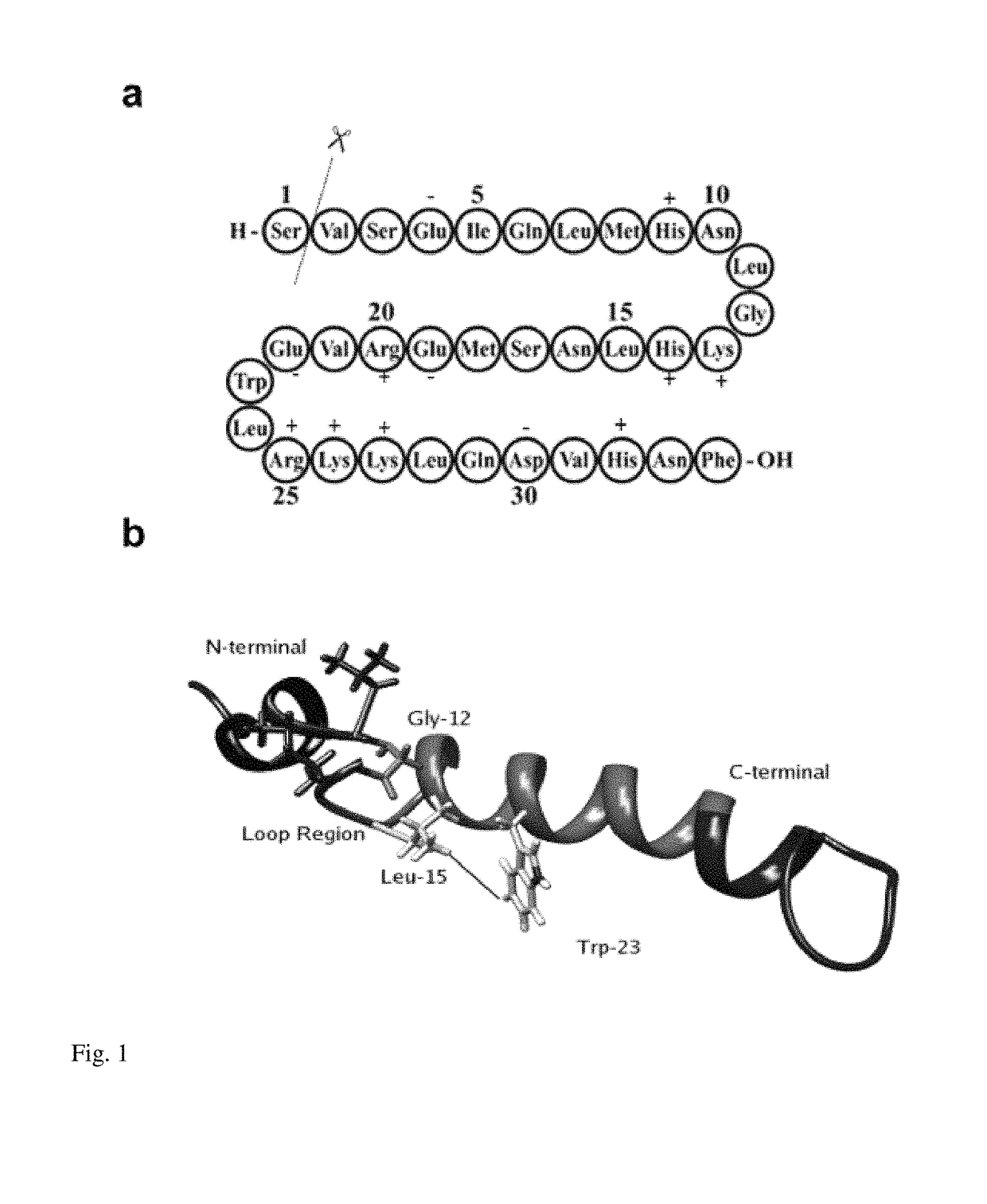
Novel parathyroid hormone (PTH) mimetic peptide based on protein domain reconstruction, and application of novel PTH mimetic peptide
ActiveCN109320600AGood differentiation effectThe osteoclastogenic effect is not highPeptide/protein ingredientsSkeletal disorderTyrosineAnti osteoporosis
Owner:北京兆来医药科技有限公司
Application of LncRNAGAS5 to treatment on primary osteoporosis
InactiveCN111568916AHigh acceptanceImprove comfortOrganic active ingredientsMicrobiological testing/measurementDiseasePharmacologic action
The invention provides application of long-chain non-coding RNAGAS5 to preparation of a medicament for treating skeleton system diseases. A GAS5 adenovirus is applied to carry out treatment on osteoporosis, and the pharmacological action of the GAS5 adenovirus is similar with that of teriparatide, and both the GAS5 adenovirus and the teriparatide are medicaments for promoting bone formation. The difference is that the GAS5 is naturally expressed in various types of cells in vivo, has no specific glandular secretion and cannot cause corresponding inhibition from endocrine glands of the human body; and the GAS5 only promotes MSCs to be differentiated into bone cells in vivo, and has no obvious influence on osteoclast. Therefore, the long-chain non-coding RNAGAS5 is higher in osteoporosis resistance, and has no similar problem of small dosage range of the teriparatide. In addition, due to the unique action mode of the adenovirus, the GAS5 can take an acting effect of resisting to osteoporosis only by injecting in vitro for once, and acceptability and comfort of a patient can be greatly promoted.
Owner:SUN YAT SEN UNIV
Oral pharmaceutical composition containing teriparatide and preparation method thereof
PendingCN114599388AImprove permeabilityImprove bioavailabilityPeptide/protein ingredientsSkeletal disorderCholic acidAcyl group
The present invention relates to an oral pharmaceutical composition and a preparation method therefor, the oral pharmaceutical composition comprising an ionic bond complex consisting of teriparatide, deoxycholic acid, N [alpha]-deoxycholyl-L-lysyl methyl ester (N [alpha]-deoxycholyl-L-lysyl methyl ester, DCK), and D-[alpha]-tocopherol polyethylene glycol 1000 succinate (D-[alpha]-tocopherol polyethylene glycol 1000 succinate), the ionic bond complex comprising at least one compound selected from the group consisting of N [alpha]-deoxycholyl-L-lysyl methyl ester, N [alpha]-tocopherol polyethylene glycol 1000 succinate, N [alpha]-tocopherol polyethylene glycol 1000 succinate, N [alpha]-tocopherol polyethylene glycol 1000 succinate, N [alpha]-tocopherol polyethylene glycol 1000 succinate, N [alpha]- The oral pharmaceutical composition provided by the invention can improve the intestinal mucosa permeability and oral administration bioavailability of teriparatide and improve patient compliance, and can be used for treating osteoporosis.
Owner:ICURE BNP CO LTD
Application of higenamine hydrochloride in preparation of medicine for treating osteoporosis
ActiveCN113827595AContribute to riskContribute to other side effectsOrganic active ingredientsSkeletal disorderDiseasePhosphorylation
The invention relates to application of higenamine hydrochloride in preparation of a medicine for treating osteoporosis, and belongs to the technical field of chemical medicine development. It is found for the first time that higenamine hydrochloride can activate a Smad2 / 3 pathway and promote Smad2 / 3 phosphorylation by targeting IQGAP1 protein, so that osteogenic differentiation is promoted, and the higenamine hydrochloride has the potential to become a new medicine for treating osteoporosis. At present, the osteoporosis is clinically treated mainly by anti-bone resorption medicines such as diphosphates and the like, however, with different causes and development of disease courses of the osteoporosis, pure anti-bone resorption cannot meet the requirements of patients, while the unique osteogenesis promoting medicine teriparatide has long-term osteosarcoma risk and other side effects and limitations; and the higenamine hydrochloride has the characteristics of oral administration and injection, small side effect, obvious osteogenesis promoting effect and the like, and provides a new choice for clinical treatment of osteoporosis.
Owner:JINAN CENTER HOSPITAL
A method for synthesizing teriparatide
ActiveCN104910269BHigh yieldNo harmPeptide preparation methodsParathyroid hormonesAcid hydrolysisCombinatorial chemistry
Owner:CHENGDU SHENGNUO BIOTEC CO LTD
Teriparatide sustained-release microsphere and preparation method thereof
ActiveCN103157096BHigh drug loadingUniform particle size distributionPeptide/protein ingredientsSolution deliveryPolyesterMicrosphere
The invention relates to the technical field of medicines, and in particular relates to a teriparatide sustained-release microsphere and a preparation method thereof. The teriparatide sustained-release microsphere comprises a teriparatide and a carrier, wherein the particle size of the teriparatide is 30 micrometers-50 micrometers; and the carrier is a mixture of one or two of polyester, starch, gelatin, Arabic gum, albumin, alginate and chitosan. The teriparatide microsphere is prepared by adjusting the constitution and molecular weight of the carrier and the medicine-loading rate and particle size of the microsphere according to the properties of the teriparatide. The prepared microsphere is stable in releasing and small in sudden releasing, the dose administration times can be reduced, the probability of infection is decreased, patients can conveniently take the microsphere, and the compliance of the patients is improved. Experiments show that the teriparatide has uniform particle size distribution, high encapsulation efficiency and large medicine-loading capacity. The sustained-release microsphere can be externally released for 10 days.
Owner:HYBIO PHARMA
A method of preparing teriparatide
InactiveCN104017064BPromote resultsLow purityPeptide preparation methodsParathyroid hormonesSide chainCoupling
Owner:ADLAI NORTYE BIOPHARMA CO LTD
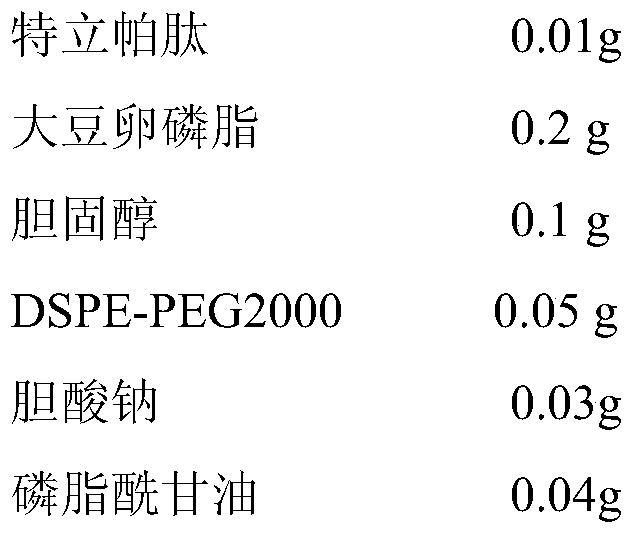
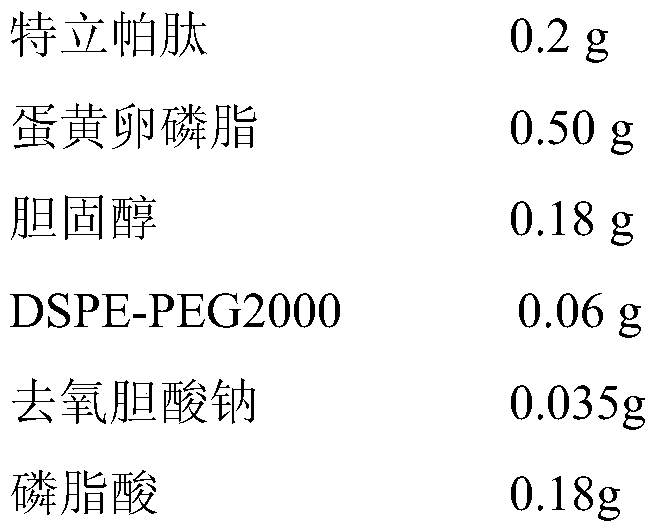
A liposome preparation for nasal administration of teriparatide and its preparation method
ActiveCN106110306BImprove bioadhesionExtended stayPeptide/protein ingredientsSkeletal disorderNasal cavitySide effect
The invention discloses a teriparatide nasal administration liposome preparation and a preparation method thereof, and belongs to the field of pharmaceutical preparations. The teriparatide nasal administration liposome preparation is mainly prepared from 0.01-0.5g of teriparatide, 0.1-1.5g of phospholipid, 0.015-0.6g of cholesterol, 0.015-0.2g of DSPE-PE2000, 0.012-0.5g of a membrane softener and 0.02-0.3g of a stabilizing agent. The teriparatide is encapsulated, and the liposome is controllable in grain size from 20nm to 100nm and uniform in distribution, the encapsulation efficiency of the liposome is higher than 90% and the activity of the liposome is stabilized above 90%. The nasal administration liposome preparation can achieve the non-injection administration of the teriparatide, and the liposome preparation can improve the bioavailability of the teriparatide, reduce the toxic and side effects of the teriparatide and improve patient's medication compliance.
Owner:南京星银药业集团有限公司
Application of higenamine hydrochloride in preparation of medicine for treating osteoporosis
ActiveCN113827595BLittle side effectsOsteogenic effect is obviousOrganic active ingredientsSkeletal disorderPhosphorylationTeriparatide
The invention relates to the application of higenamine hydrochloride in the preparation of medicines for treating osteoporosis, and belongs to the technical field of chemical medicine development. The present invention discovers for the first time that higenamine hydrochloride can target IQGAP1 protein, activate Smad2 / 3 pathway, promote Smad2 / 3 phosphorylation, thereby promoting osteogenic differentiation, and has the potential to become a new drug for treating osteoporosis. At present, the clinical treatment of osteoporosis is mainly based on anti-bone resorption drugs: such as bisphosphonates, etc., but with the different causes of osteoporosis and the development of the disease course, simple anti-bone resorption drugs can no longer meet the needs of patients , and the only bone-promoting drug, teriparatide, has long-term osteosarcoma risk and other side effects and limitations, while higenamine hydrochloride has the characteristics of oral and injectable, small side effects, and obvious osteogenesis-promoting effect. Treating osteoporosis offers new options.
Owner:JINAN CENTER HOSPITAL
Oral mucosal delivery systems comprising monophasic concentrate of teriparatide
PendingUS20210251886A1Peptide/protein ingredientsSkeletal disorderDigestive canalOral mucous membrane
This invention comprises a water free liquid composition comprising Teriparatide for transmucosal delivery that adheres to mucosa after coming in contact with mucosal surface and a system of making dosage form from the same and delivering to mucous membrane at various locations though liquid drops, capsule or tablets. The liquid composition is characterized by being a monophasic composition. The liquid monophasic composition comprises a non-aqueous liquid as carrier, a penetration enhancer / permeation enhancer, stabilizer and a surfactant. The liquid composition of Teriparatide intended to be dispensed to a mucosal membrane of oral / buccal cavity as liquid drops, or of oral / buccal cavity or nasal cavity as an oral or nasal spray or of oral / buccal cavity as an oral film; or of a part of alimentary canal as incorporated in a capsule or a tablet as an ingredient.
Owner:ZIM LAB LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com