Production method of recombination human interleukin-4
An interleukin and cell technology, applied in the field of genetic engineering, can solve the problems of low expression, low yield of IL-4, and large loss of target protein
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] The construction of embodiment 1 expression plasmid and the acquisition of high expression engineering strain
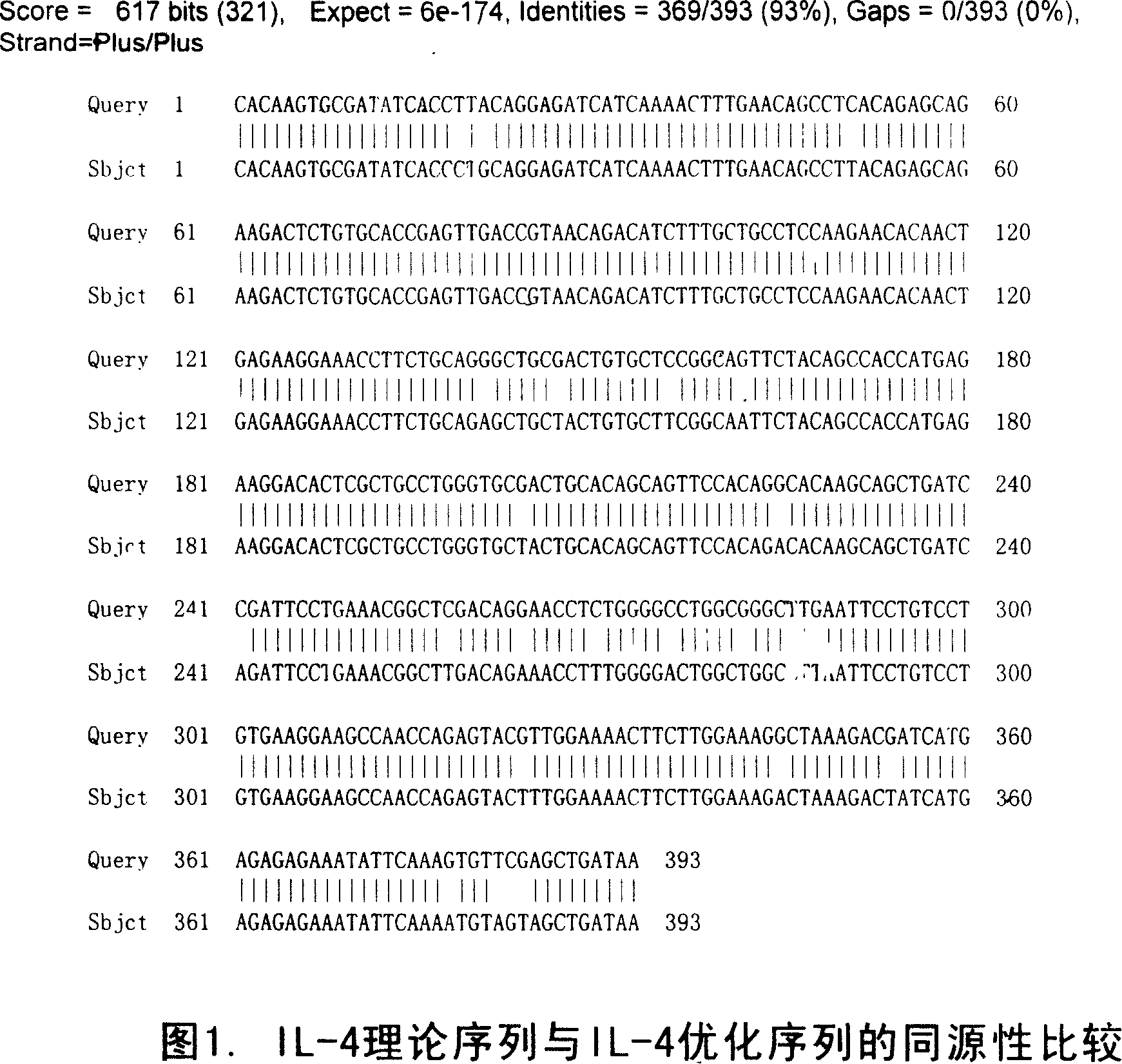
[0061] According to the natural amino acid sequence of human interleukin-4, according to the codon preference, under the condition of not changing the amino acid sequence, the target gene sequence (SEQ ID NO: 1) of the recombinant human interleukin-4 protein is synthesized completely. The homology between the optimized IL-4 gene sequence and the natural IL-4 gene sequence is 93% (see FIG. 1 ). The gene was cloned into pET-30a(+) and verified by sequencing.
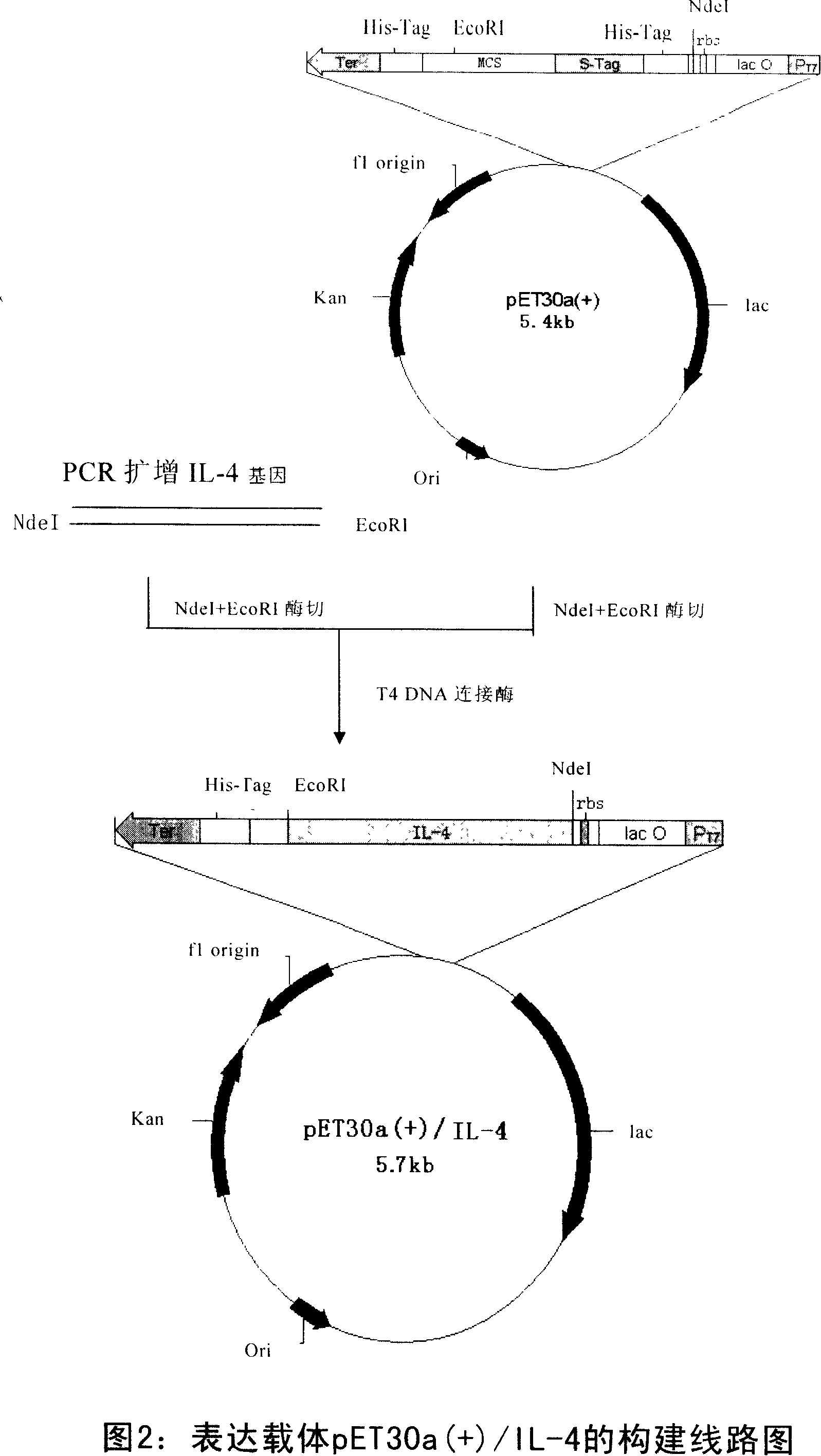
[0062] The expression vector construction method is shown in Figure 2. The target human interleukin-4 gene was amplified by PCR, and the PCR recovery product of the gene IL-4 and the plasmid pET30a(+) were respectively treated with Nde I and EcoRI double digestion, and the corresponding fragments were recovered after digestion and ligated with T4 DNA Enzymes were ligated overnight at 16°C. The ligation ...
Embodiment 2
[0064] Example 2 Expression of Recombinant Human Interleukin-4 and Determination of Expression Form
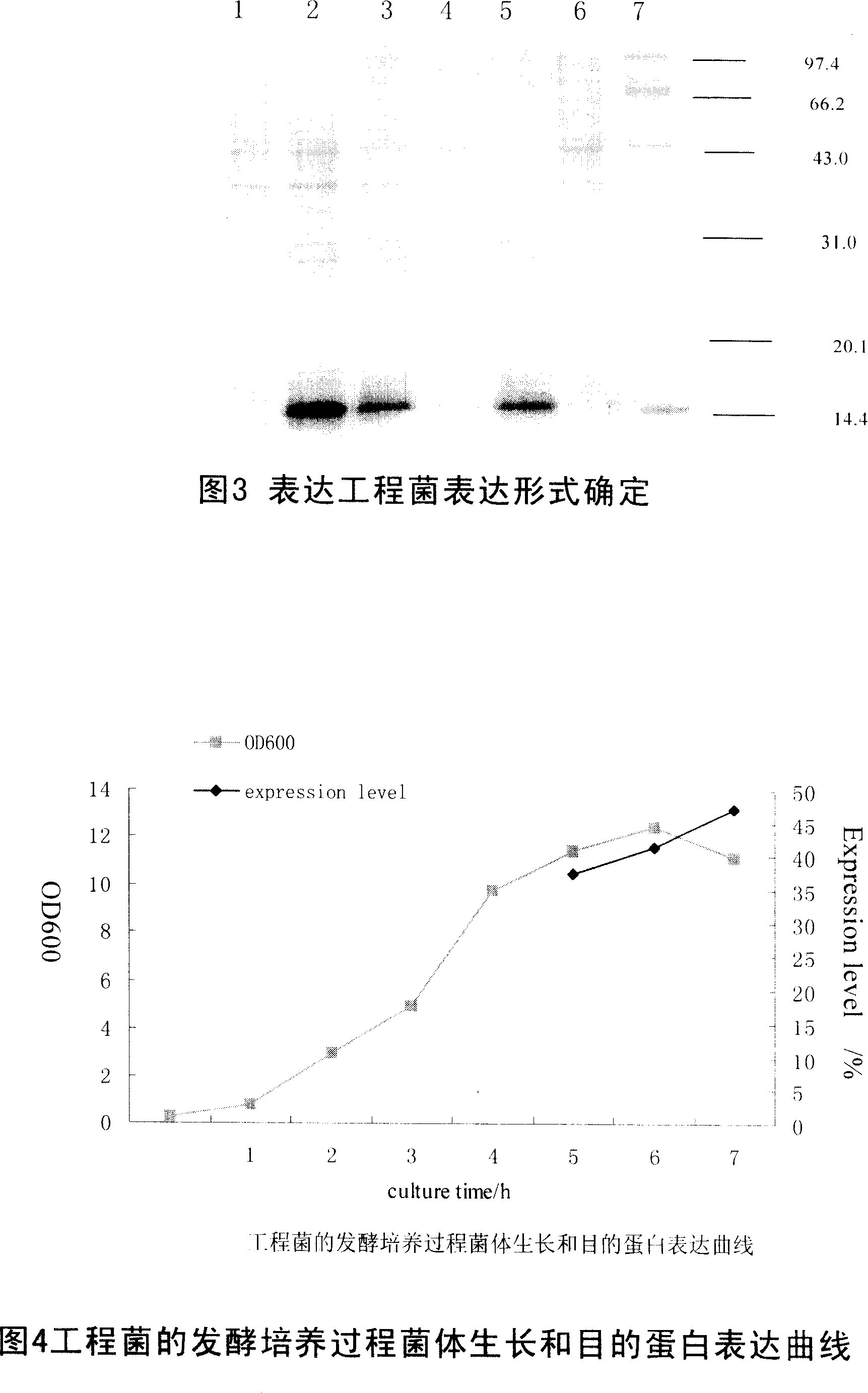
[0065] Select a well-confirmed expression engineering strain BL21(DE3) / pET30a(+) / IL-4, culture it with LB medium and induce expression with IPTG, detect whether there is any target band by SDS-PAGE electrophoresis, and analyze its expression. A part of the fermented bacterial liquid was centrifuged, resuspended in pH 8.0, 20 mM Tris buffer, ultrasonically disrupted in an ice bath, centrifuged to collect ultrasonic supernatant and ultrasonic precipitation, detected by SDS-PAGE electrophoresis, and determined its expression form. Analysis of the ultrasonic supernatant and ultrasonic precipitation revealed that the target protein was mostly in the precipitate, indicating that the target protein was expressed in the form of inclusion bodies (see Figure 3).
Embodiment 3
[0066] Example 3 Large-scale fermentation of recombinant human interleukin-4
[0067] Get the expression engineered bacteria BL21(DE3) / pET30a(+) / rhIL-4 identified as positive clones, culture it in LB containing 30ug / ml kanamycin for 14-16 hours; to OD 600 1 to 3, as the secondary seed solution. Prepare 2.96L of improved M9 fermentation medium (Glucose 1%, Yeast extract 0.5%, K 2 HPO 1 0.5%, KH 2 PO 4 0.35%, (NH 4 ) 2 HPO 4 0.35%, MgSO 4 0.025%, CaCl 2 0.1%), pour it into a cleaned fermenter, after autoclaving the tank, cool to 30-40°C, put the cultured secondary seed liquid into the fermenter at 1.5% inoculum, cultivate at 37°C, and Constantly adjust the ventilation flow rate and stirring speed according to the dissolved oxygen value, and control the dissolved oxygen above 40%. Sampling every hour to measure OD 600 value. Grow to OD 600 When it reaches about 5.0, the induction starts. Add IPTG to the final concentration of 1mM in the fermenter for induction....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com