Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
87 results about "Human Parathyroid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Antibody against human parathormone related peptides
InactiveUS6903194B1Improve hypophosphatemiaHigh activityPeptide/protein ingredientsAntibody mimetics/scaffoldsThyroid hormonesA-DNA
Owner:CHUGAI PHARMA CO LTD
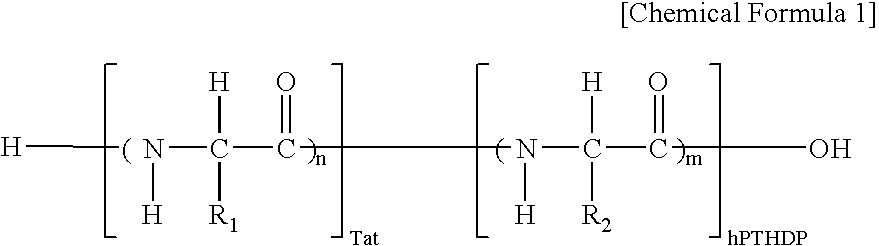
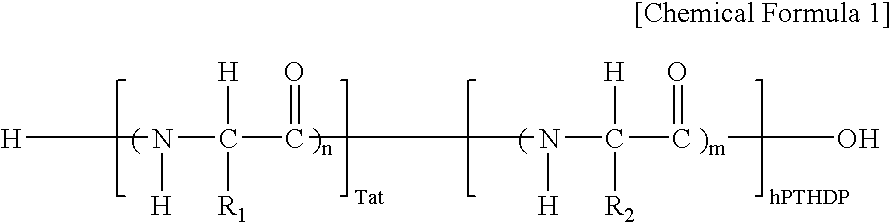
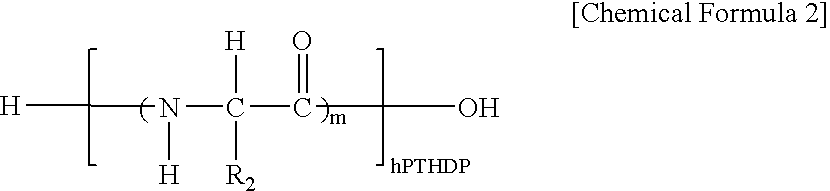
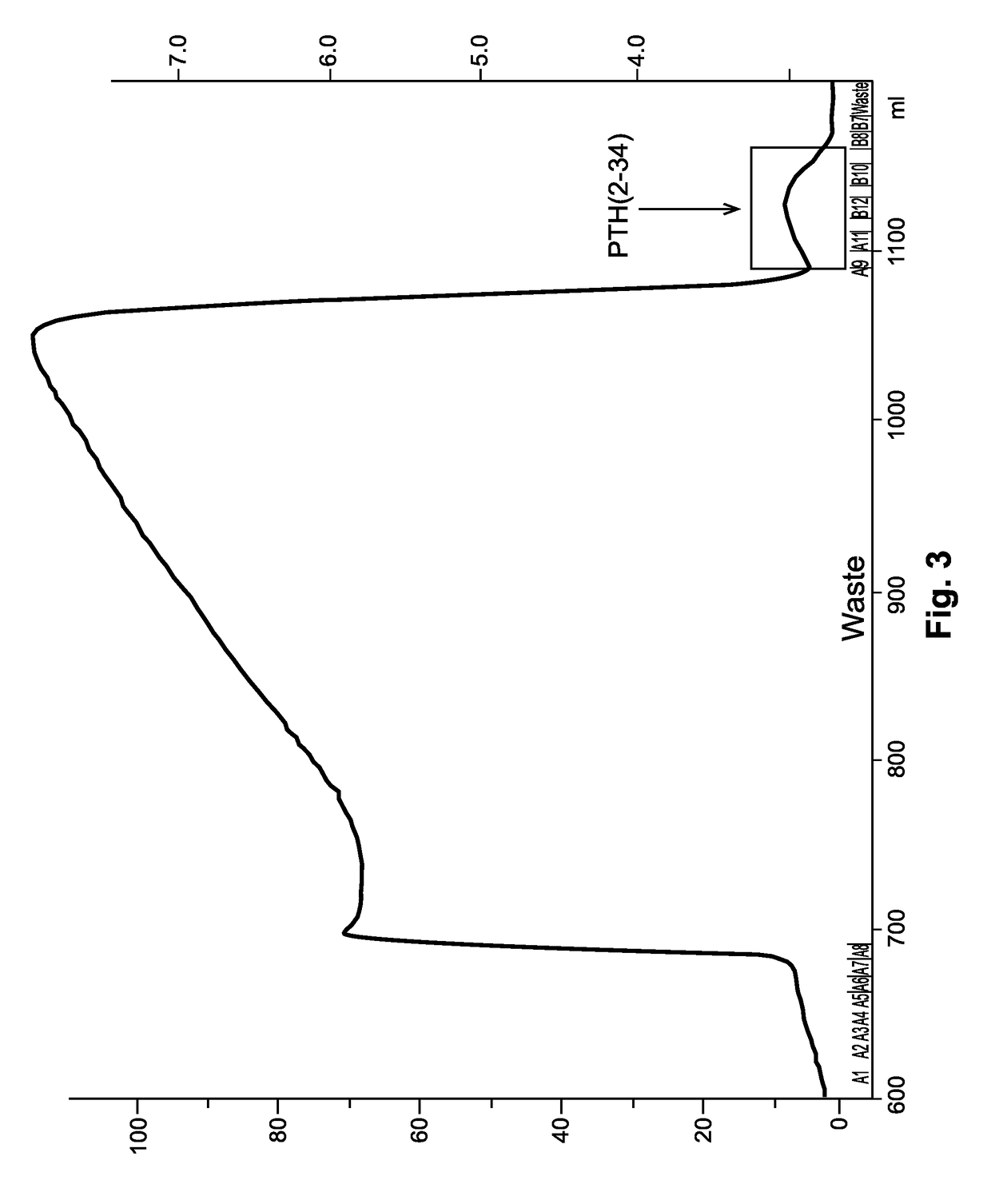
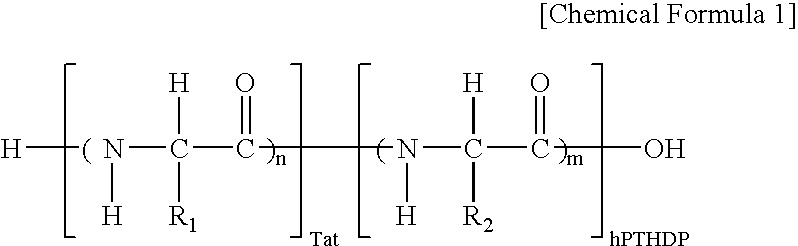
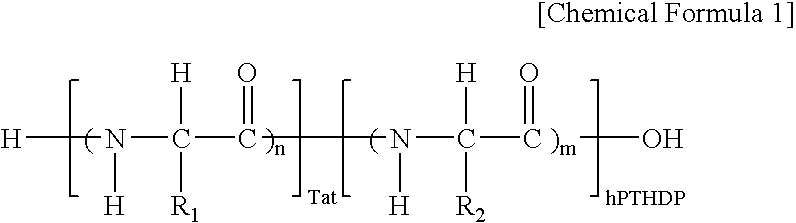
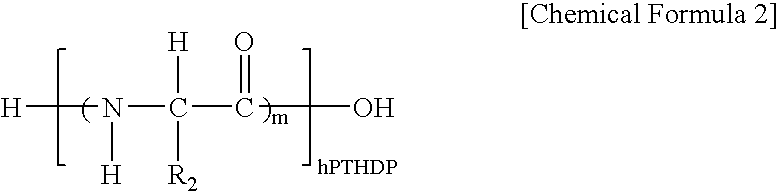
Fusion peptide of human parathyroid hormone derived peptide and tat peptide, preparation thereof, and skin slimming cosmetic composition comprising the same
InactiveUS20050048629A1EasilySafely penetrates into the integument and endotheliumCosmetic preparationsPeptide/protein ingredientsTat peptideIncreased Lipolysis
The present invention relates to a fusion peptide wherein a self cell-penetrating Tat peptide having a self penetrating signal is bound to a human parathyroid hormone-derived peptide, a preparation thereof, and a skin slimming cosmetic composition comprising the same. Since the fusion peptide wherein the Tat peptide is bound to the human parathyroid hormone-derived peptide has high stability and superior skin absorption, the present invention provides a skin slimming agent having superior lipolysis effects and improved durability of the effects.
Owner:LG HOUSEHOLD & HEALTH CARE LTD
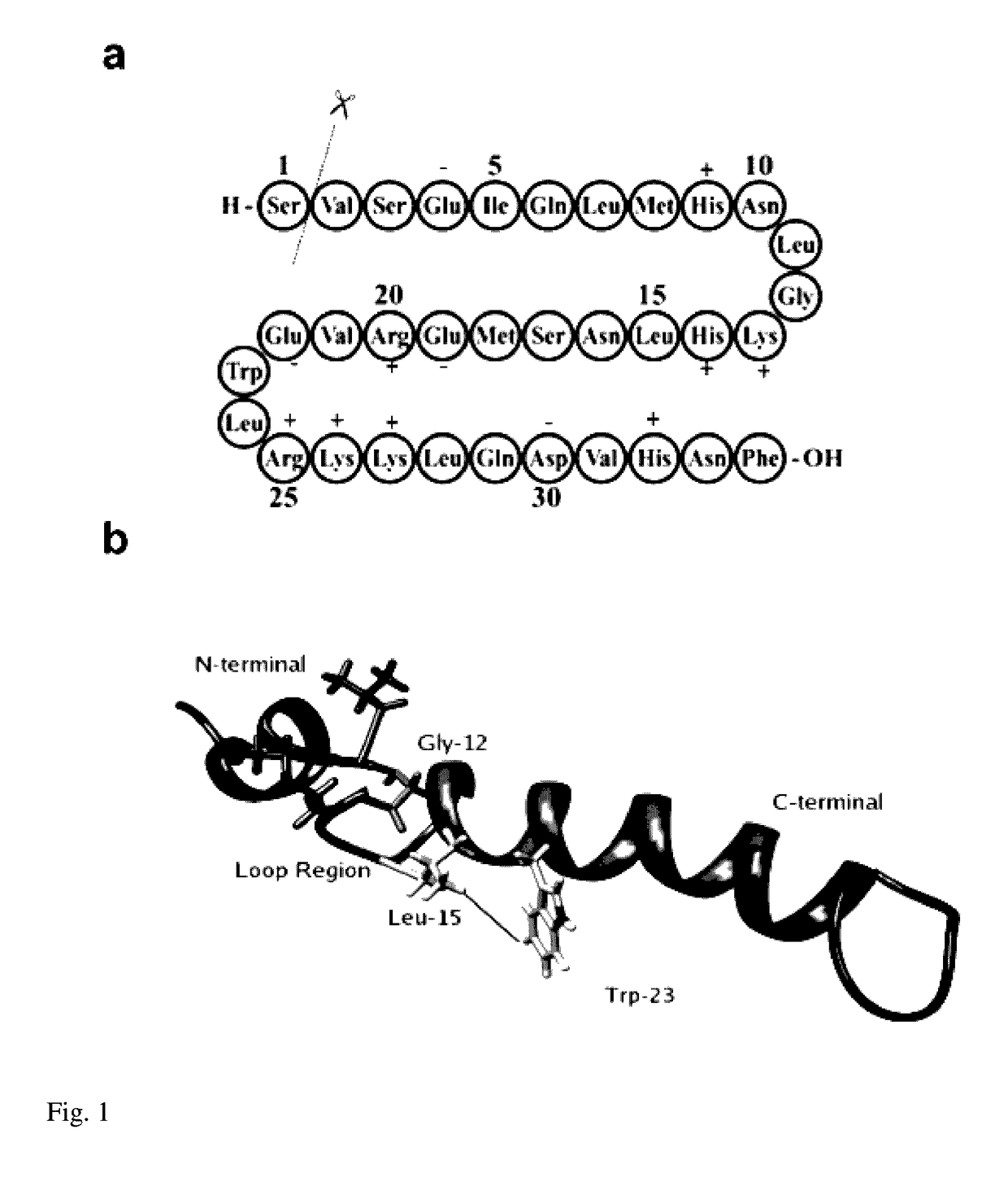
Human parathyroid hormone modifications, preparation and use
The present invention is related to synthetic and / or recombinant biologically active peptide derivatives of PTH(1-28). Some of the peptides of the invention are at least 90% identical to a peptide consisting essentially of the amino acid sequence X01ValSerGluIleGlnLeuMetHis AsnLeuGlyLysHisLeuAsnSer MetX02ArgValGluTrpLeuArgLysLysLeu (SEQ ID NO:1), wherein X01 is Ser, Ala or Gly; and X02 is Glu or Arg.
Owner:THE GENERAL HOSPITAL CORP
Amidated parathyroid hormone fragments and uses thereof
InactiveUS20050215476A1Easy to manufactureImprove bioavailabilityPeptide/protein ingredientsSkeletal disorderHormone analogDisease
C-terminal amidated human parathyroid hormone analogs PTH 1-32-NH2 and PTH 1-33-NH2 are biologically active and can be used for the treatment of various bone related diseases and conditions.
Owner:UNIGENE LABORATORIES +1
Human parathyroid hormone modifications, preparation and use
InactiveUS20050203012A1Improve efficacyHigh potencyFungiBacteriaHormones regulationBioactive peptide
Synthetic and / or recombinant biologically active peptide derivatives of PTH(1-28), comprising a biologically active peptide at least 90% identical to a peptide consisting essentially of the formula: (a)X01ValSerGluIleGlnLeuMetHisAsn(SEQ ID NO:1);LeuGlyLysHisLeuAsnSerMetX02ArgValGluTrpLeuArgLysLysLeu(b) fragments thereof containing amino acids 1-24, 1-25, 1-26, or 1-27; (c) pharmaceutically acceptable salts thereof; or (d) N- or C-derivatives thereof; wherein: X01 is Ser, Ala or Gly; and X02 is Glu or Arg, provided that said peptide is not hPTH(1-26)NH2, hPTH(1-27)NH2 or hPTH(1-28)NH2.
Owner:THE GENERAL HOSPITAL CORP
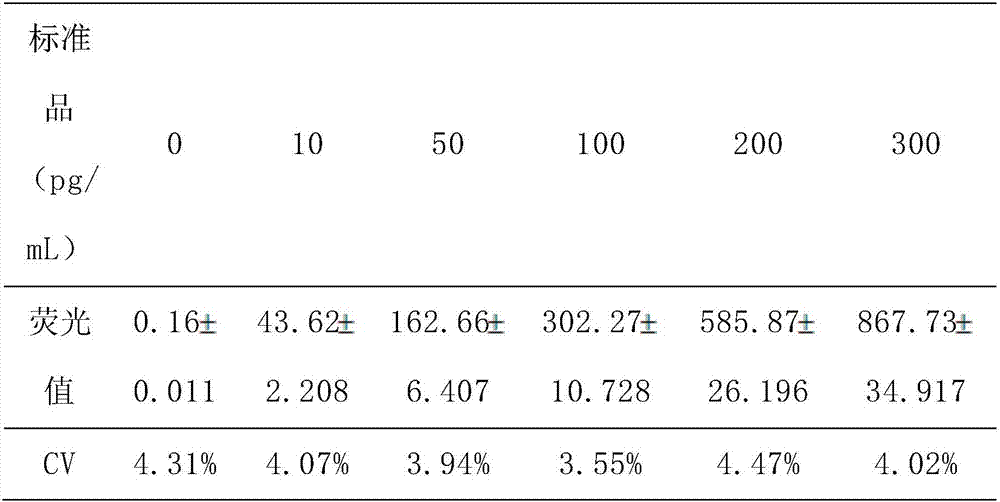
Fluorescence immunochromatography test paper for quantitatively detecting human parathyroid hormone and preparation method of fluorescence immunochromatography test paper
The invention discloses fluorescence immunochromatography test paper for achieving intraoperative judgment of parathyroid tissues through quantitatively detecting a human parathyroid hormone (PTH) and a preparation method of the fluorescence immunochromatography test paper. The test paper capable of detecting the human PTH through a double-antibody sandwich method and a fluorescence immunochromatography; the antibody is prepared by using a specific antigen peptide. The human PTH in a to-be-detected matter can be quickly and accurately detected, so that the fluorescence immunochromatography test paper is simple, convenient and fast to operate, wide in detection range, high in specificity and good in sensitivity.
Owner:JIANGSU INST OF NUCLEAR MEDICINE +1
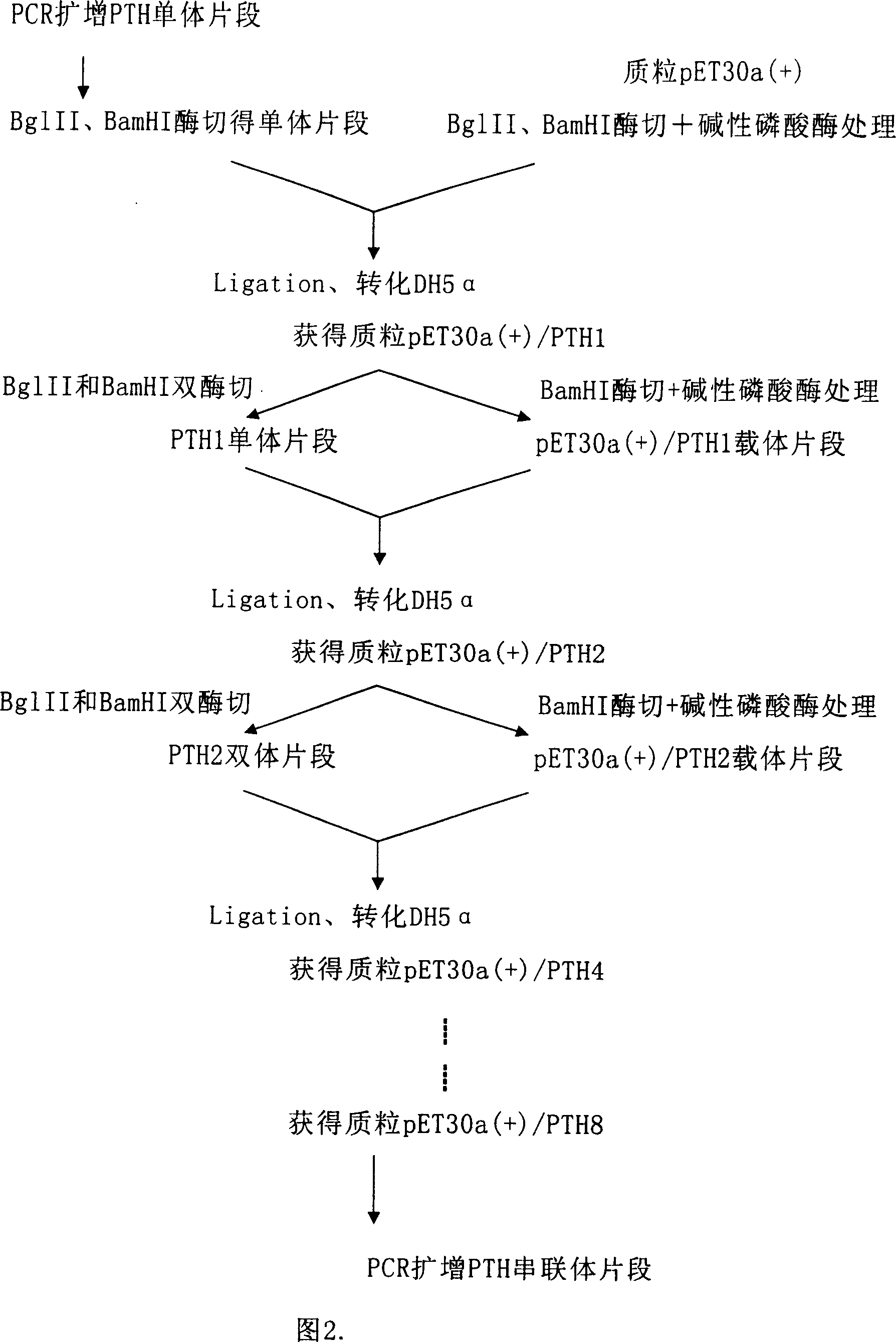
Production of reorganized human parathyroid hormone 1-34 peptide
A process for preparing recombinant human parathyroxin 1-34 peptide includes such steps as culturing host cell in proper condition, separating Gly-Ser-Pro-PTH 1-34 peptide, severing by Pro endopeptidase to form PTH 1-34 peptide and separating and purifying PTH 1-34 peptide. The relative coding sequence, carrier and host cell are also disclosed. Its advantages are simple process, and high purity and activity.
Owner:中国科学院上海生物工程研究中心
Protein formulations
InactiveUS20050209144A1Extended half-lifeLess discomfortPowder deliveryPeptide/protein ingredientsTonicityPharmaceutical formulation
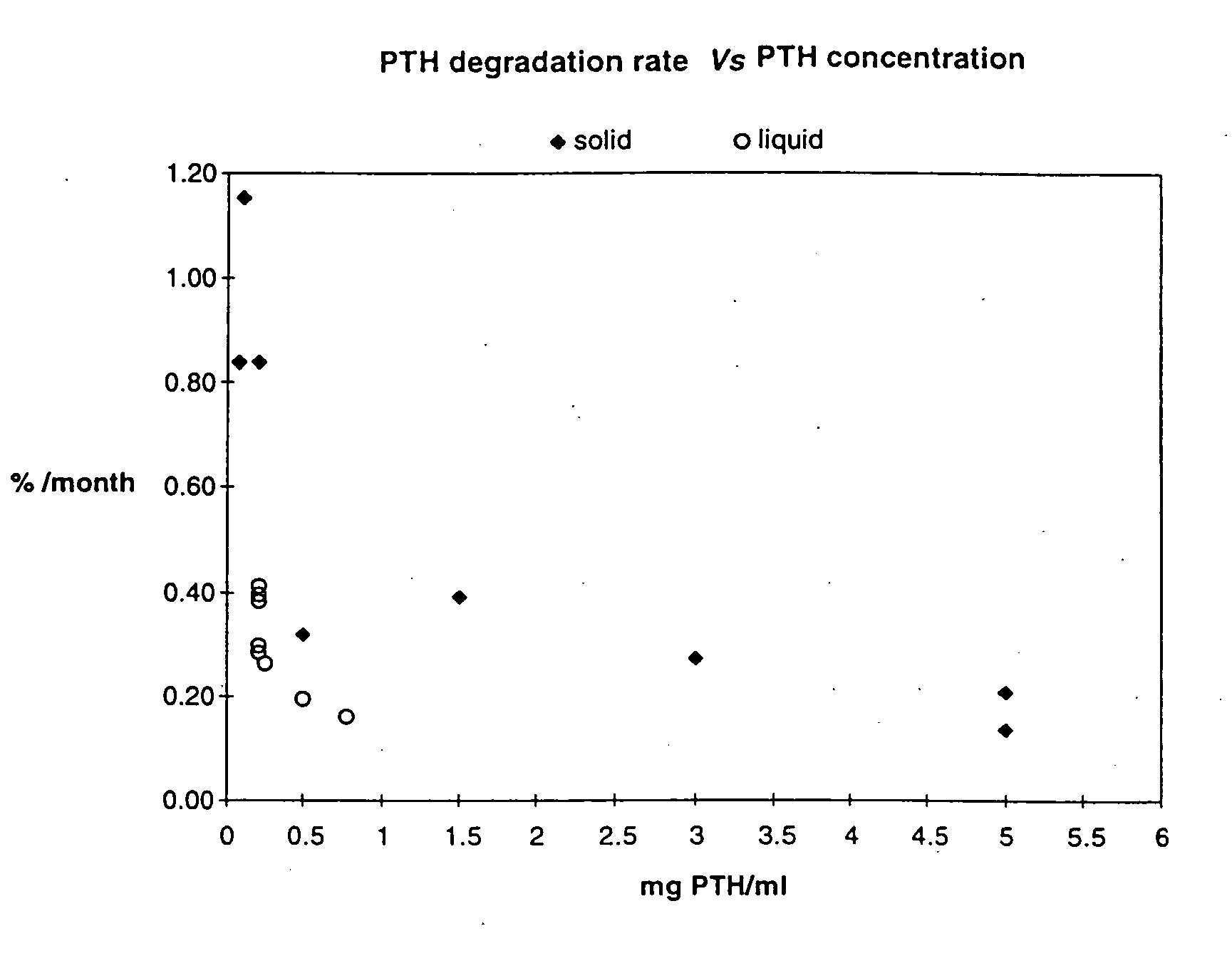
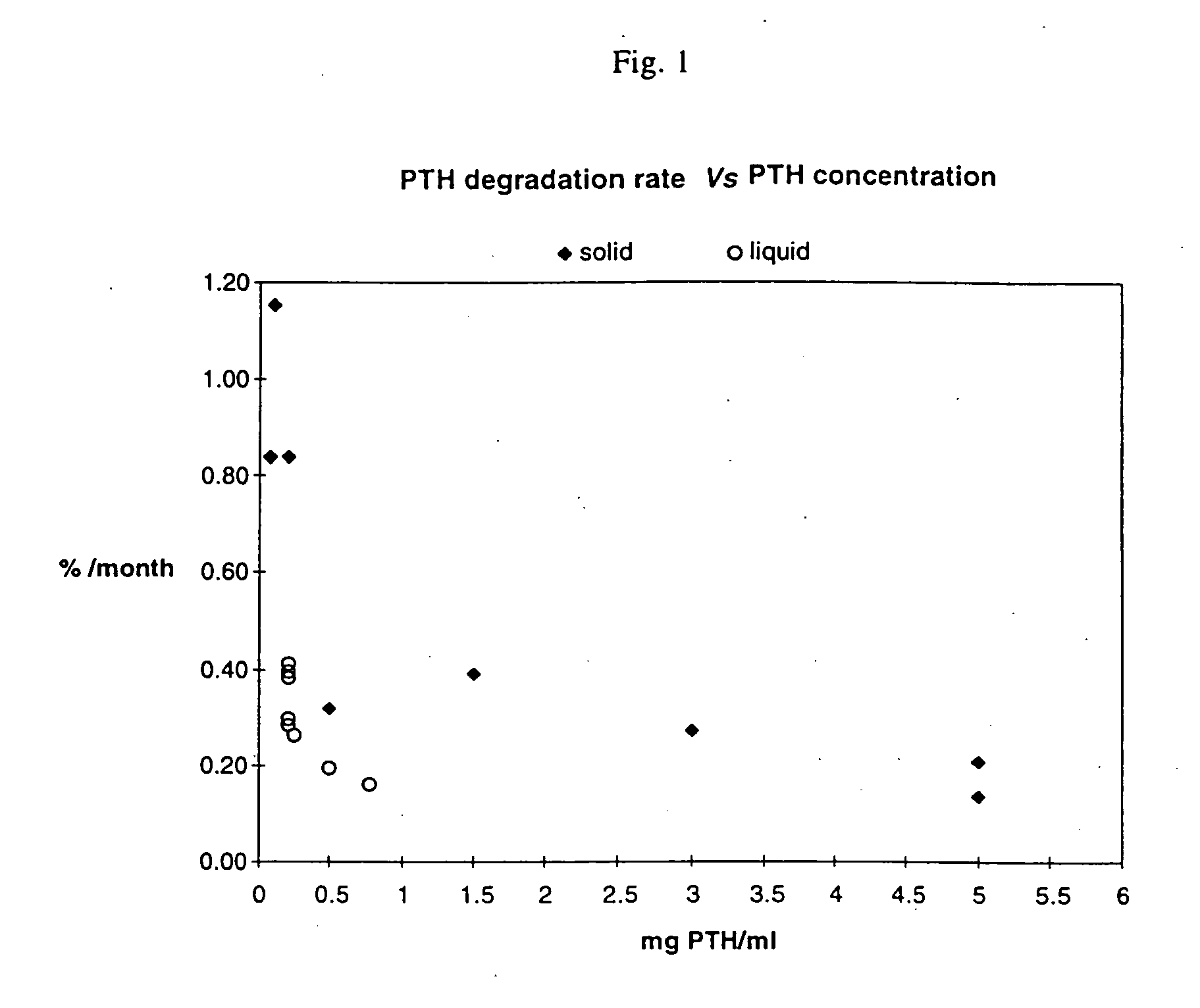
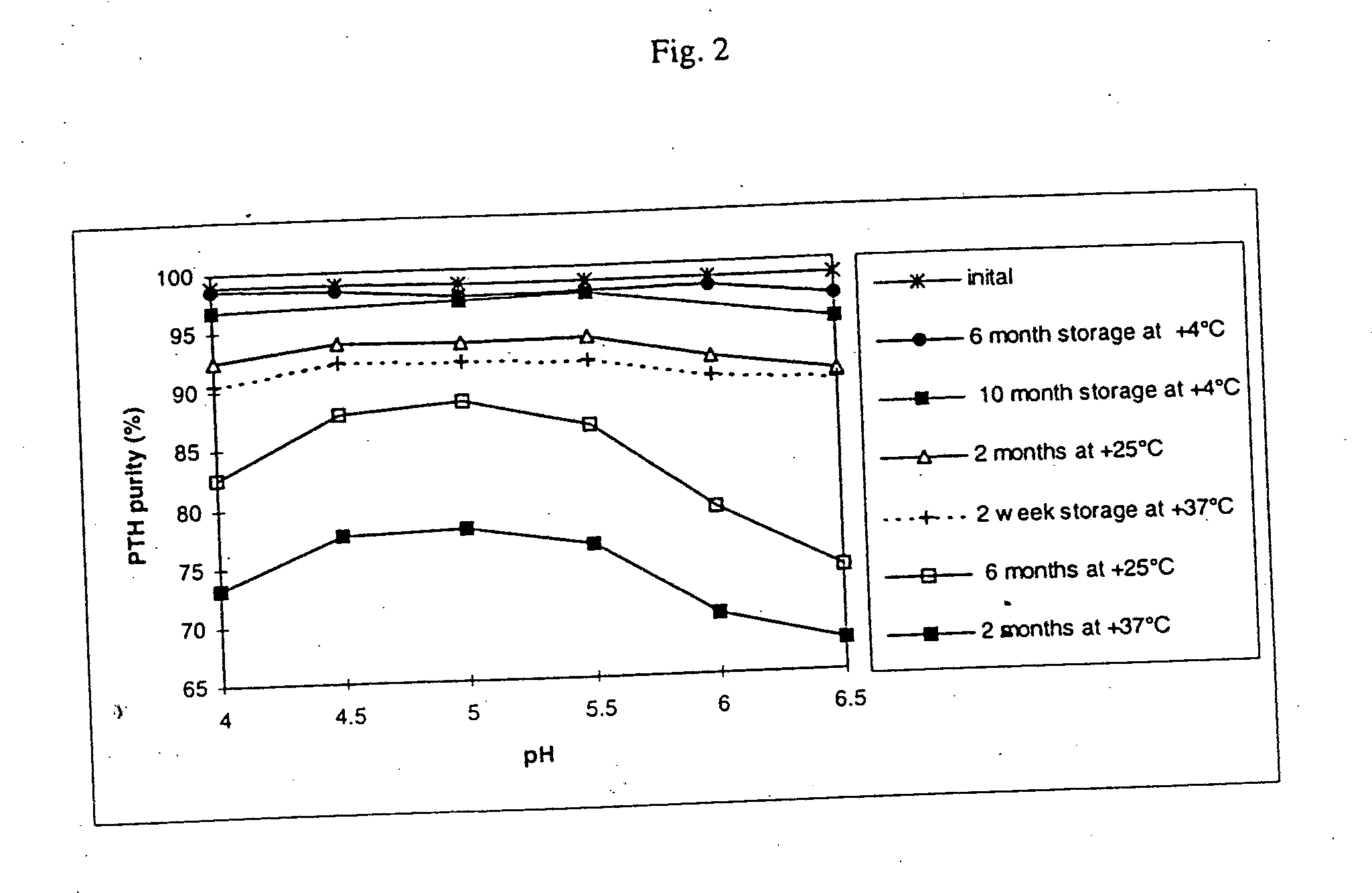
The present invention relates to pharmaceutical formulations comprising human parathyroid hormone at a concentration from 0.3 to 10 mg / ml, a pharmaceutically acceptable buffer having a pH from 4 to 6, and at least one tonicity modifier. The said pharmaceutical formulations are useful for the treatment of bone related disorders such as osteoporosis.
Owner:NPS ALLELIX CORP
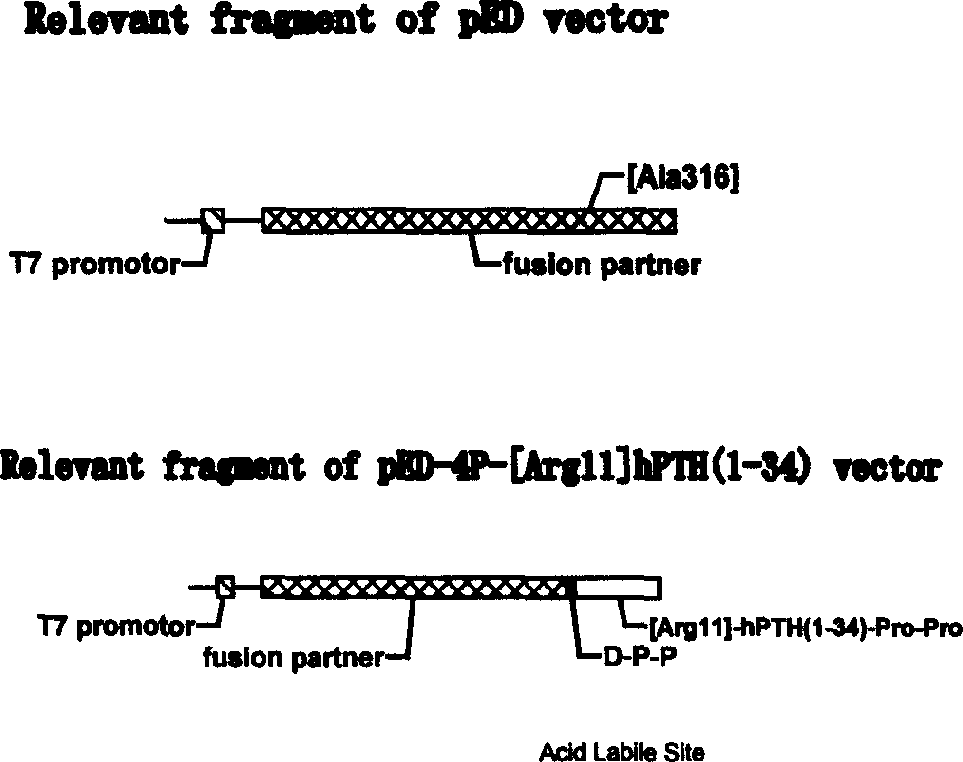
Human parathyrin 1.34 peptide related peptide-Pro-Pro-[Arg11 hPTH (1.34)-Pro-Pro
A human parathyroxni 1-34 peptide associated peptide Pro-Pro-[Arg11]hPTH(1-34)-Pro-Pro. It is a precursor peptide, whose two terminal Proscan be excised in human body to become bioactive [Arg11]hPTH(1-34)-Pro-Pro. Its preparing process includes such steps as culturing the host cell of pED-4P-[Arg11]hPTH(1-34), separating the fusion protein of Pro-Pro-[Arg11]hPTH(1-34)-Pro-Pro from the cultured substance, and excising two Pros by dipeptidase in body to obtain [Arg11]hPTH(1-34)-Pro-Pro.
Owner:CHINA PHARM UNIV
Prepn of recombinant human parathyroid hormone PTH (1-34)
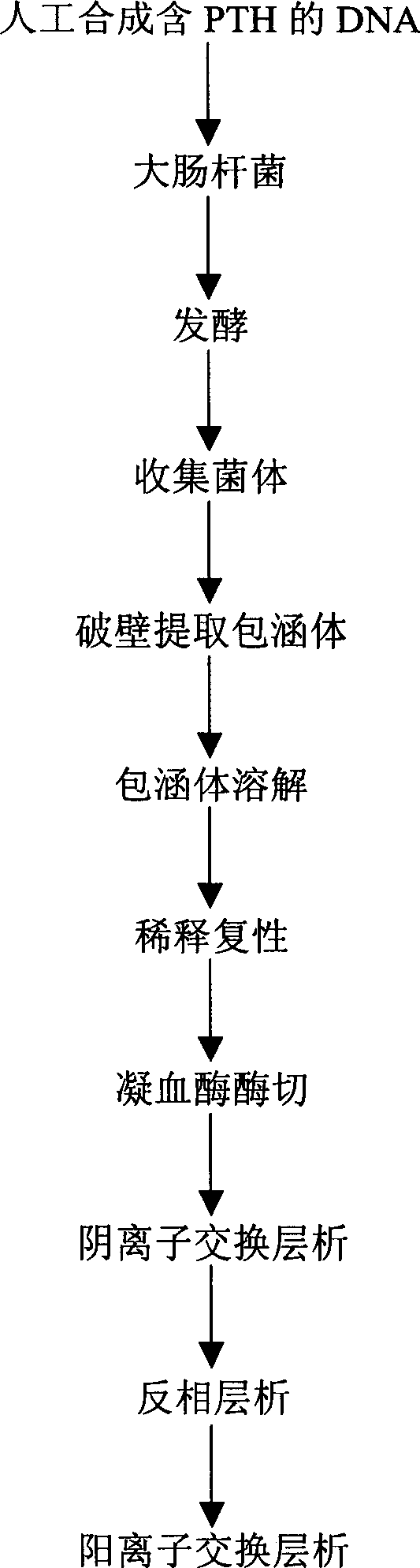
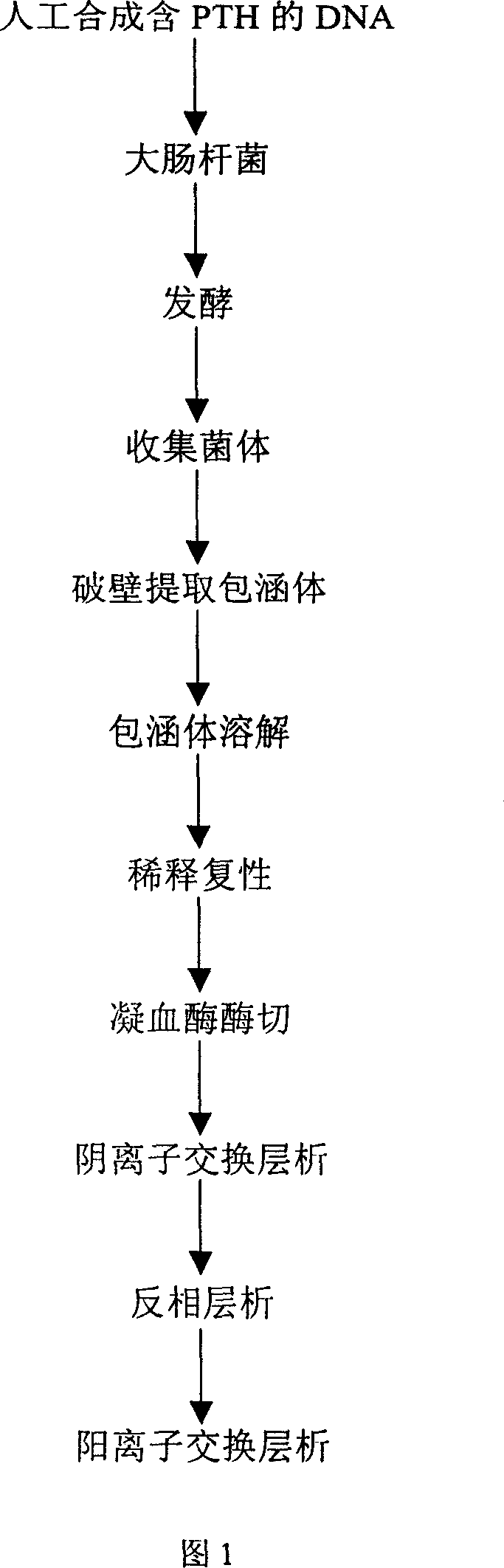
The present invention relates to a preparation process of recombinant human parathyroid hormone PTH(1-34). The process constitutes a new engineering strain to result in high expression amount, simplepurifying course and low cost.The present invention adopts colibacillus degenerate codon modifying sequence corresponding cDNA, the basic sequence is L-V-P-R-PTH(1-34), where L-V-P-R is the enzyme incision site of thrombin. To the end of the nucleotides sequence, colibacillus terminator codon TAA is added; and in order to clone easily to the 5'-terminal enzyme incision site of EcoR I is added, and to the 3'-terminal, enzyme incision site of Sal I is added. High-purity recombinant huamn parathyroid hormone PTH(1-34) is obtained through fermentation of the engineering bacteria, occlusion body extraction, diluting renaturation, thrombase incision, anionic exchange chromatography and other steps.
Owner:西南生物工程产业化中试基地有限公司
Preparation process of human recombined parathyroid hormone 1 84
InactiveCN1861790AGood effectOmit refolding stepBacteriaMicroorganism based processesEscherichia coliIon exchange
The invention discloses a preparing method of the recombination human parathyroid hormone 1-84 by the gene engineering technology. It uses the codon for E.Coli and synthesizes the encoding sequence of the rhPTH1-84 and constructs the carrier and engineering bacteria. The invention is to express the soluble fusion protein contain the whole sequence of the hPTh 1-84 in the E.Coli cytoplasm, then the protein is purified by the chelate chromatography and cut by the enterokinase, next to purify by the ionic exchange and the gel chromatography to get the rhPTH 1-84 which the purity is more than 80%, the yield can reach 300mg / L.
Owner:EAST CHINA UNIV OF SCI & TECH
Medicinal components comprising human parathyroid hormone and medicinal compositions for nasal administration containing the components
InactiveUS20050107292A1Easy to useLong-term usePowder deliveryPeptide/protein ingredientsAcetic acidCompound (substance)
A medical component comprising a human parathyroid hormone peptide or its derivative, and acetic acid contained at a concentration less than its chemical equivalent with respect to the human parathyroid hormone peptide or to its derivative. Since in the medical component acetic acid, which is present as a salt of or attached to the peptide or its derivative, has been reduced to an amount less than chemical equivalent with respect to the human parathyroid hormone peptide or its derivative, a medical component, which is highly stable and will ensure an excellent use feeling when introduced into a pharmaceutical composition, is obtained.
Owner:ASUBIO PHARMA
Man's serum albumin with man's parathormone (1-34) fusion protein and its application
InactiveCN1597965AEasy to purifyEasy to usePeptide/protein ingredientsSerum albuminHalf-lifePlant cell
The invention discloses a fusion protein of human sperm albumin and human parathyroid hormone (1-34), and DNA sequence coding it as well as bacteria, yeast, animal cells and plant cells that carry the DNA sequence. It contains a first region with at least 85% sequence isogeny with human sperm albumin and a second region with at least 85% sequence isogeny with hPTH (1-34), and can substitute, delete or add several aminophenol residues on the premise of not changing its own property; there is a peptide linkage between the two regions; it not only retains the functional actions of hPTH (1-34) to activate receptor and stimulate bon reconstruction but also remarkably prolongs in vivo half life, and it is a medicinal protein that can cure osteoporosis.
Owner:浙江优诺金生物工程有限公司
Construction, expression and purification method of recombinant human parathyroid hormone in colibacillus
InactiveCN1706947AIncrease productionAvoid the renaturation stepPeptide/protein ingredientsParathyroid hormonesCelluloseEscherichia coli
The present invention relates to medicine biological engineering technology, and is cDNA of small peptide encoding human parathyroid hormone and the method of preparing human parathyroid hormone with its expression vector. Through PCR amplification of chemically synthesized recombinant human human parathyroid hormone (1-34) gene and cloning to expression vector pET-35b(+), recombinant human human parathyroid hormone (1-34) is fused to the carboxyl end of cellulose combining structure domain (CBDclose) and efficient expressed. The fusion protein is treated through cellulose resin affinity chromatographic purification, Factor Xa schizolysis to release rhPTH(1-34), the second cellulose resin affinity chromatography, and C4 reverse efficient liquid chromatographic purification to obtain pure rhPTH(1-34). The sample is tested to have molecular weight of 4117.0 Da.
Owner:南京大学生物制药工程研究中心
Method for preparing human serum albumin-human parathyroid hormone
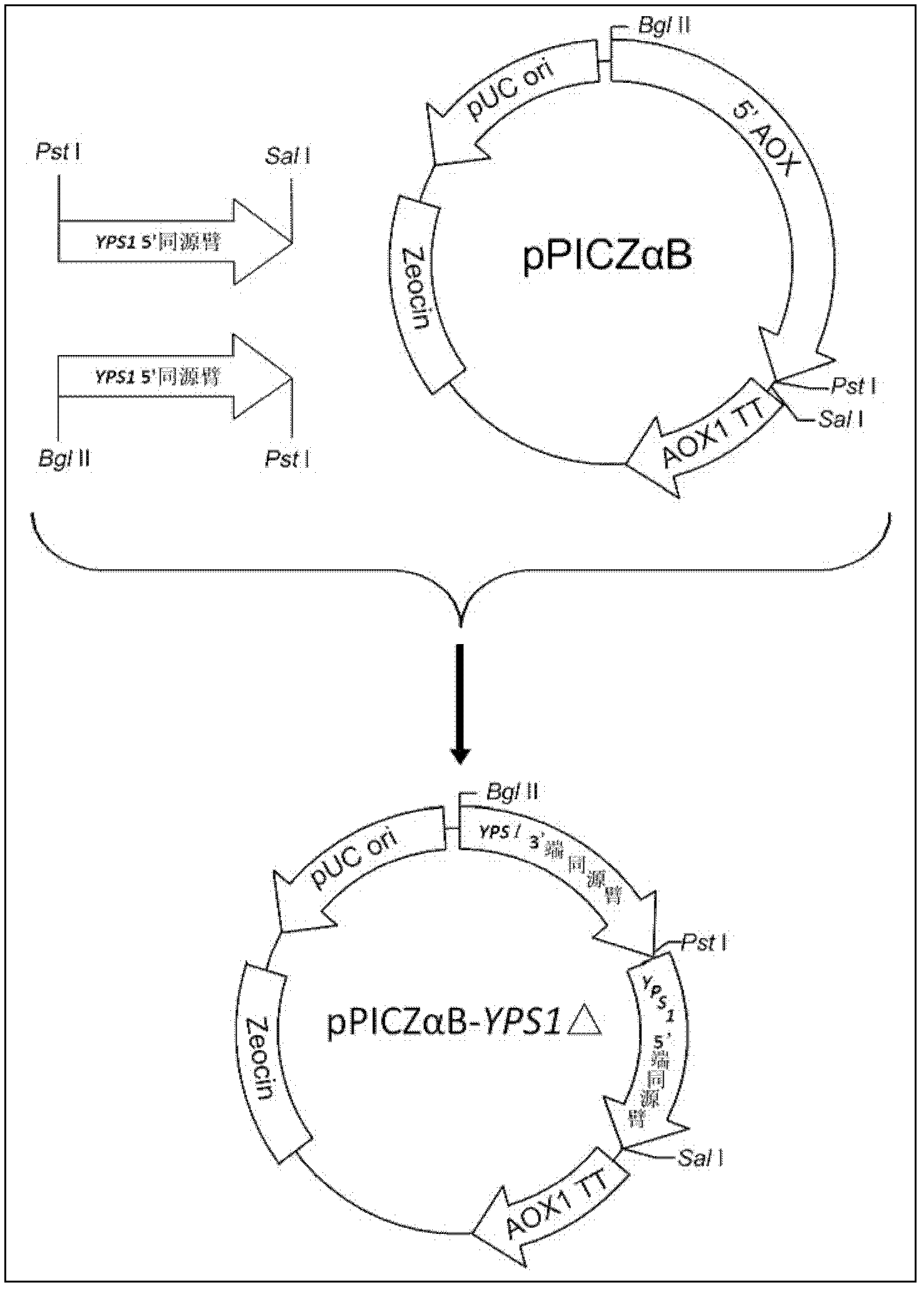
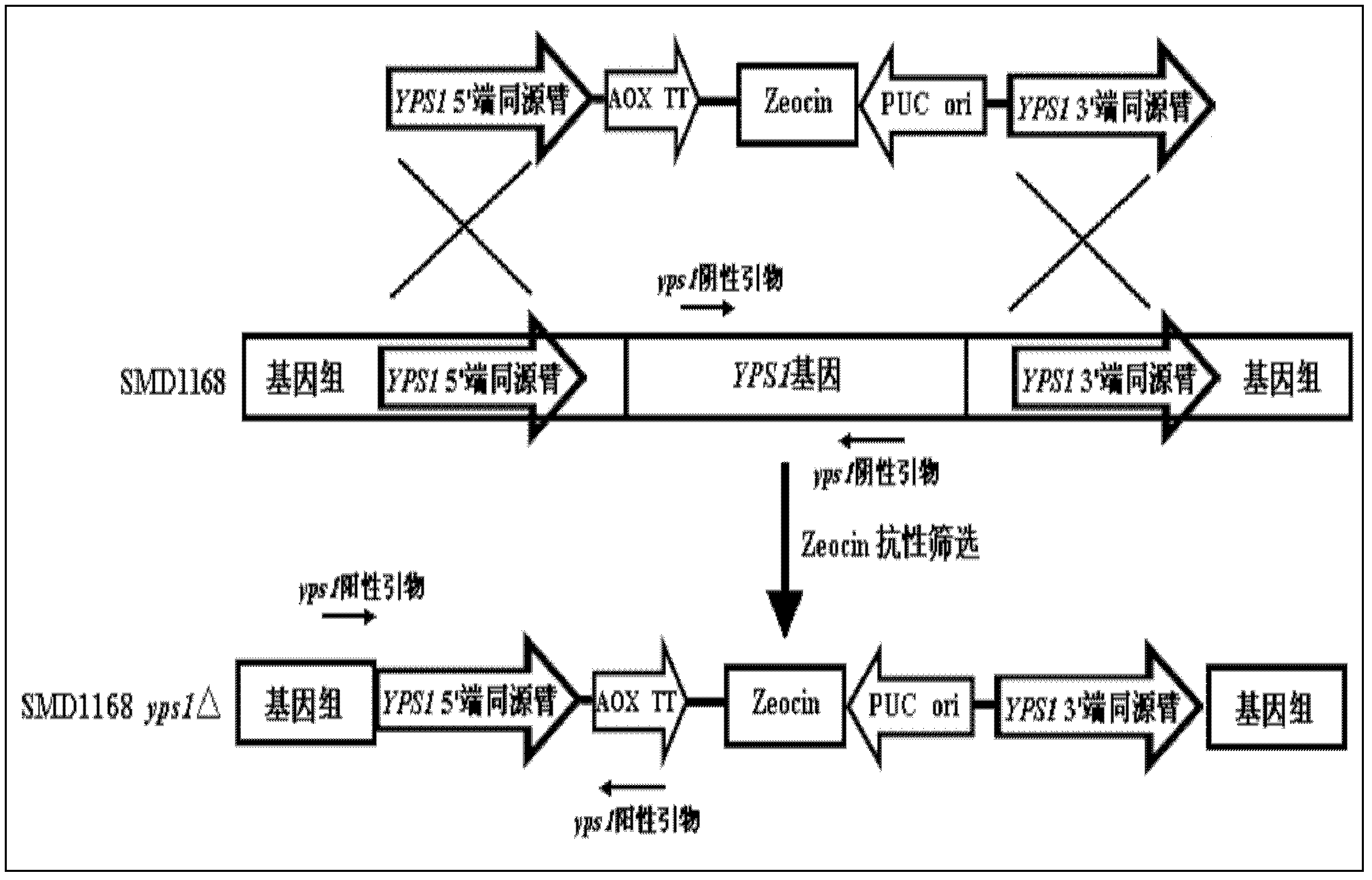
The invention provides a method for preparing human serum albumin-human parathyroid hormone. By means of transforming a yeast host SMD1168, yapson1 coded sequences on the SMD 1168 can be knocked out. On the basis of the SMD 1168, coded gene YPS1 of yapson1 is further knocked out, namely double knockouts of a proteinase A and yapsin1 are achieved, and the transformed host is named as SMD1168yps1 delta. By the aid of the transformed host, a recombination expression of fusion proteins of human serum albumin-human parathyroid hormone (1-34) is optimized, so that the degradation of interest proteins in the expressing process can be reduced, and downstream mass production costs and difficulties of separation and purification are lowered. According to the method, the optimized the human serum albumin-human parathyroid hormone (1-34) fusion proteins can be applied to prepare parathyroid hormone (PTH) (1-34) medicines which are used for treating osteoporosis.
Owner:ZHEJIANG UNIV
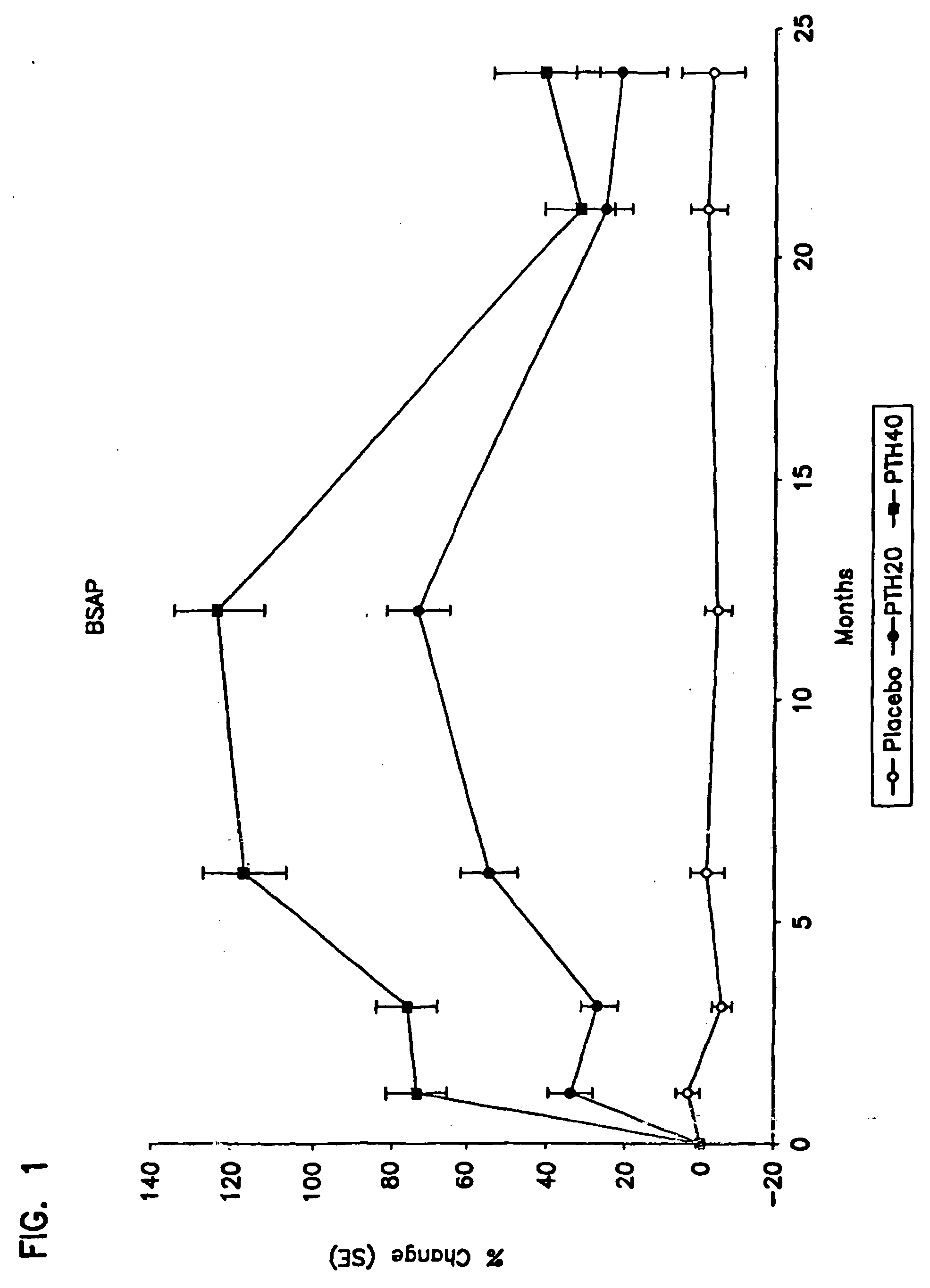
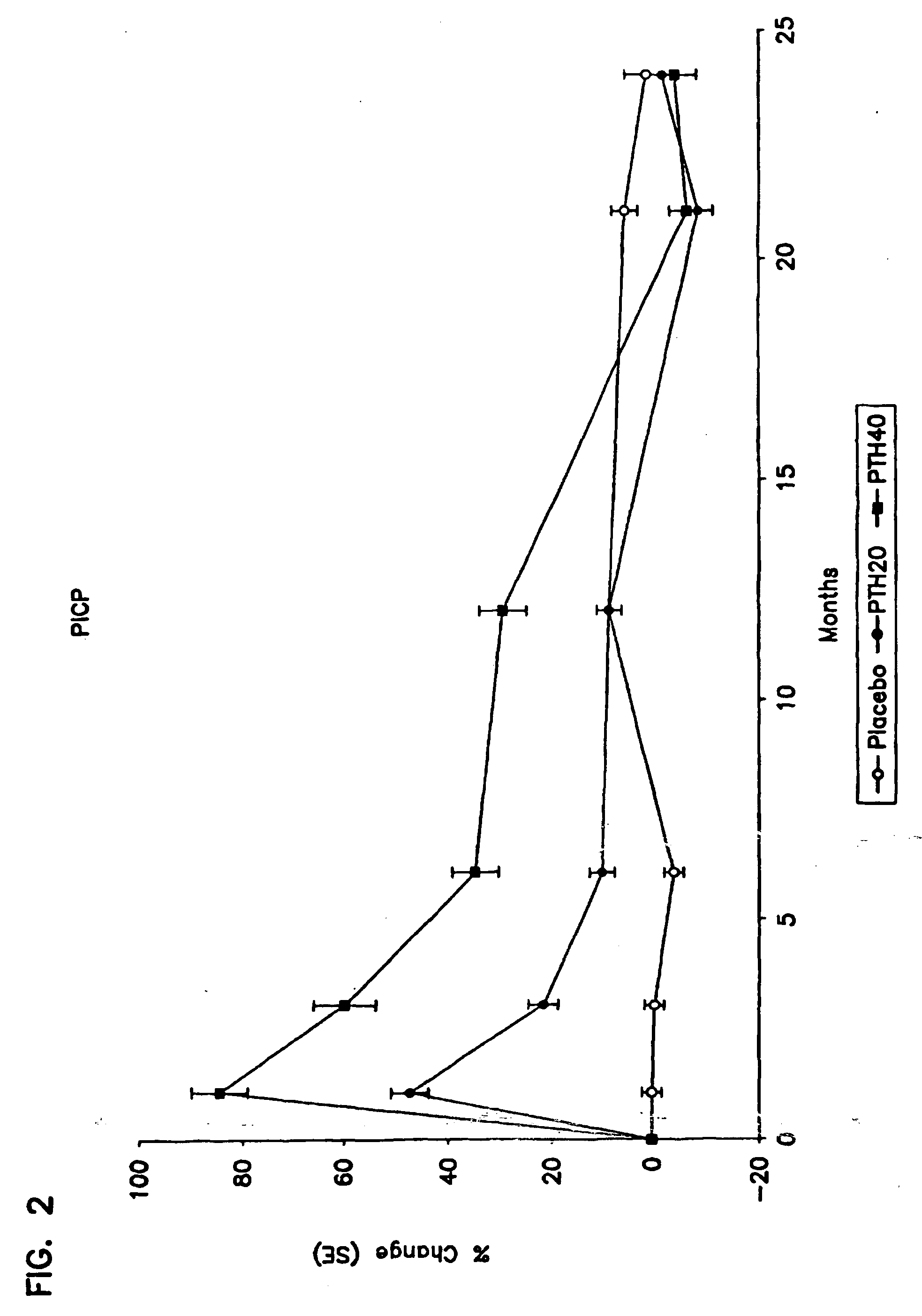
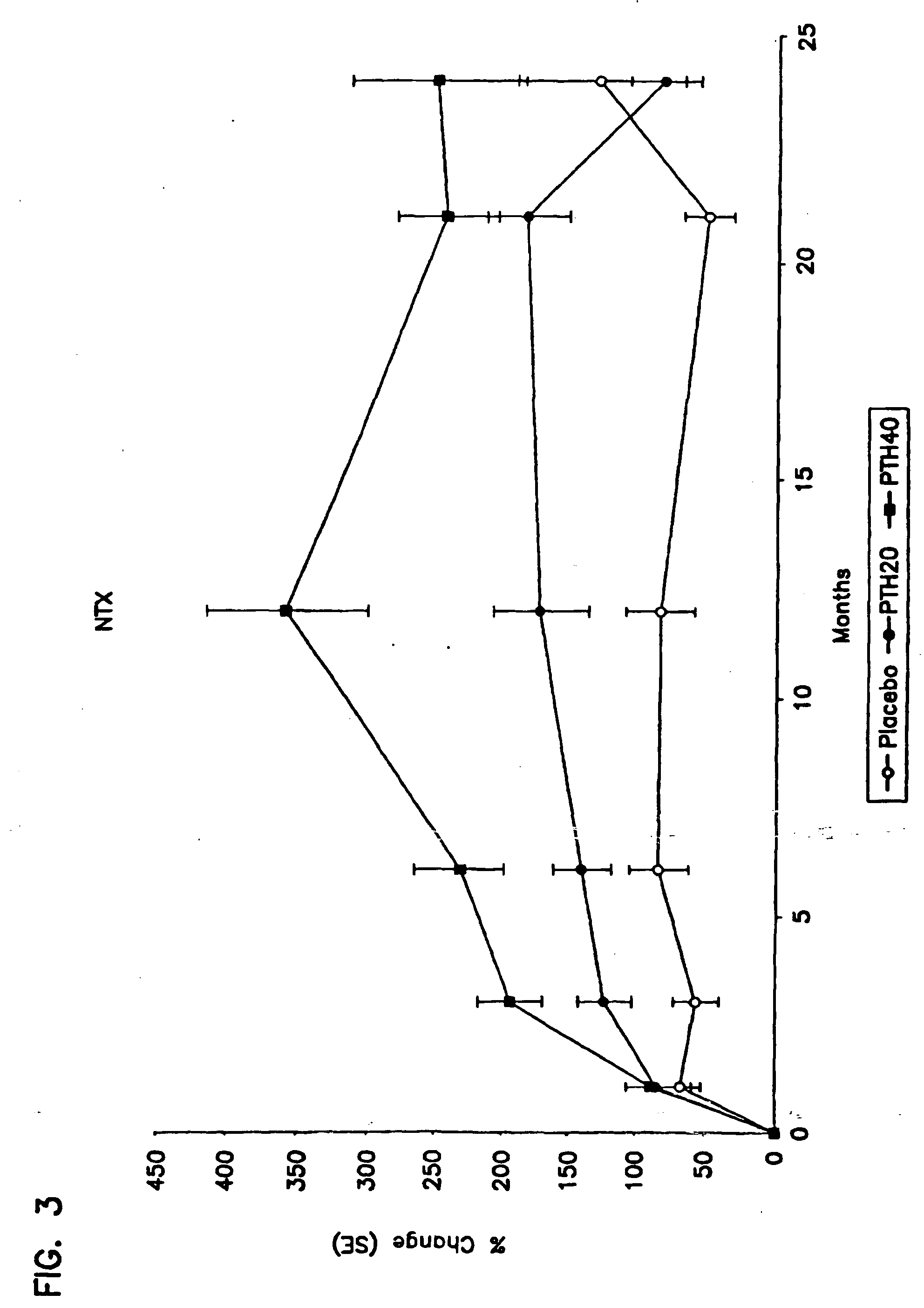
Method for monitoring treatment with a parathyroid hormone
InactiveUS20050255537A1Increase stiffnessImprove toughnessOrganic active ingredientsBiocideProcollagen iOsteoblast
The present invention relates to a method for monitoring effects of administration of a parathyroid hormone by determining levels of one or more markers of an activity of this hormone. Suitable markers of bone formation include one or more enzymes indicative of osteoblastic processes of bone formation, preferably bone specific alkaline phosphatase, and / or one or more products of collagen biosynthesis, preferably a procollagen I C-terminal propetide. Suitable markers of bone resorption and turnover include one or more products of collagen degradation, preferably an N-terminal telopeptide (NTX). In addition, methods for concurrently reducing the risk of both vertebral and non-vertebral bone fracture in a male human subject at risk of or having ossteoporosis are also disclosed, involving administration of human parathyroid hormone (amino acid sequence 1-34) without concurrent administration of an antiseropositive agent other than vitamin D or calcium.
Owner:HOCK JANET MARY +1
Method for preparing human parathyroid hormone 1-34
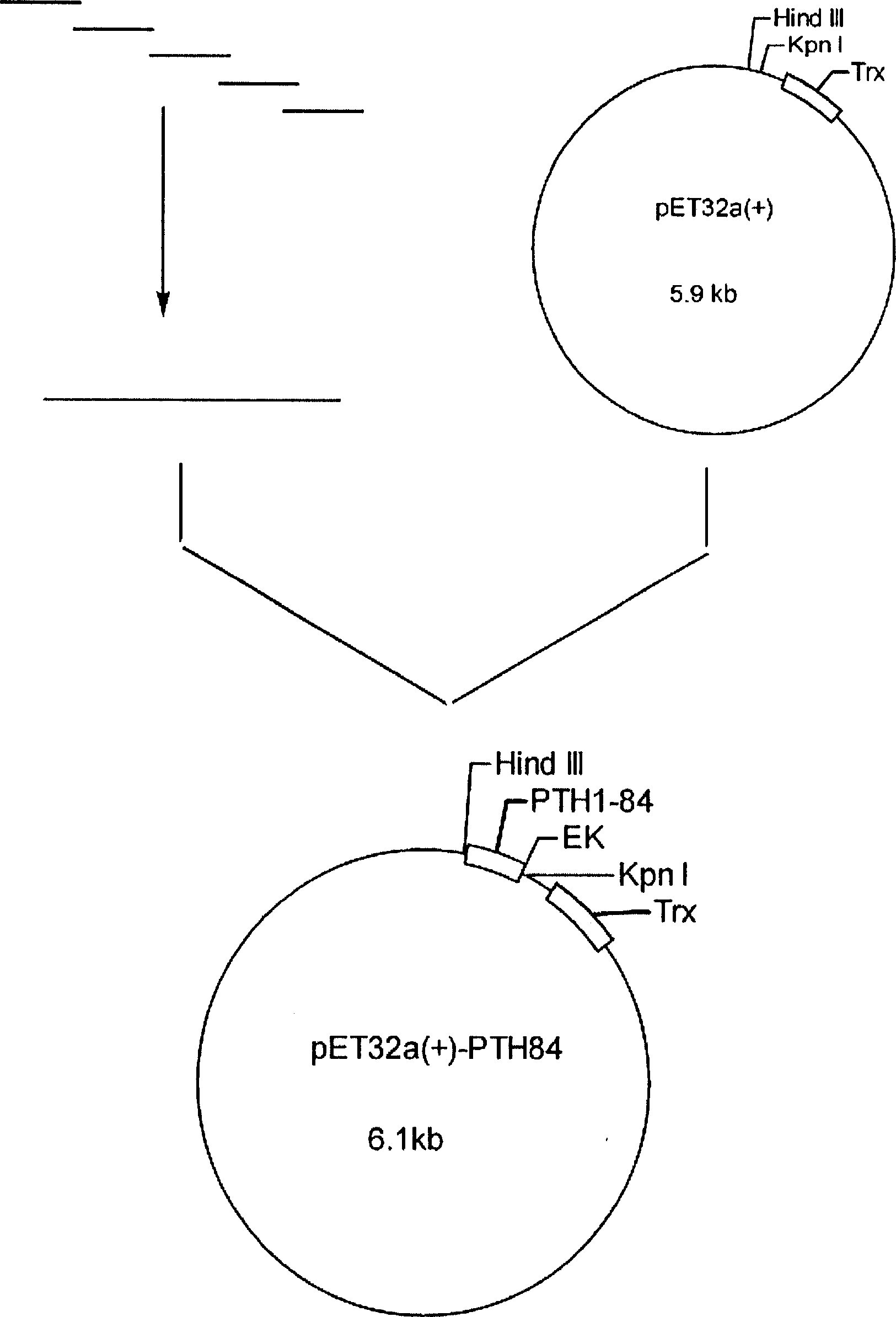
InactiveCN102304518APromotes high-yield extracellular accumulationAvoid attackPeptide preparation methodsParathyroid hormonesProtein targetThioredoxin
The invention relates to the technical field of biomedical engineering, and relates to a method for preparing human parathyroid hormone (1-34) through fermenting and purifying human parathyroid hormone 1-34 recombinant engineering bacteria constructed by a gene engineering method. The method provided by the invention comprises the following steps: fusing and expressing the genes for coding human parathyroid hormone and a thioredoxin label, and further constructing and finishing genetic engineering bacteria capable of highly expressing human parathyroid hormone; adding IPTG (isopropyl beta D thiogalactopy ranoside) and triton X-100 in the fermentation process by adopting a chemosmosis fermentation technology so as to improve the release of proteins extracellularly in the fermentation process, which is good for the continuous synthesis of proteins, and avoiding the attack of endoproteinase, thus protein high-yield extracellular accumulation is promoted; and simultaneously carrying out heat treatment on a fermentation liquid when the fermentation reaches the final point aiming at the heat stability of thioredoxin fusion, thus initially purified target proteins are directly recycled and obtained from a fermentation liquor supernatant after centrifugation.
Owner:中国科学院上海生命科学研究院湖州工业生物技术中心
Method for preparing parathormone 1-34
This invention provides a method for preparing recombinant human parathyroid hormone 1-34 (rhPTH (1-34)). The method comprised: (1) expressing with an expression vector capable of expressing a fusion protein with an amino acid sequence (from N-terminal to C-terminal) containing thioredoxin, (His) 6, enterokinase recognition site and parathyroid hormone 1-34 peptide; (2) purifying the fusion protein by nickel ion complexation affinity chromatography; (3) cutting the purified fusion protein with enterokinase to release the rhPTH (1-34) from the fusion protein. By this method, rhPTH (1-34) can be purified rapidly, simply and efficiently, and the recovery yield is largely increased.
Owner:DONGGUAN TAILI BIOTECH
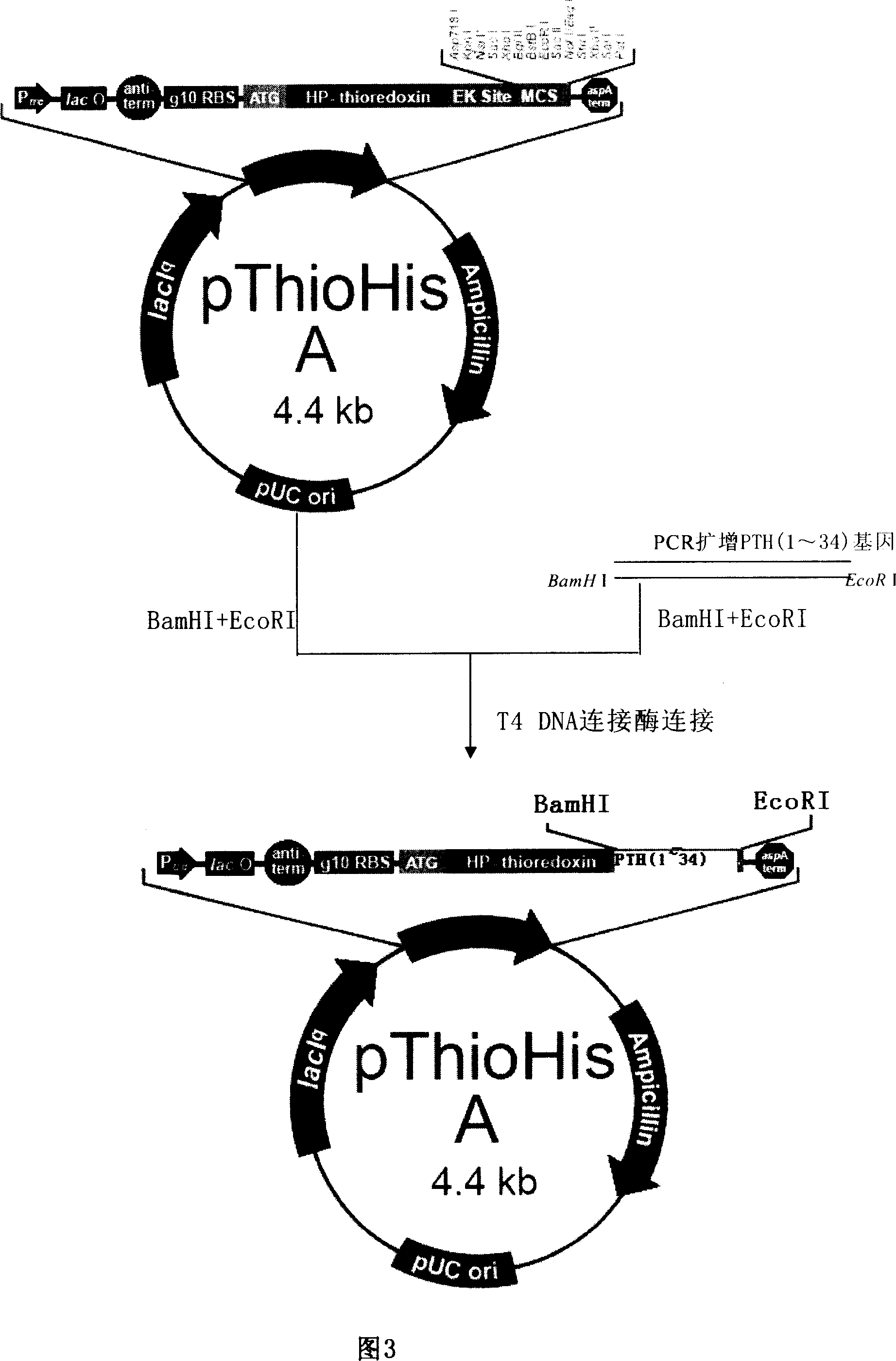
Fusion protein containing haman parathyroxin 1-34 and its expression vector
ActiveCN1900120AAntibody mimetics/scaffoldsFusions for enhanced expression stability/foldingThioredoxinProteolytic enzymes
The present invention provides a kind of fusion protein, which possesses thioredoxin sequence and parathormone peptide 1-34 located in the downstream of the thioredoxin. Preferably, the fusion protein possesses also connecting peptide containing proteolytic enzyme recognizing site and located between the thioredoxin and the parathormone peptide 1-34. From the fusion protein, human parathormone peptide 1-34 may be prepared.
Owner:BEIJING GENETECH PHARML
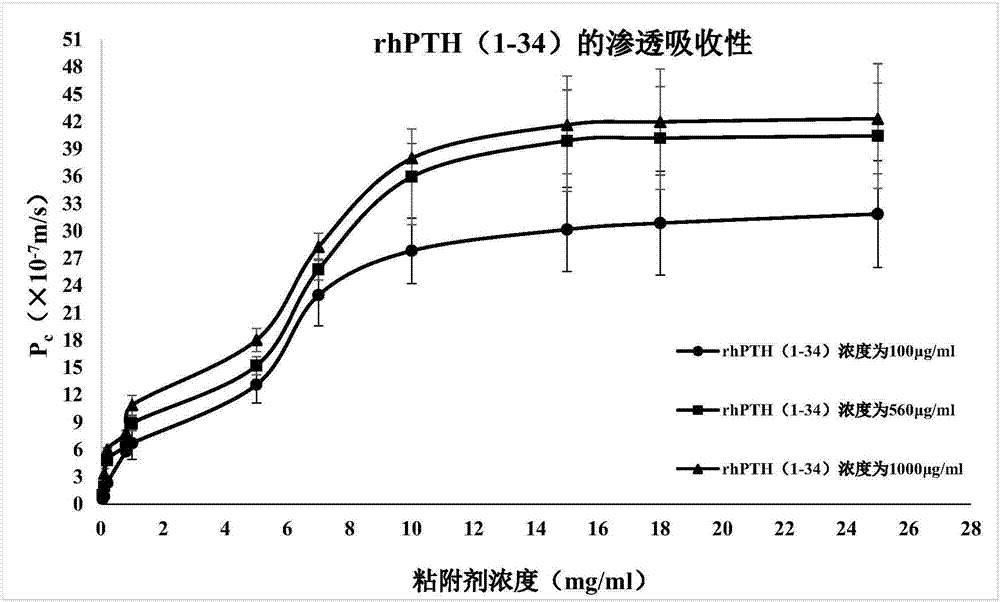
Human parathyroid hormone containing medicine composition delivered via oral mucosa
ActiveCN106924721AGuaranteed stabilityImprove compliancePeptide/protein ingredientsPharmaceutical delivery mechanismHigh concentrationAntioxidant
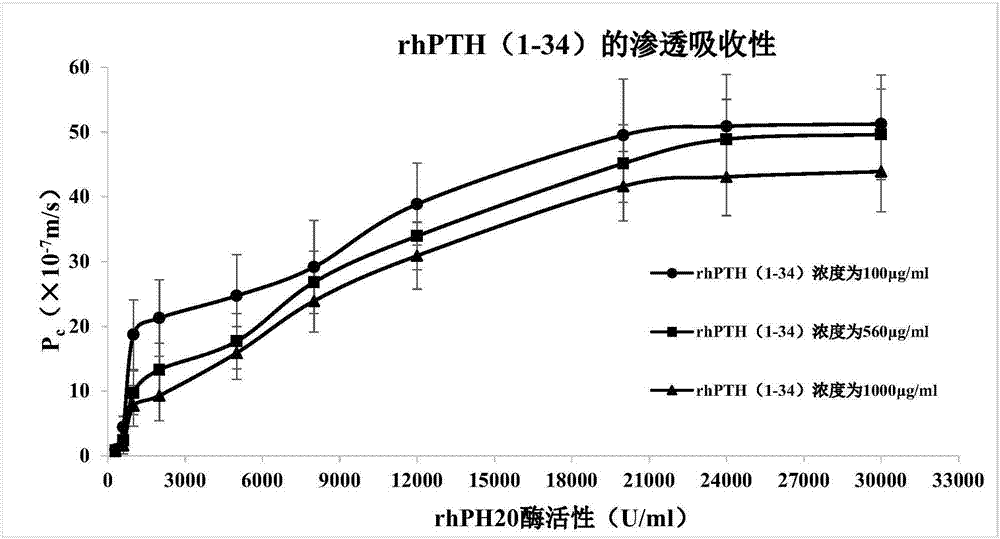
The invention relates to a human parathyroid hormone containing medicine composition delivered via an oral mucosa. The medicine composition comprises high-concentration human parathyroid hormone, an absorption penetrating promoter, a mucosa absorbent, a thickening agent, a buffer solution, an antioxidant and a stabilizing agent. The medicine composition has excellent stability, permeation absorbency and biological activity and is high in bioavailability and safe and nontoxic in formula.The invention further provides a preparation method of the medicine composition, and application of the medicine composition in preparing a medicine for treating and / or preventing osteoporosis. The medicine composition is convenient to use, has no toxicity or stimulation in a using process, takes effect quickly, and can significantly improve clinical medication compliance of a patient.
Owner:CHENGDU JINKAI BIOTECH CO LTD
Antibodies against human parathyroid hormone related protein
InactiveUS20050136057A1High activityEasy to useCosmetic preparationsPeptide/protein ingredientsThyroid hormonesDna encoding
Owner:CHUGAI PHARMA CO LTD
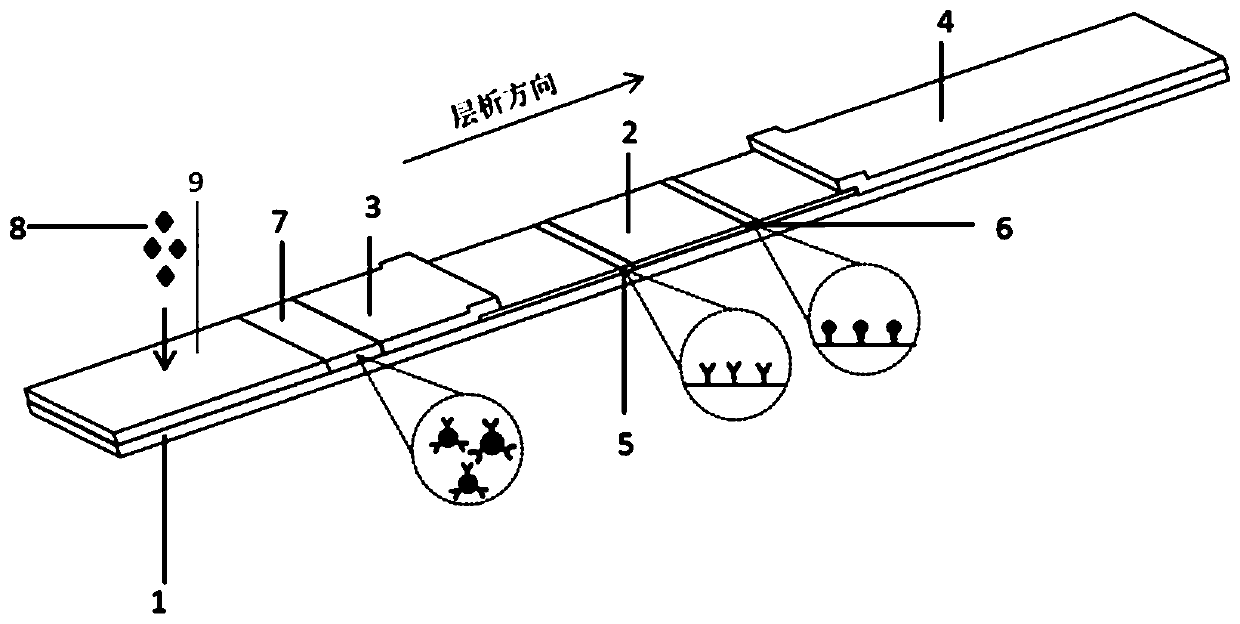
Parathyroid hormone kit for detecting peripheral blood, and preparation method and application thereof
InactiveCN110488003ASolve the rare problem of blood collectionSmall amount of sampleMaterial analysisMonoclonal antibodyPolyclonal antibodies
The invention relates to a parathyroid hormone kit for detecting peripheral blood, and a preparation method and an application thereof. The parathyroid hormone kit comprises a parathyroid hormone immunochromatographic detection card and a release agent, wherein the parathyroid hormone immunochromatographic detection card comprises a test strip, the test strip comprises a detection line and a quality control line, the detection line is coated with a mouse-anti-human parathyroid hormone monoclonal antibody, and the quality control line is coated with a goat-anti-rabbit polyclonal antibody. The parathyroid hormone kit provided by the invention is high in sensitivity, high in stability and wide in linear range, and has excellent accuracy and precision.
Owner:BEIJING ELCOTEQ BIO TECH
Prepn. of recombinant human parathyroid hormone PTH(1-34)
The present invention relates to a preparation process of recombinant human parathyroid hormone PTH(1-34). The process constitutes a new engineering strain to result in high expression amount, simple purifying course and low cost.The present invention adopts colibacillus degenerate codon modifying sequence corresponding cDNA, the basic sequence is L-V-P-R-PTH(1-34), where L-V-P-R is the enzyme incision site of thrombin. To the end of the nucleotides sequence, colibacillus terminator codon TAA is added; and in order to clone easily to the 5'-terminal enzyme incision site of EcoR I is added, and to the 3'-terminal, enzyme incision site of Sal I is added. High-purity recombinant huamn parathyroid hormone PTH(1-34) is obtained through fermentation of the engineering bacteria, occlusion body extraction, diluting renaturation, thrombase incision, anionic exchange chromatography and other steps.
Owner:西南生物工程产业化中试基地有限公司
Method for rapidly identifying human parathyroid glands
The invention discloses a method for rapidly identifying human parathyroid glands. The method is realized by using parathyroid hormone (PTH) fluorescence immunochromatography test paper to determine the content of PTH in liquid to be detected; the test paper is used for detecting human PTH by using a double-antibody sandwich method and a fluorescence immunochromatography; the antibodies are prepared by using specific antigenic epitope peptide. The method can be used for accurately detecting the human PTH in a matter to be detected, and is simple and convenient to operate, wide in detection range, high in specificity and good in sensitivity.
Owner:无锡市江原实业技贸有限公司
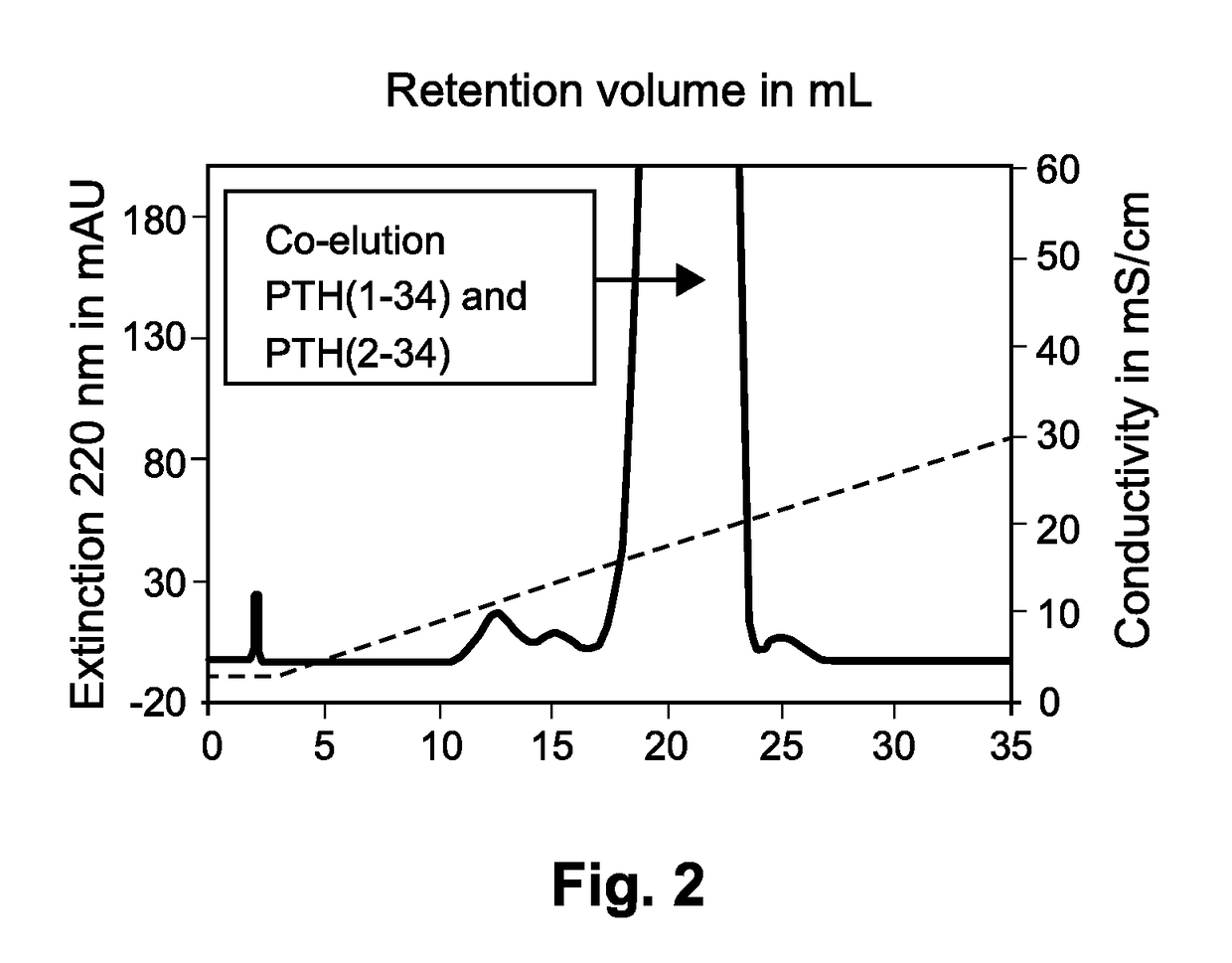
Method for purifying teriparatide
The present invention relates to a novel method for purifying teriparatide, a therapeutically active polypeptide fragment of full-length human parathyroid hormone. The method is based on ion exchange chromatography and is effective in separating the therapeutically active polypeptide from undesirable variants, such as truncated polypeptides. The method of the invention can be used at a preparative scale which allows it to be implemented into the production process for teriparatide. Accordingly, the invention also provides a method for the production of teriparatide which includes a step in which teriparatide is purified by the novel ion exchange chromatography method of the invention.
Owner:RICHTER HELM BIO TEC
Fusion peptide of human parathyroid hormone derived peptide and tat peptide, preparation thereof, and skin slimming cosmetic composition comprising the same
InactiveUS7060673B2EasilySafely penetrates into the integument and endotheliumCosmetic preparationsPeptide/protein ingredientsTat peptideIncreased Lipolysis
The present invention relates to a fusion peptide wherein a self cell-penetrating Tat peptide having a self penetrating signal is bound to a human parathyroid hormone-derived peptide, a preparation thereof, and a skin slimming cosmetic composition comprising the same. Since the fusion peptide wherein the Tat peptide is bound to the human parathyroid hormone-derived peptide has high stability and superior skin absorption, the present invention provides a skin slimming agent having superior lipolysis effects and improved durability of the effects.
Owner:LG HOUSEHOLD & HEALTH CARE LTD
Cascade expression method of recombinant human glandulae parathyroideae (1 to 34 peptide)
InactiveCN101134963AEfficient and/or easy productionBacteriaParathyroid hormonesHormone productNucleotide
The present invention provides one nucleotide sequence of human parathyroid hormone, the serial expression process for producing recombinant human parathyroid hormone in high efficiency, and relevant engineering cell constituting, expressing and purifying process. The purified recombinant human parathyroid hormone protein gene is especially suitable for serial expression in prokaryotic cell, and has the advantages of high expression quantity and high stability after being optimized through a fermenting and purifying process. The present invention can obtain pure recombinant human parathyroid hormone product simply in high efficiency and low cost.
Owner:SHANGHAI NEWSUMMIT BIOPHARMA +1
Method for purifying human parathyroid hormone (1 to 34)
InactiveCN101781367AEasy to operateHigh purityPeptide preparation methodsLeech-based protease inhibitorsPurification methodsPhosphate
The invention relates to a method for purifying human parathyroid hormone (1 to 34). The invention provides a method suitable to industrially purify lepirudin, which comprises the following steps: (1) using octadecylsilane chemically bonded silica as a solid phase, using a phosphate buffered solution the pH value of which is between 2.5 and 3.5 as an A phase, using acetonitrile as a B phase and carrying out gradient elution on crude peptide solution, wherein the gradient B percent is between 17 and 32 percent; (2) and adopting the reversed phase high performance liquid chromatography to convert lepirudin into acetate. The method for purifying lepirudin, which is provided by the invention, has simple operation, high product purity and good yield and reaches the industrialization requirements.
Owner:HYBIO PHARMA
Novel orthogonal process for purification of recombinant human parathyroid hormone (RHPTH) (1-34)
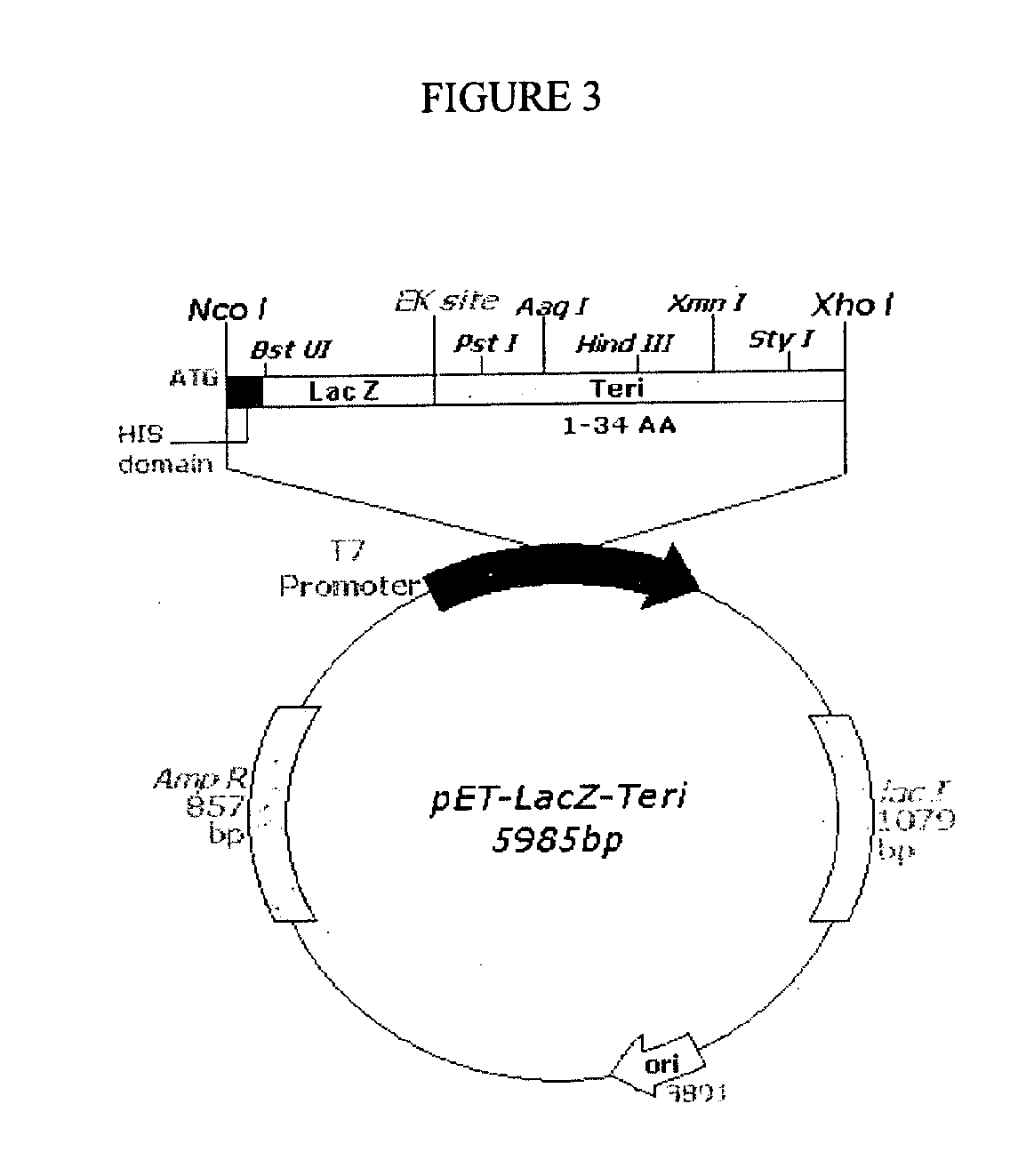
The present invention discloses a process for the preparation of rhPTH (1-34) also known as teriparatide by con-struction of a novel nucleotide, as an NcoI.IXhoI fragemt as set forth in SEQ. ID. No.:1 encoding a chimeric fusion protein as set forth in SEQ.ID. No.:2 comprising of a fusion partner consisting of 41 amino acids belonging to Escherichia coli β-galactosidase (LacZ) gene, an endopeptidase cleavage site, rhPTH (1-34) gene fragment, cloning the said nucleotide in an expression vector under the control of T7 promoter, transforming Escherichia coli with the said vector and expressing the chimeric fusion protein in fed batch fermentation. The present invention further discloses a low feed rate lactose induction for optimized expression of rhPTH (1-34) in Escherichia coli. The present invention also discloses an unique, novel two step orthogonal purification process for rhPTH (1-34) comprising of cation exchange chromatography optionally followed by preparative chromatography selected from HIC or RP-HPLC to yield a target protein of ≧99% purity. The present invention discloses a simple, cost-effective, environmentally benign method of producing high purity rhPTH (1-34).
Owner:USV LTD
Methods of screening for apoptosis-controlling agents for bone anabolic therapies and uses thereof
InactiveUS20050070473A9Extend your lifeQuality improvementCompounds screening/testingCompound screeningAnabolic EffectPhysiology
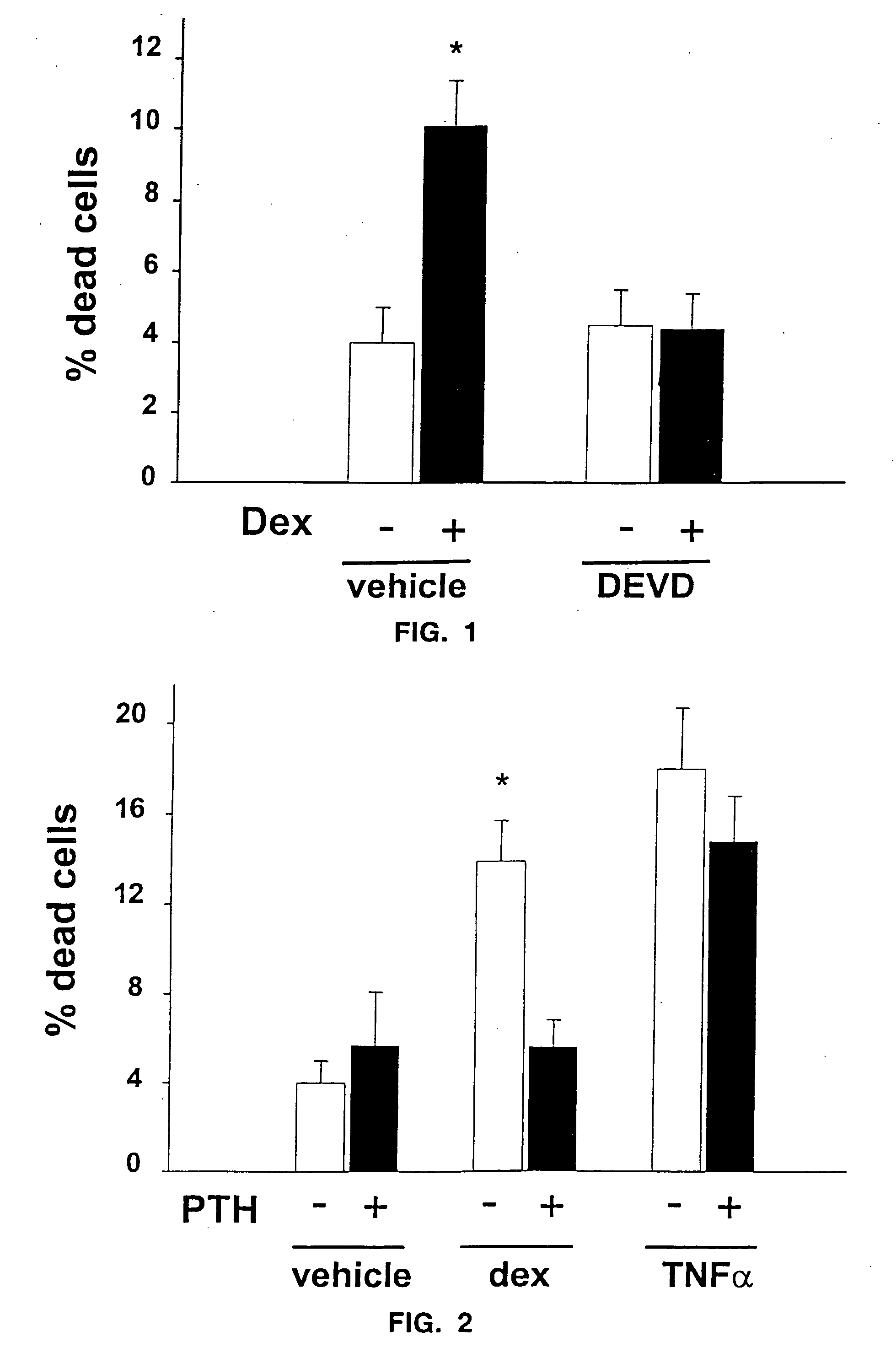
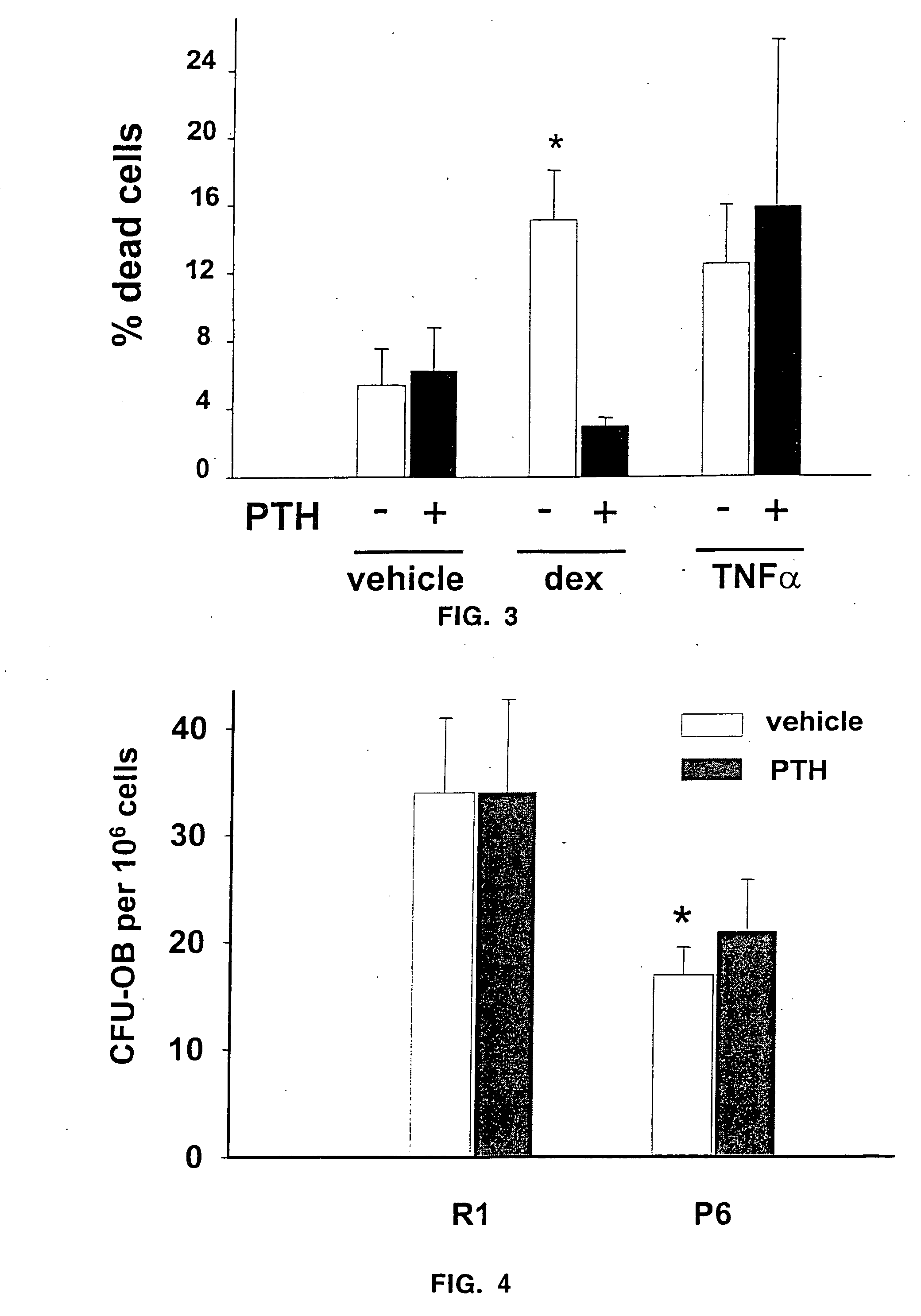
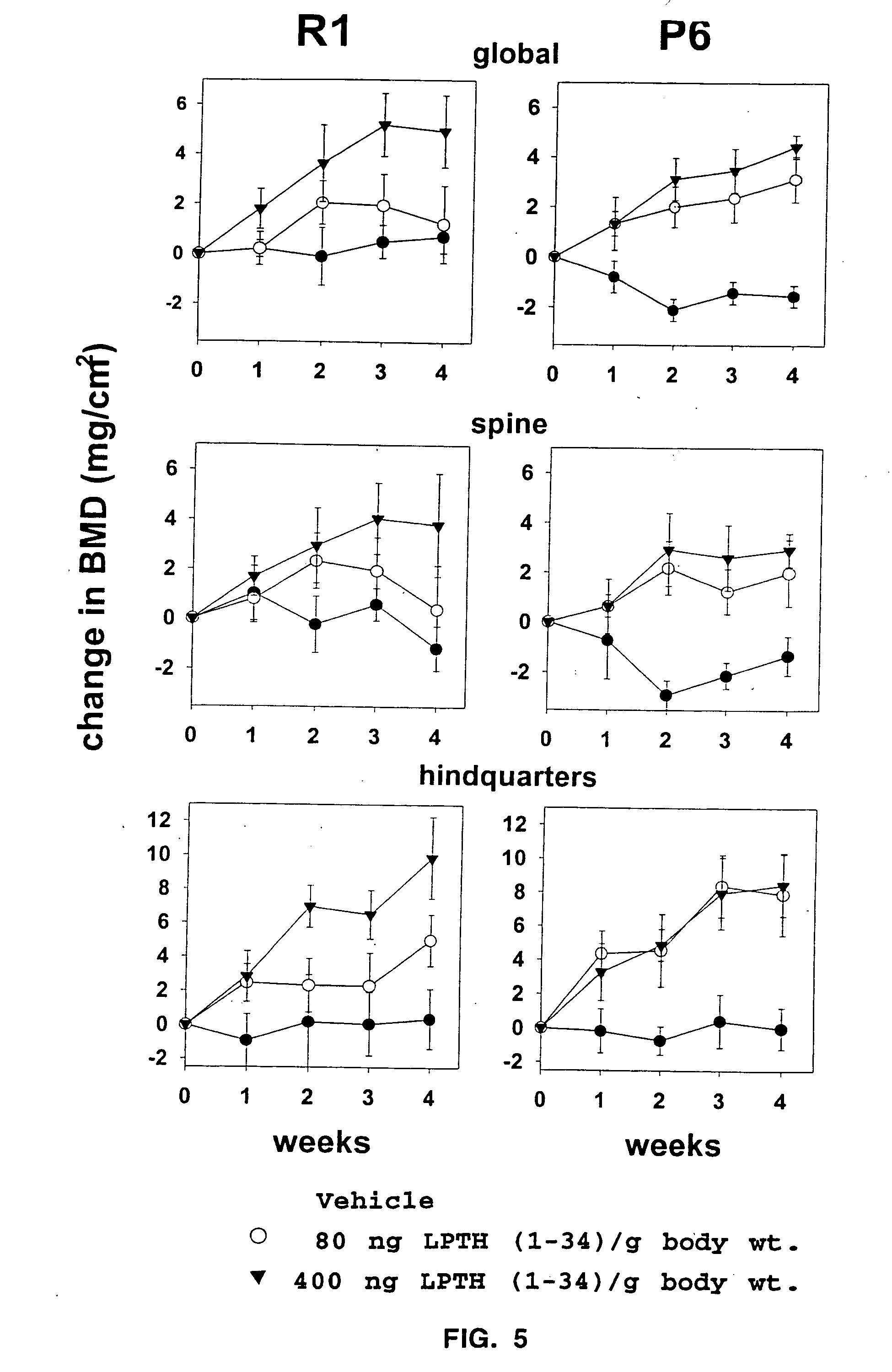
The present invention demonstrates that human parathyroid hormone 1-34 [hPTH(1-34)] exerts anti-apoptotic effects on osteoblasts when administered in an intermittent fashion to mice in vivo. The present invention further demonstrates that bovine PTH(1-34) [bPTH(1-34)] prevents glucocorticoid-induced apoptosis of osteoblastic and osteocytic cells in vitro. Therefore, the present invention demonstrates that the previously established anabolic effects of PTH on the skeleton are mediated by its ability to postpone osteoblast apoptosis, as opposed to a stimulatory effect on osteoblastogenesis. The present invention provides methods of screening agents for anti-apoptotic effects on osteoblasts, wherein such agents stimulate and / or restore bone in osteopenic individuals, or prevent bone loss caused by agents such as glucocorticoids.
Owner:MANOLAGAS STAVROS C +3
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com