Cascade expression method of recombinant human glandulae parathyroideae (1 to 34 peptide)
A parathyroid hormone and expression vector technology, applied in the field of genetic engineering, can solve the problems of low PTH expression, unstable mRNA, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Example 1 Construction of expression plasmids and acquisition of high-expression engineering strains
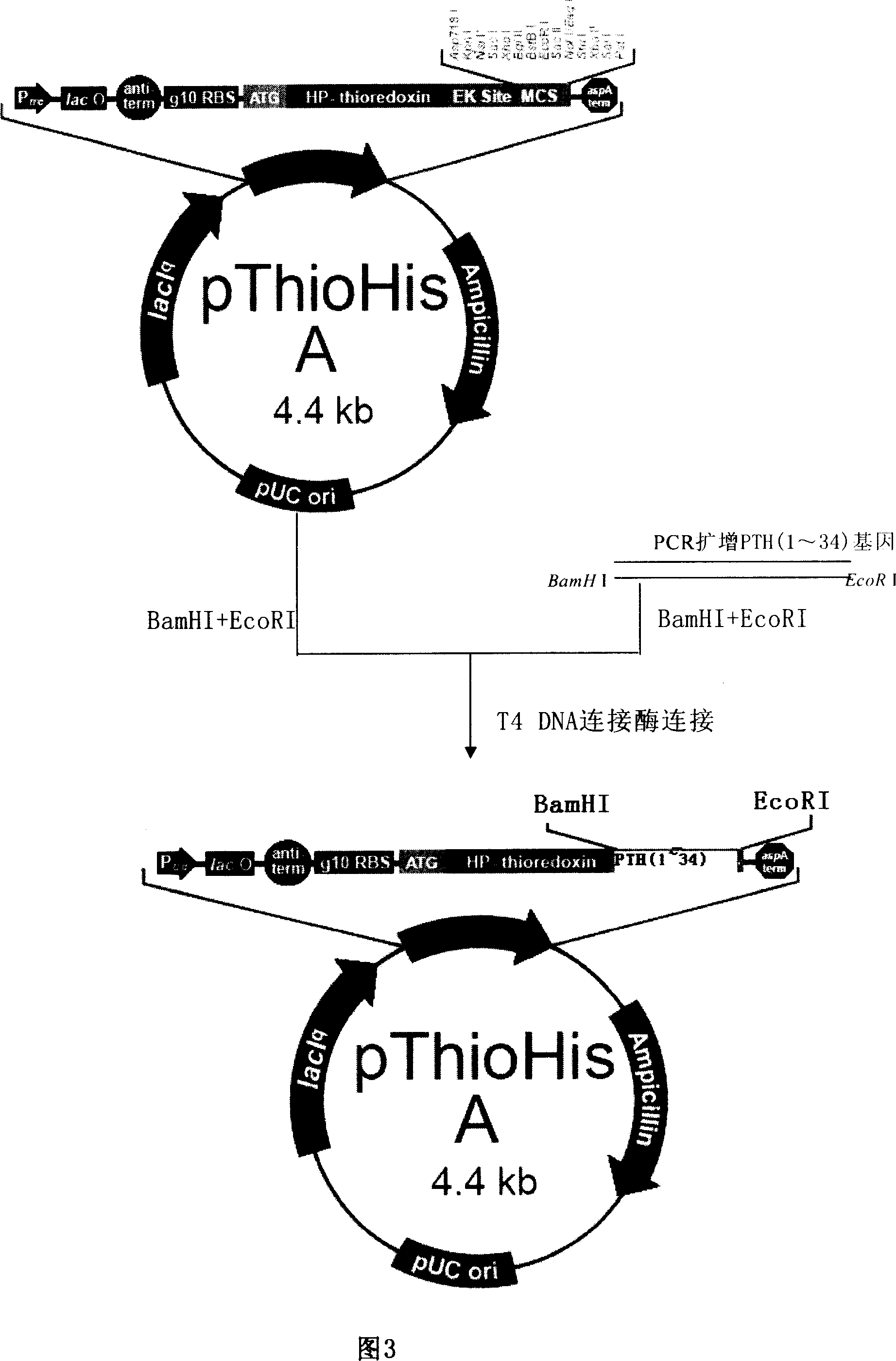
[0062] According to the natural amino acid sequence of human parathyroid hormone (1-34 peptide), according to codon preference, under the condition of not changing the amino acid sequence, the target gene of recombinant human parathyroid hormone (1-34 peptide) protein is synthesized from the whole gene Sequence (SEQ ID NO: 1), the homology between the optimized PTH gene sequence and the natural PTH gene sequence is 85% (see Figure 1). The gene was cloned into pThiHisA and verified by sequencing.
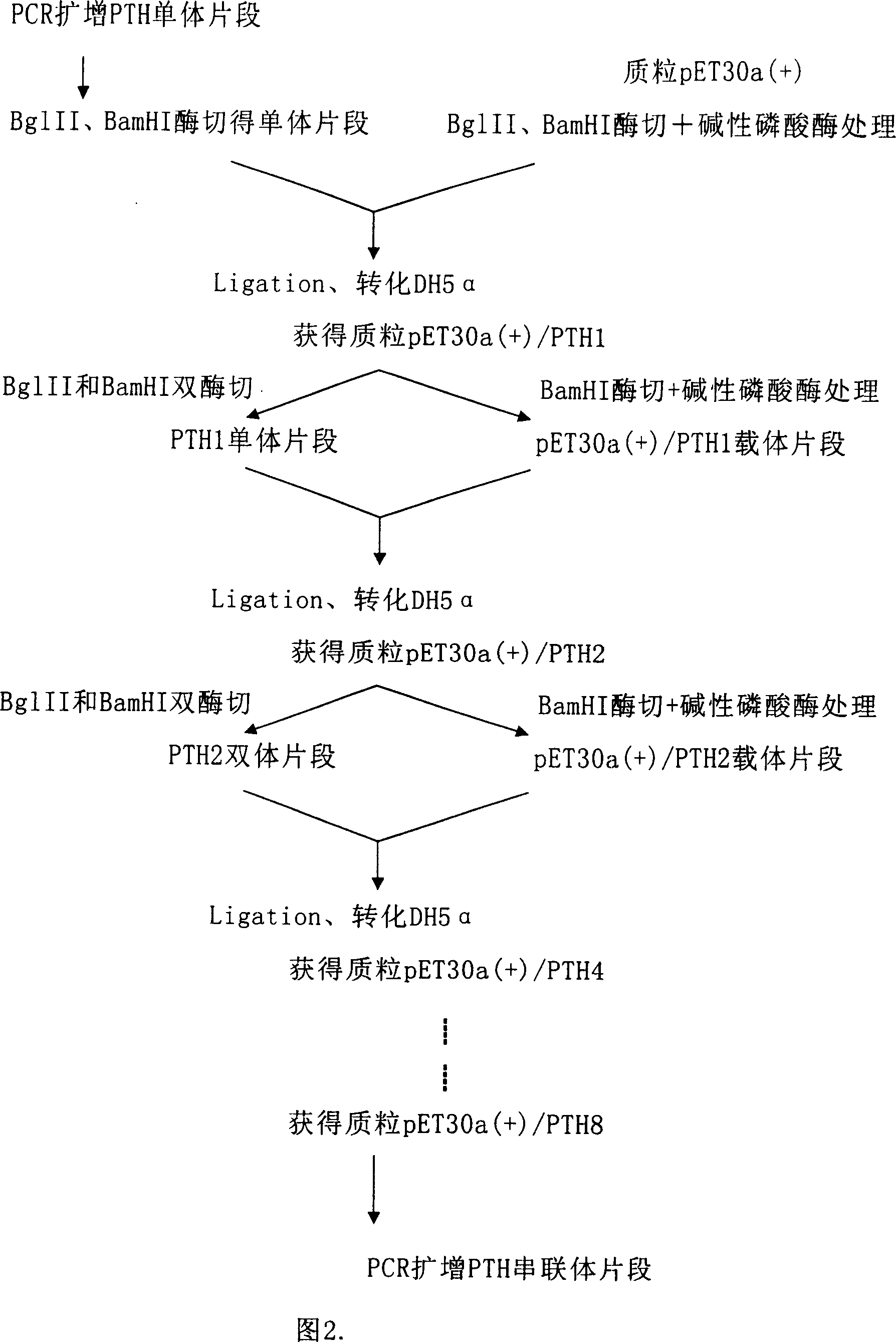
[0063] The expression vector construction method is shown in Figure 2. Introduce a Lys(K), Arg(R) and BglII restriction site at the front 5' end of each monomeric gene, and introduce a Lys(K), Arg(R) and BamHI restriction site at its 3' end . The target human parathyroid hormone (1-34 peptide) gene was amplified by PCR, and the PCR product was double digested with BglII an...
Embodiment 2
[0066]Select a well-confirmed expression engineering strain BL21(DE3) / pThiHisA / PTH-fusion, culture it with LB medium and induce expression with IPTG, detect whether there is any target band by SDS-PAGE electrophoresis, and analyze its expression level . Take a part of the fermented bacterial liquid and centrifuge, resuspend in pH 7.4, 20mM PB, + 2.5mM EDTA buffer, ultrasonically destroy the bacteria in an ice bath, collect the ultrasonic supernatant and ultrasonic precipitation by centrifugation, detect by SDS-PAGE electrophoresis, and determine its ways of expression. Analysis of the ultrasonic supernatant and ultrasonic precipitation showed that most of the target protein was in the ultrasonic supernatant, indicating that the target protein was expressed in a soluble form.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com