Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
275 results about "Parathyroid gland" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Parathyroid glands are small endocrine glands in the neck of humans and other tetrapods. Humans usually have four parathyroid glands, located on the back of the thyroid gland in variable locations. The parathyroid gland produces and secretes parathyroid hormone in response to a low blood calcium, which plays a key role in regulating the amount of calcium in the blood and within the bones.
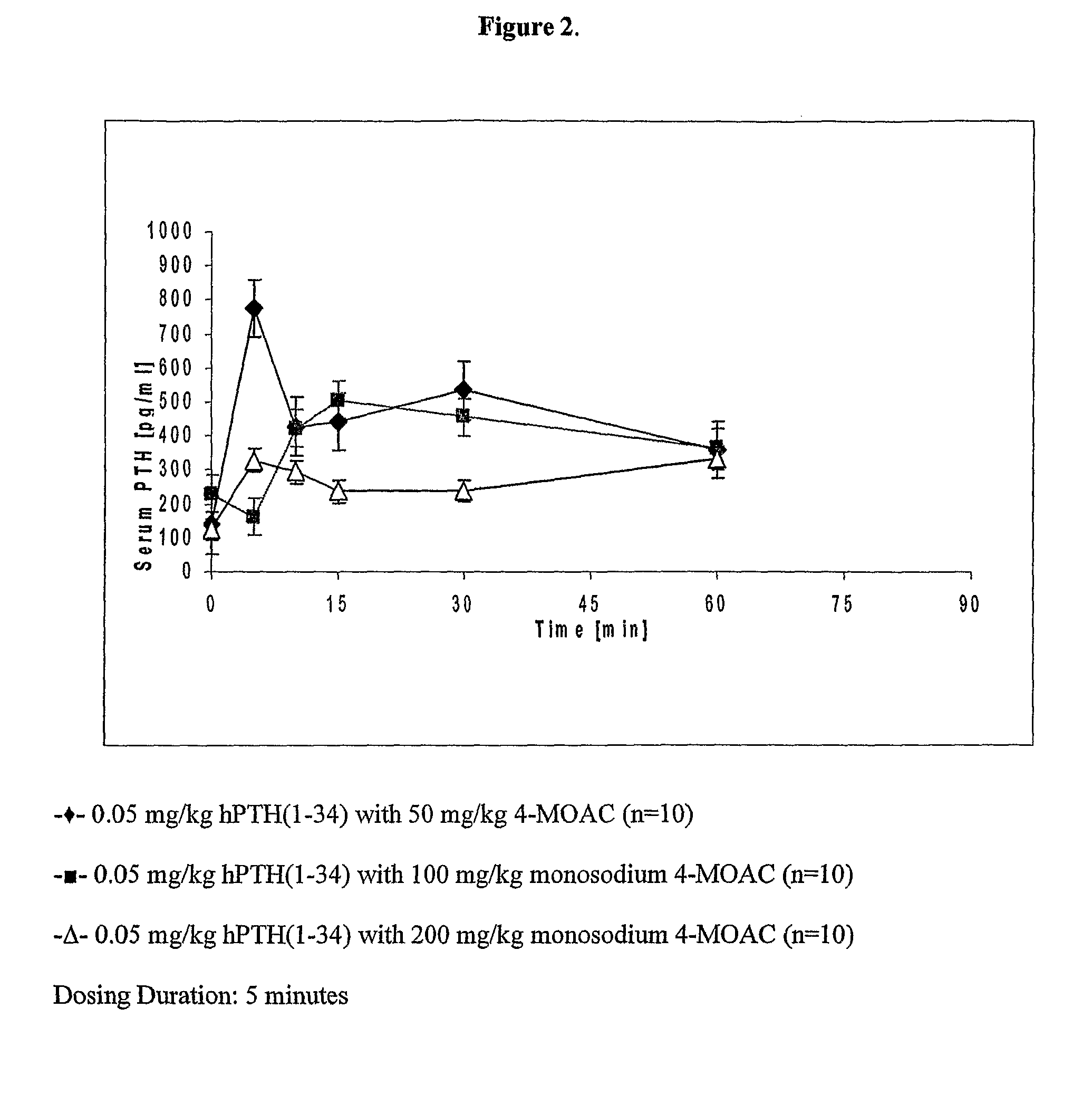
Method and device for the controlled delivery of parathyroid hormone
InactiveUS7497855B2Efficiently providePeptide/protein ingredientsMicromachined deliveryOsteopoikilosisMedical device
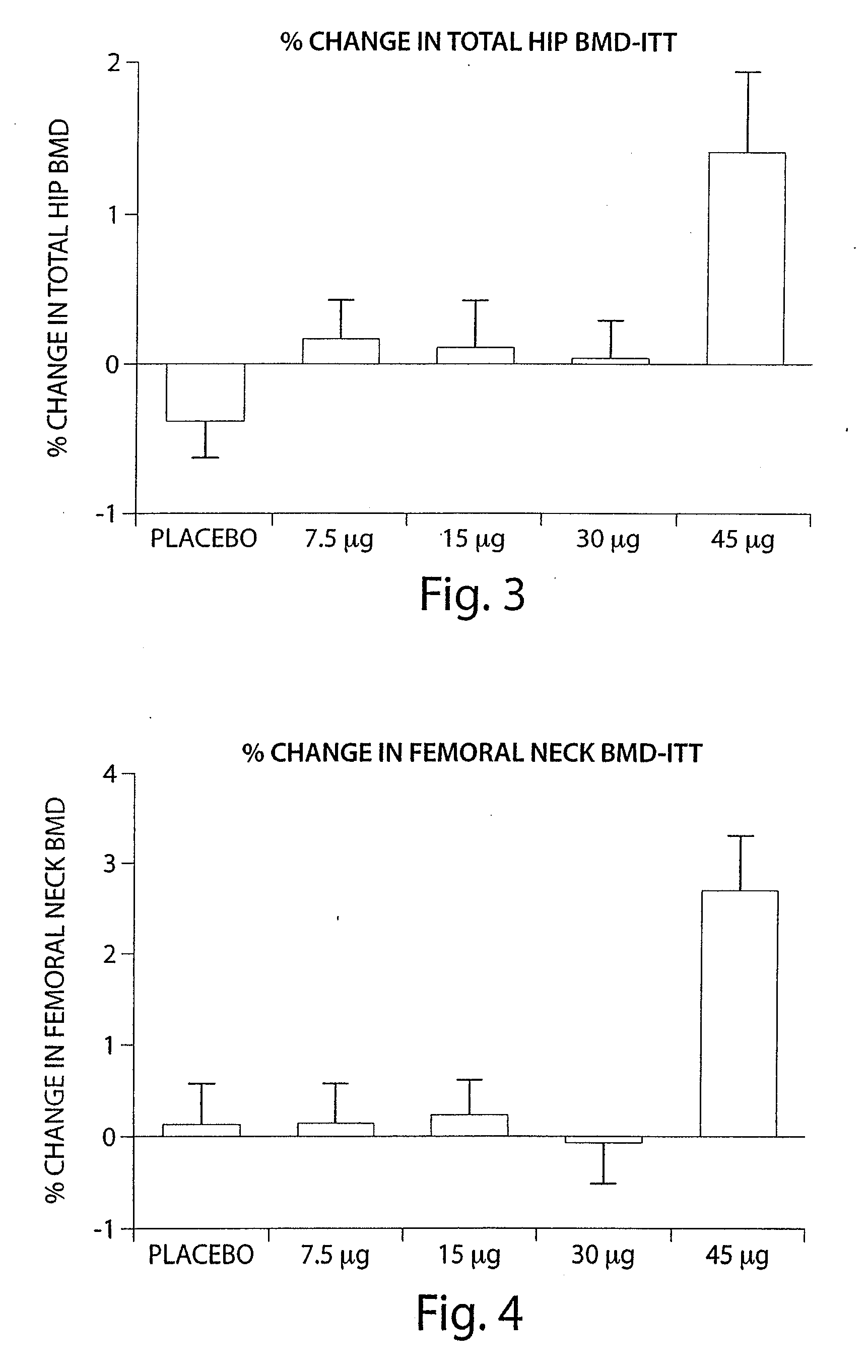
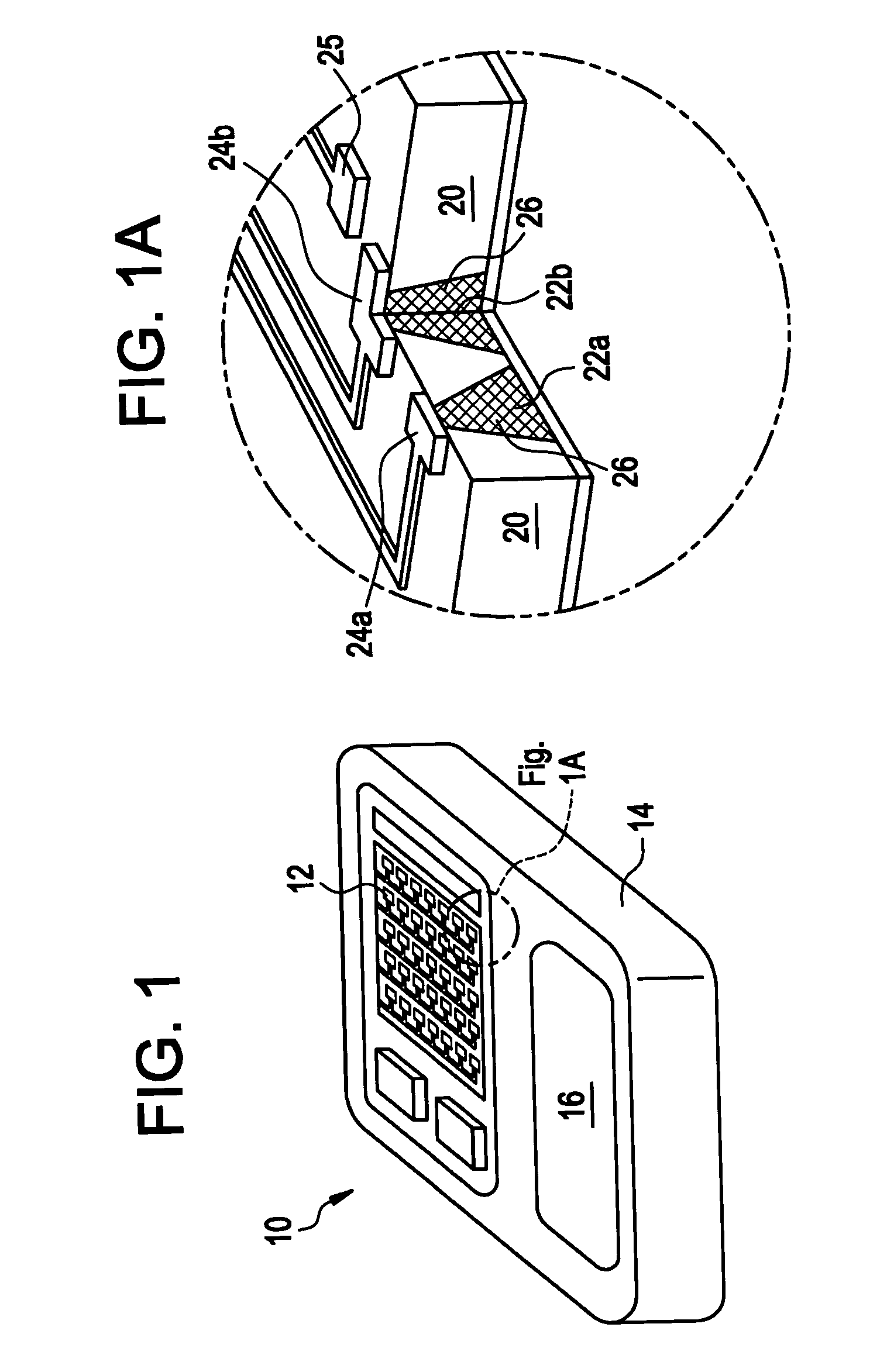
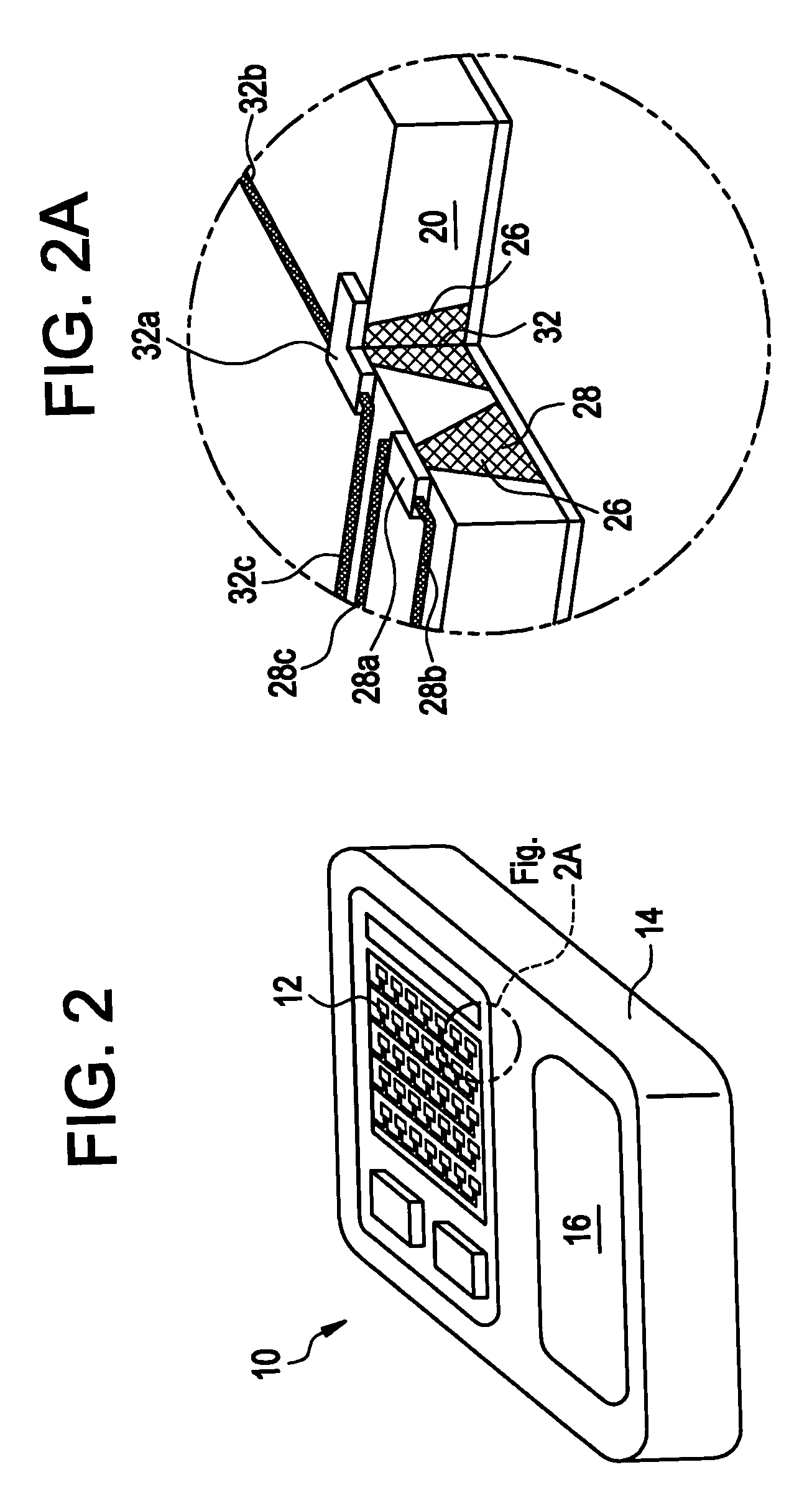
Method and devices are provided for extended and controlled delivery of parathyroid hormone to a patient. The method includes implanting a medical device into the patient, the medical device comprising a substrate, a plurality of reservoirs in the substrate, a release system contained in each of the reservoirs, wherein the release system comprises parathyroid hormone; and controllably releasing a pharmaceutically effective amount of the parathyroid hormone from the reservoirs. The parathyroid hormone can be released intermittently, such as once daily over an extended period (e.g., two months, ten months, or more.). The device can further include reservoirs containing a bone resorption inhibitor or other drug for release. The devices are useful in delivering PTH for the treatment and prevention of bone loss, such as associated with osteoporosis.
Owner:MICROCHIPS BIOTECH INC
Parathyroid hormone (pth) containing pharmaceutical compositions for oral use
InactiveUS20070155664A1Quick releaseSufficient amountOrganic active ingredientsPeptide/protein ingredientsRegimenBlood plasma
A pharmaceutical composition for oral administration comprising PTH, wherein the in vitro release of PTH-when tested in a dissolution test of pharmacopoeia standard-is delayed with at least 2 hours and once the release starts, at least 90% w / w such as, e.g., at least 95% or at least 99% of all PTH contained in the composition is released within at the most 2 hours. The composition may also comprises a calcium containing compound and / or a vitamin, D. In particular, PTH is administered in combination with a calcium-containing compound for the treatment or prevention of bone-related diseases, so that I) an effective amount of a calcium-containing compound is administered to lower the plasma level of endogenous PTH, and II) an effective amount of PTH is administered to obtain a peak concentration of Pm once the endogeneous PTH level is lowered. This present a potential therapeutic or prophylactic regimen for bone-related disorders including osteoporosis.
Owner:NYCOMED DANMARK AS
Method for inhibiting bone resorption
ActiveUS20090074763A1Inhibiting bone resorptionLower Level RequirementsAntibacterial agentsNervous disorderBone densityIncreased bone mineral density
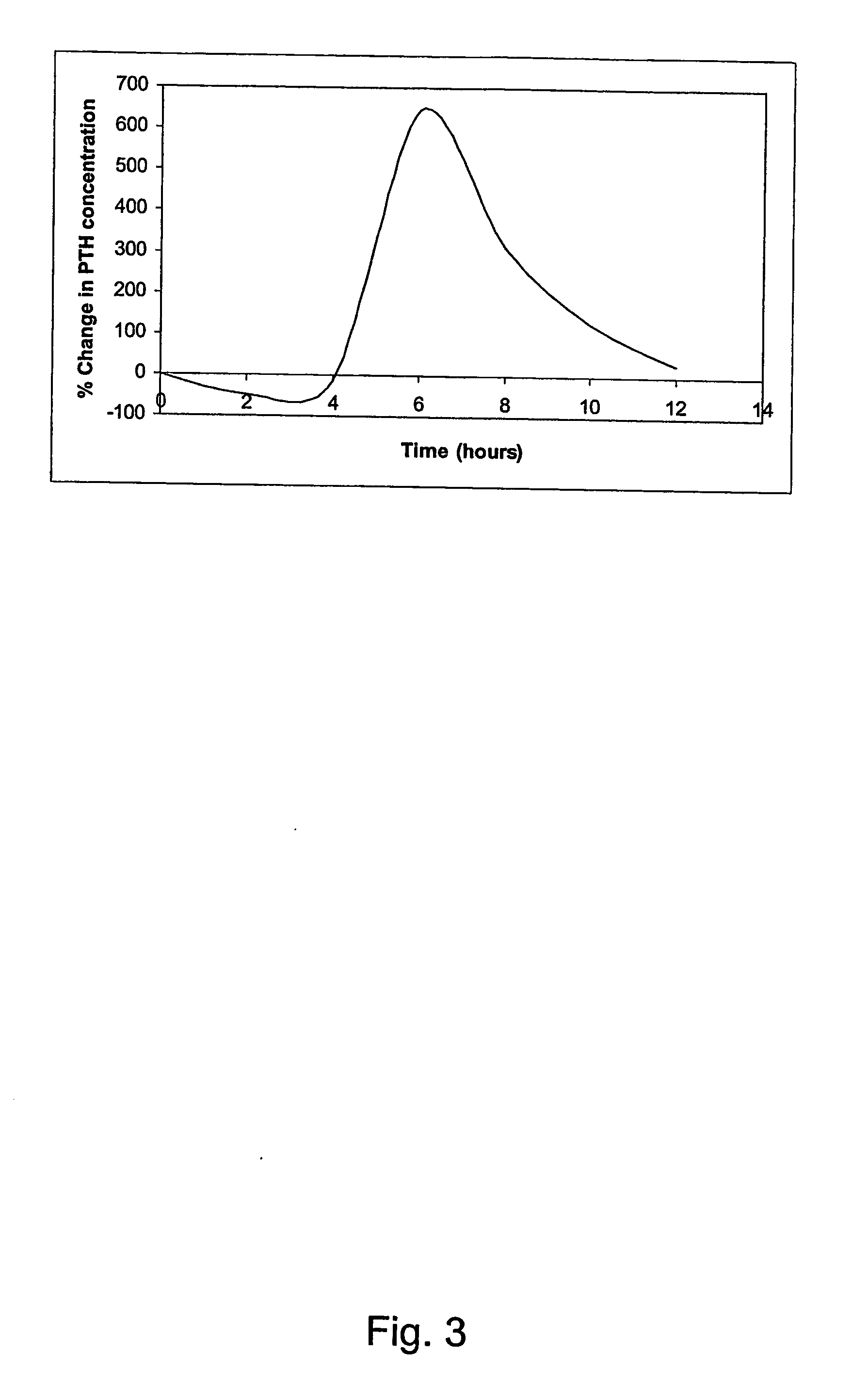
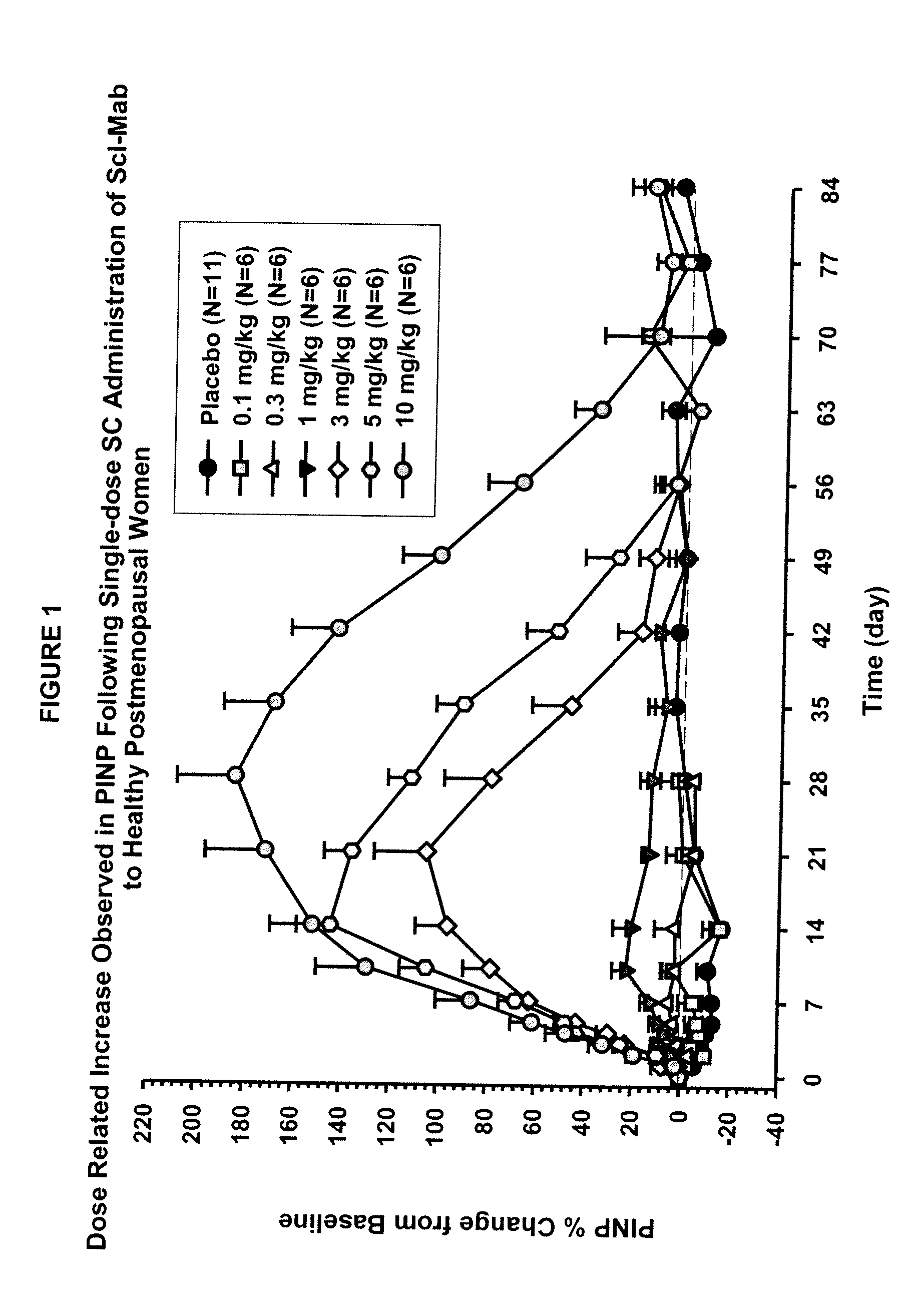
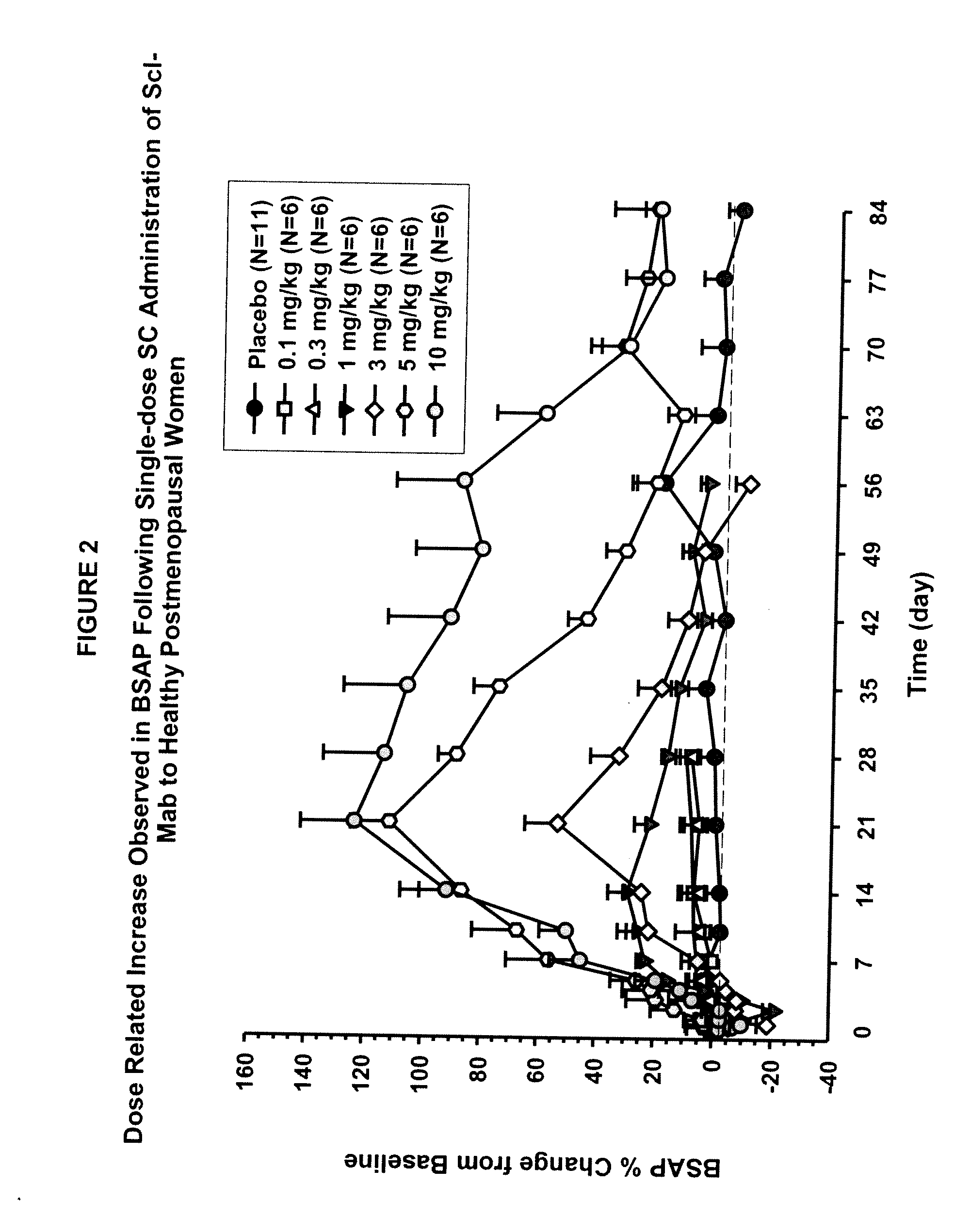
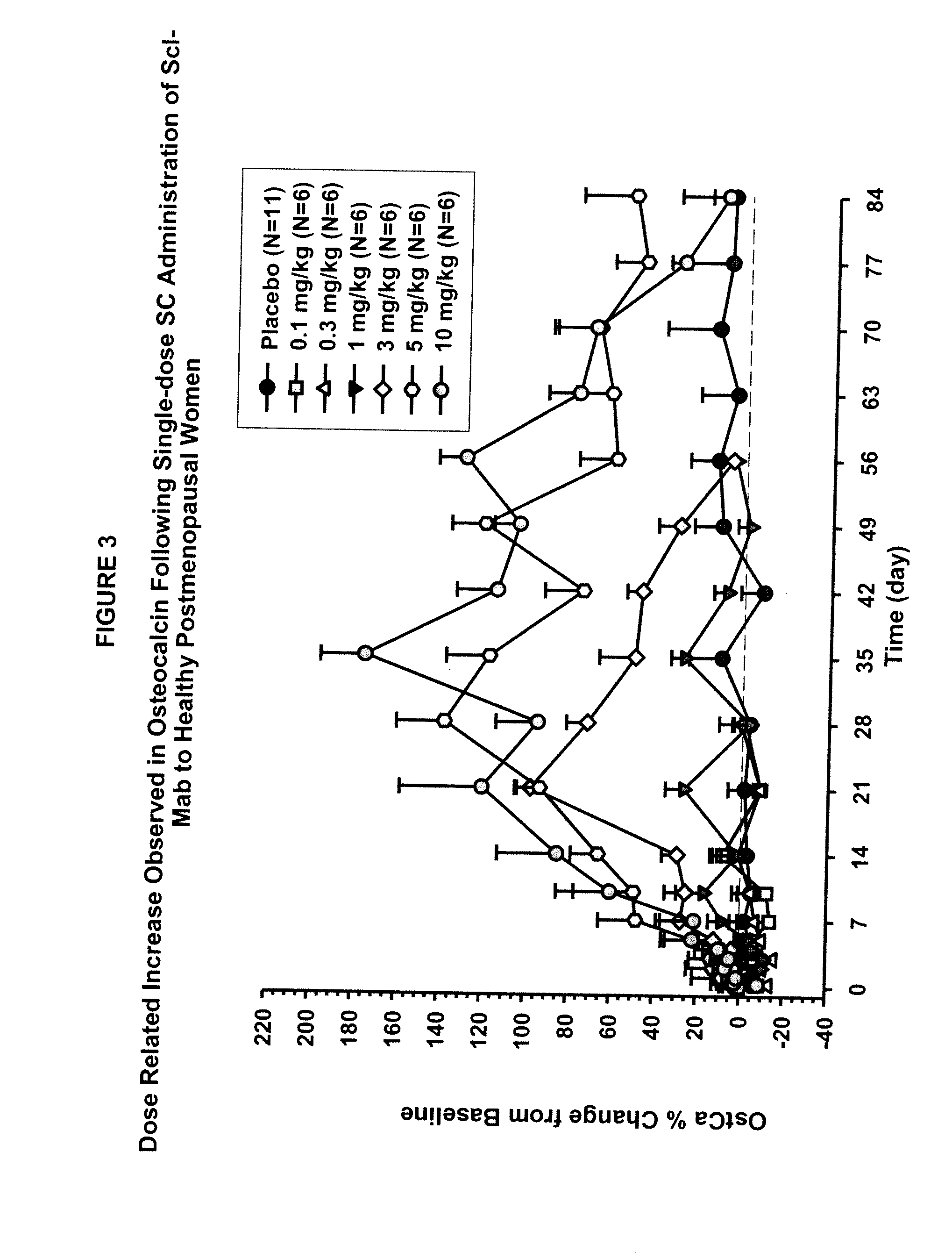
The invention is directed to a method of inhibiting bone resorption. The method comprises administering to a human an amount of sclerostin inhibitor that reduces a bone resorption marker level for at least 2 weeks. The invention also provides a method of monitoring anti-sclerostin therapy comprising measuring one or more bone resorption marker levels, administering a sclerostin binding agent, then measuring the bone resorption marker levels. Also provided is a method of increasing bone mineral density; a method of ameliorating the effects of an osteoclast-related disorder; a method of treating a bone-related disorder by maintaining bone density; and a method of treating a bone-related disorder in a human suffering from or at risk of hypocalcemia or hypercalcemia, a human in which treatment with a parathyroid hormone or analog thereof is contraindicated, or a human in which treatment with a bisphosphonate is contraindicated.
Owner:AMGEN INC
Pthr1 receptor compounds
InactiveUS20110294738A1Promote absorptionNervous disorderPeptide/protein ingredientsOsteoblastSecondary hyperparathyroidism
The invention relates generally to compounds which are allosteric modulators (e.g., negative and positive allosteric modulators, allosteric agonists, and ago-allosteric modulators) of the G protein coupled receptor PTHR1, also known as parathyroid hormone / parathyroid hormone related protein receptor. The PTHR1 compounds are derived from the intracellular loops and domains of the PTHR1 receptor. The invention also relates to the use of these PTHR1 receptor compounds and pharmaceutical compositions comprising the PTHR1 receptor compounds in the treatment of diseases and conditions associated with PTHR1 receptor modulation, such as osteoporosis; humoral hypercalcemia of malignancy; osteolytic and osteoblastic metastasis to bone; primary and secondary hyperparathyroidism associated increase in bone absorption; vascular calcification; psychiatric disorders and cognitive disorders associated with hyperparathyroidism; dermatological disorders; and excess hair growth.
Owner:REN YONG +2
PTH functional domain conjugate peptides, derivatives thereof and novel tethered ligand-receptor molecules
InactiveUS7057012B1Improve efficacyHigh potencyPeptide/protein ingredientsImmunoglobulinsAgonist drugsPharmaceutical drug
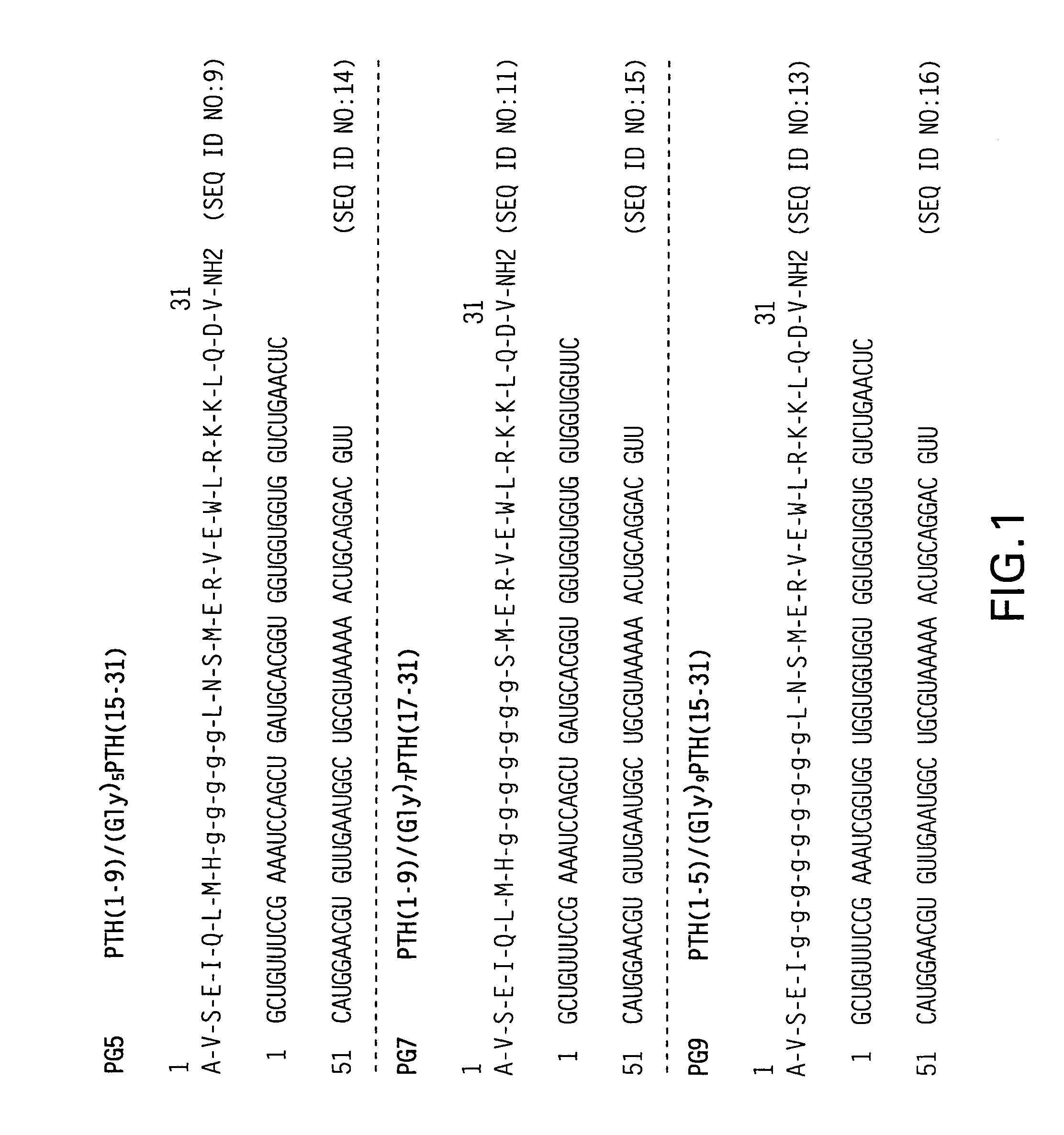
Novel parathyroid hormone (PTH) peptides and analogs thereof of the PTH(1–34) fragment are disclosed that combine the N-terminal signaling domain (residues 1–9) and the C-terminal binding domain (residues 15–31) via a linker. Nucleic acid molecules and peptides for PTH(1–9)-(Gly)5-PTH(15–31) (PG5) and (1–9)-(Gly)7-PTH(15–31) and a novel PTH receptor are disclosed. Additionally, methods of screening for PTH agonists, pharmaceutical compositions and methods of treatment are disclosed.
Owner:THE GENERAL HOSPITAL CORP
Use of gaseous nitric oxide as an anti-cancer agent
InactiveUS20070275100A1Effectively deliver gaseous NOEfficient procedureBiocideBronchoscopesCell phenotypeAnticarcinogen
The invention relates to a method for treating, controlling, or preventing cancerous cell phenotypes and growths in an animal involving the administration of gaseous nitric oxide to one or administration sites in a body. The invention generally is capable of treating cancers found in or on the adrenal gland, bladder, bones, brain, breast, cervix, colon, colorectum, esophagus, gastrointestinal tract, heart, kidney, liver, large intestine, lungs, mouth, ovaries, pancreas, parathyroid, pituitary gland, prostate, salivary gland, skin, small intestine, spleen, stomach, thymus, thyroid, testicles, urinary tract, uterus, vagina, and so forth.
Owner:PULMONOX TECH
Methods for monitoring and guiding therapeutic suppression of parathyroid hormone in renal patients having secondary hyperparathyroidism
Owner:SCANTIBODIES LAB
Methods for monitoring and guiding therapeutic suppression of parathyroid hormone in renal patients having secondary hyperparathyroidism
InactiveUS7056655B2Lower Level RequirementsMinimises levelOrganic active ingredientsBiocideNephrosisEprotirome
The present invention relates to novel methods for monitoring and guiding therapeutic suppression of parathyroid hormone in renal patients having secondary hyperparathyroidism. One determines and monitors the level of parathyroid hormone agonist and parathyroid hormone antagonist in the renal patient. The parathyroid hormone suppressing therapeutic is administered to the patient so as to minimize the level of parathyroid hormone antagonist.
Owner:SCANTIBODIES LAB
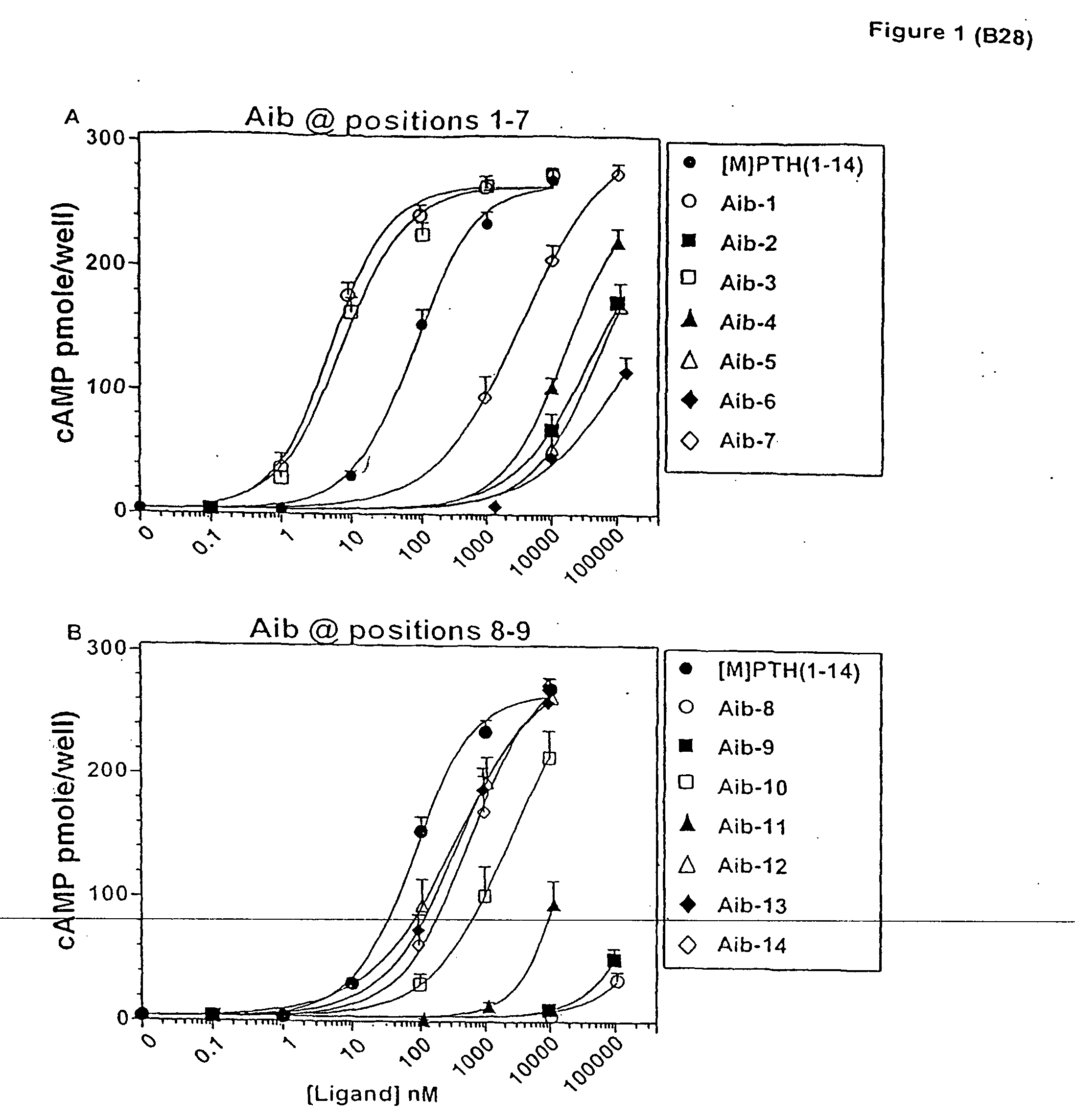
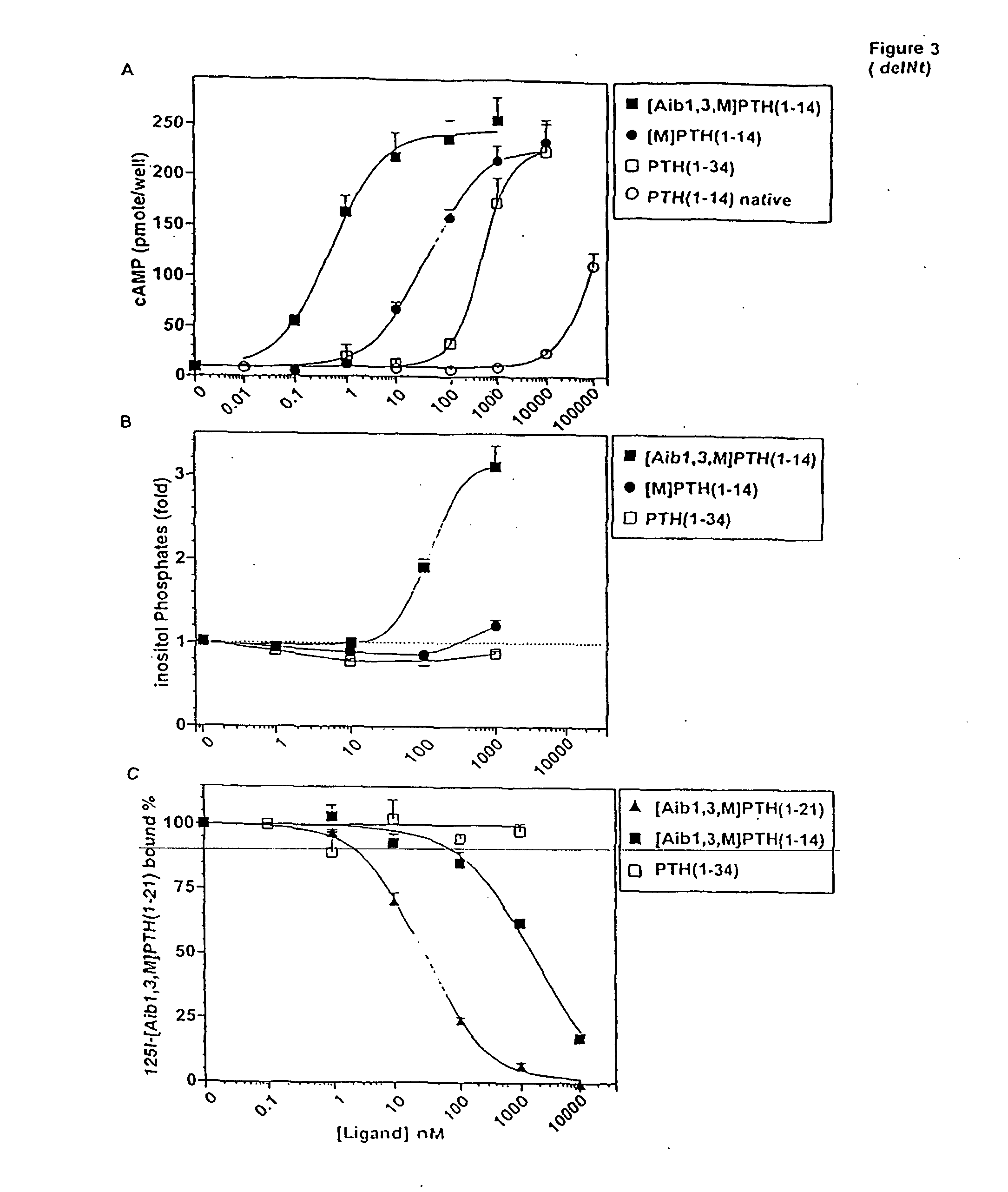
Conformationally constrained parathyroid hormone (pth) analogs
ActiveUS20050026839A1Decrease in bone massQuality improvementIn-vivo radioactive preparationsPeptide/protein ingredientsDiseaseAmino acid substitution
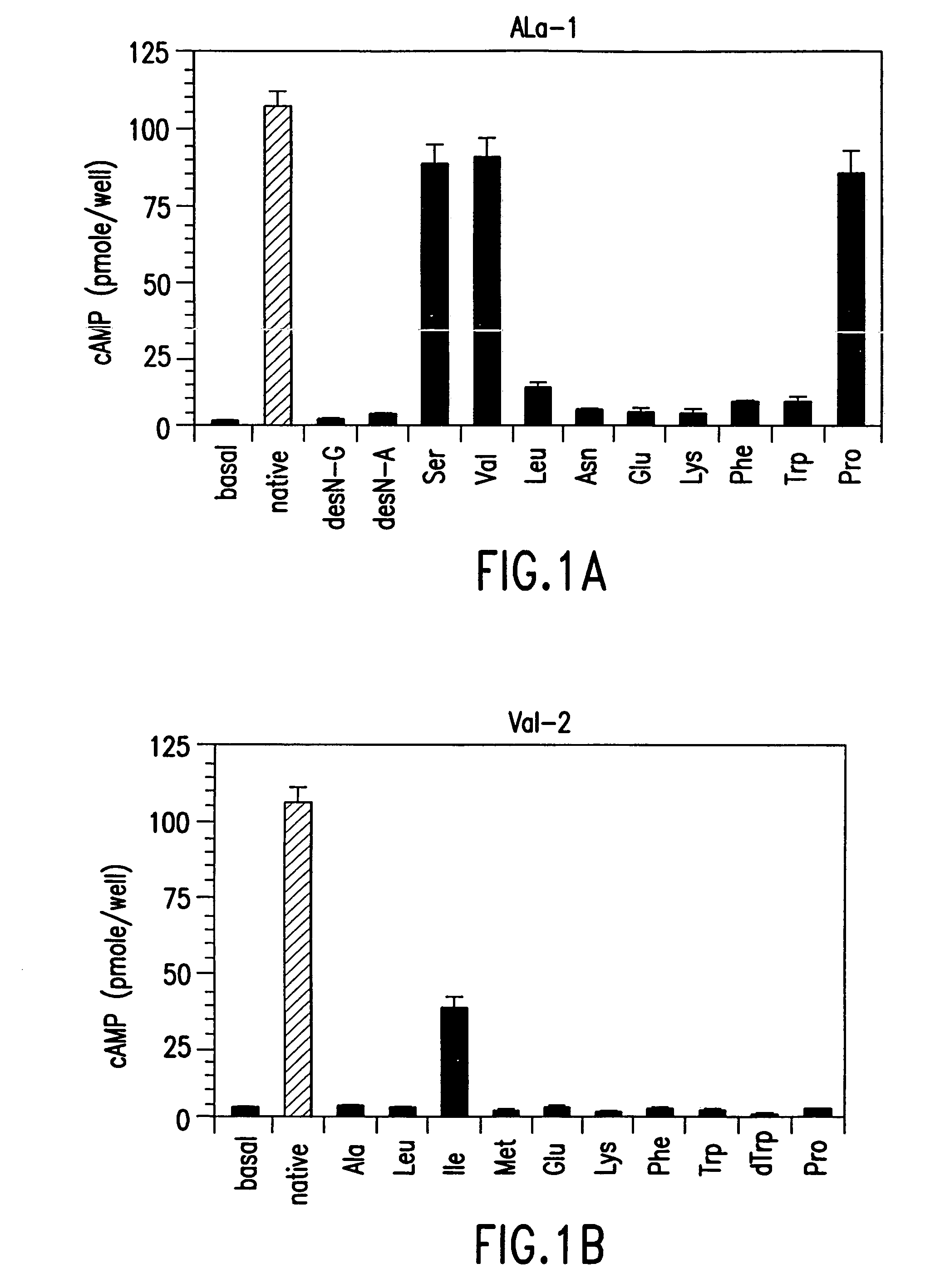
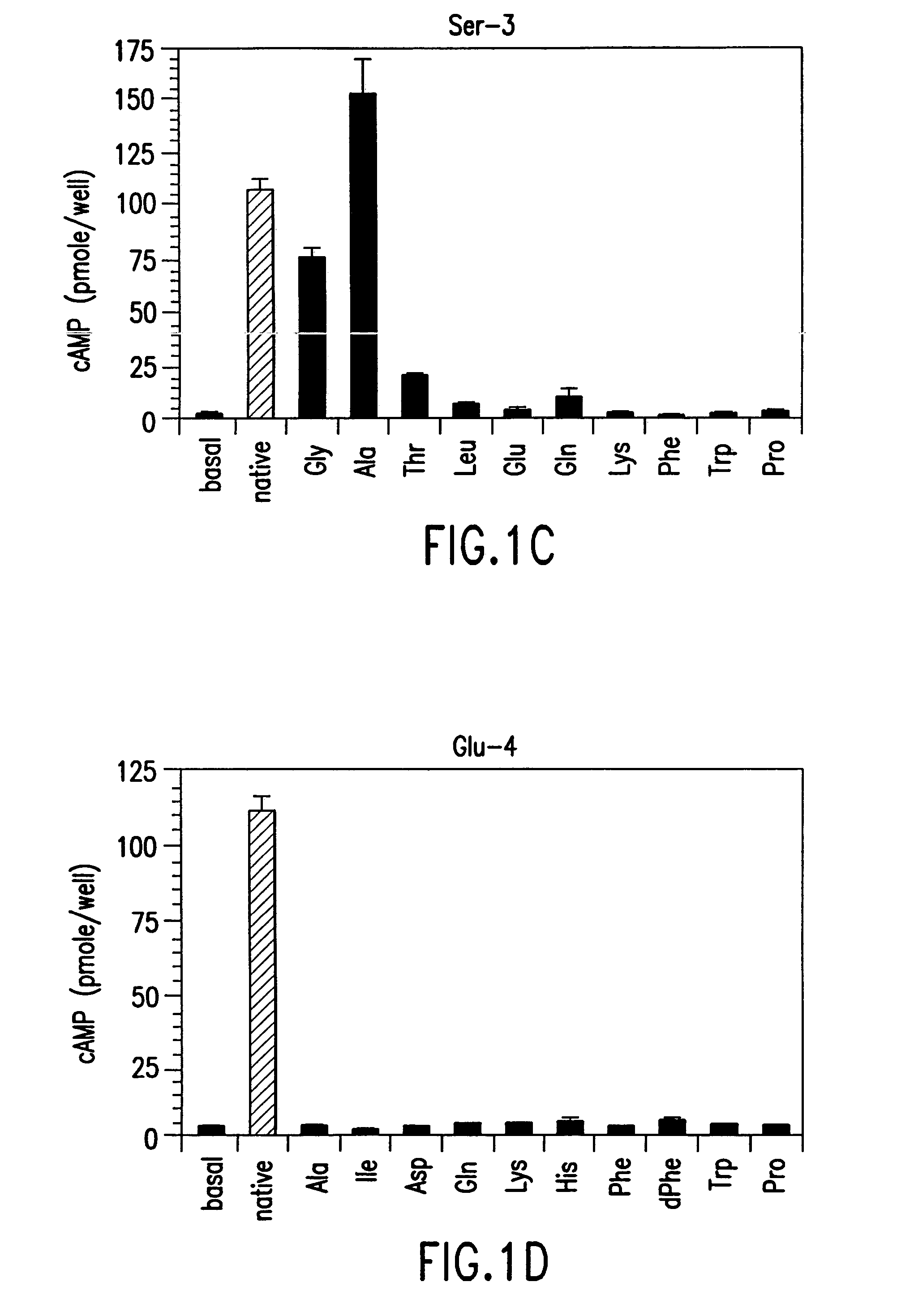
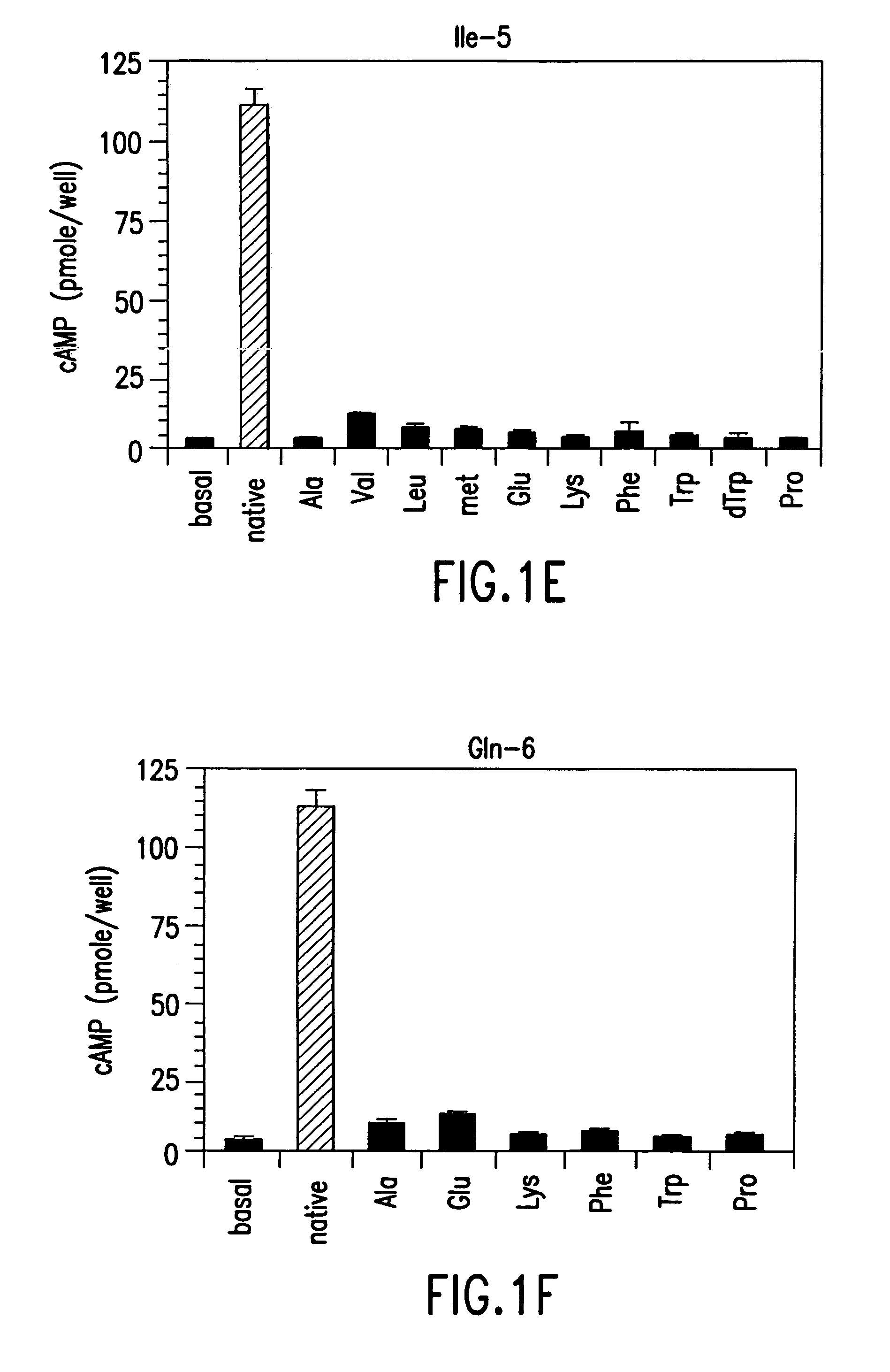
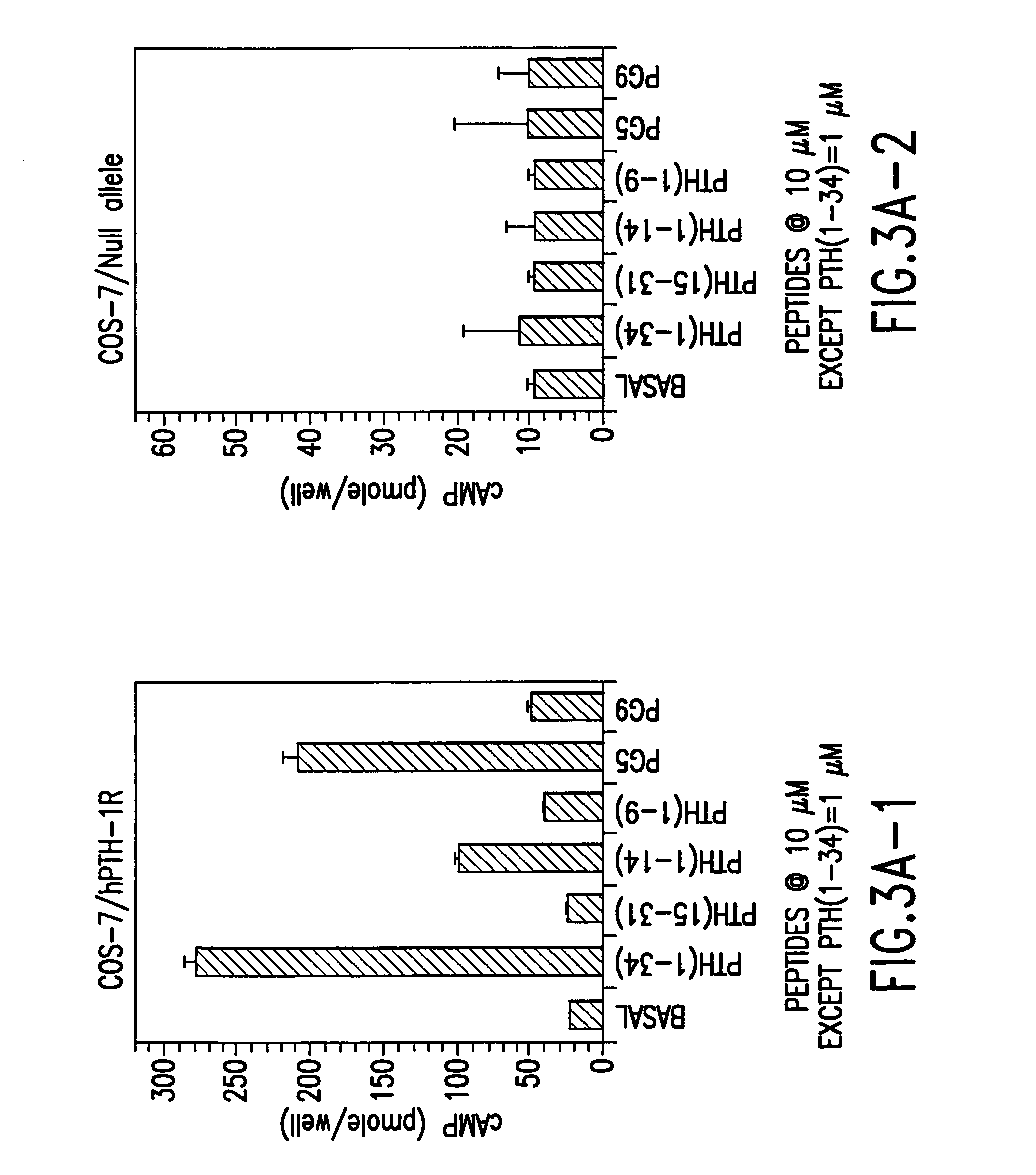
The present invention relates to conformationally constrained parathyroid hormone (PTH) analogs, and methods of preparing and using the PTH analogs. The invention provides novel PTH polypeptide derivatives containing amino acid substitutions at selected positions in the polypeptides. The invention provides derivatives of PTH (1-34), PTH(1-21), PTH(1-20), PTH(1-19), PTH(1-18), PTH(1-17), PTH(1-16), PTH(1-15), PTH(1-14), PTH(1-13), PTH(1-12), PTH(1-11) and PTH(1-1 0) polypeptides, wherein at least one residue in each polypeptide is a helix, preferably an a-helix, stabilizing residue. The invention also provides methods of making such peptides. Further, the invention encompasses compositions and methods for use in limiting undesired bone loss in a vertebrate at risk of such bone loss, in treating conditions that are characterized by undesired bone loss or by the need for bone growth, e.g. in treating fractures or cartilage disorders and for raising cAMP levels in cells where deemed necessary.
Owner:THE GENERAL HOSPITAL CORP
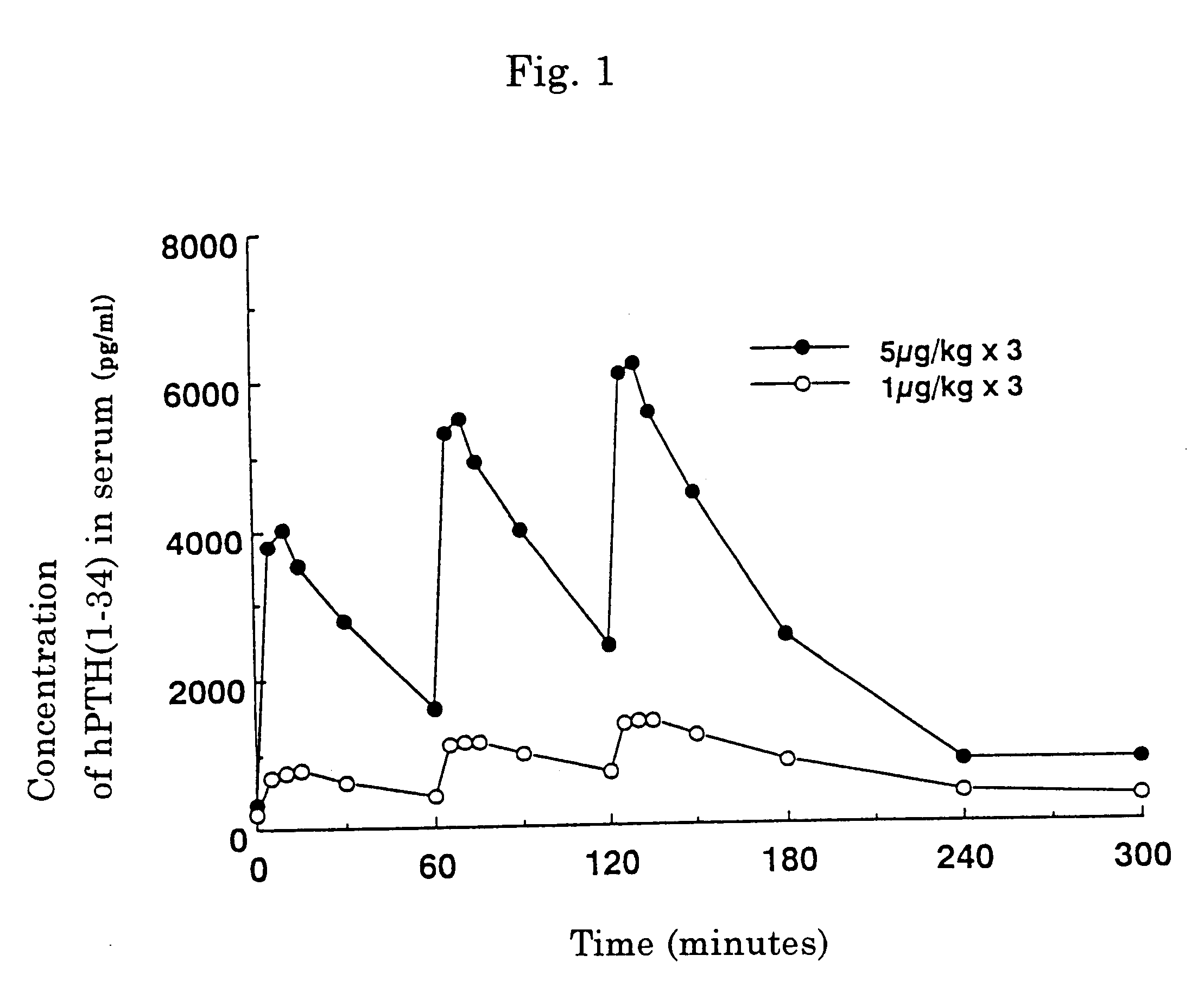
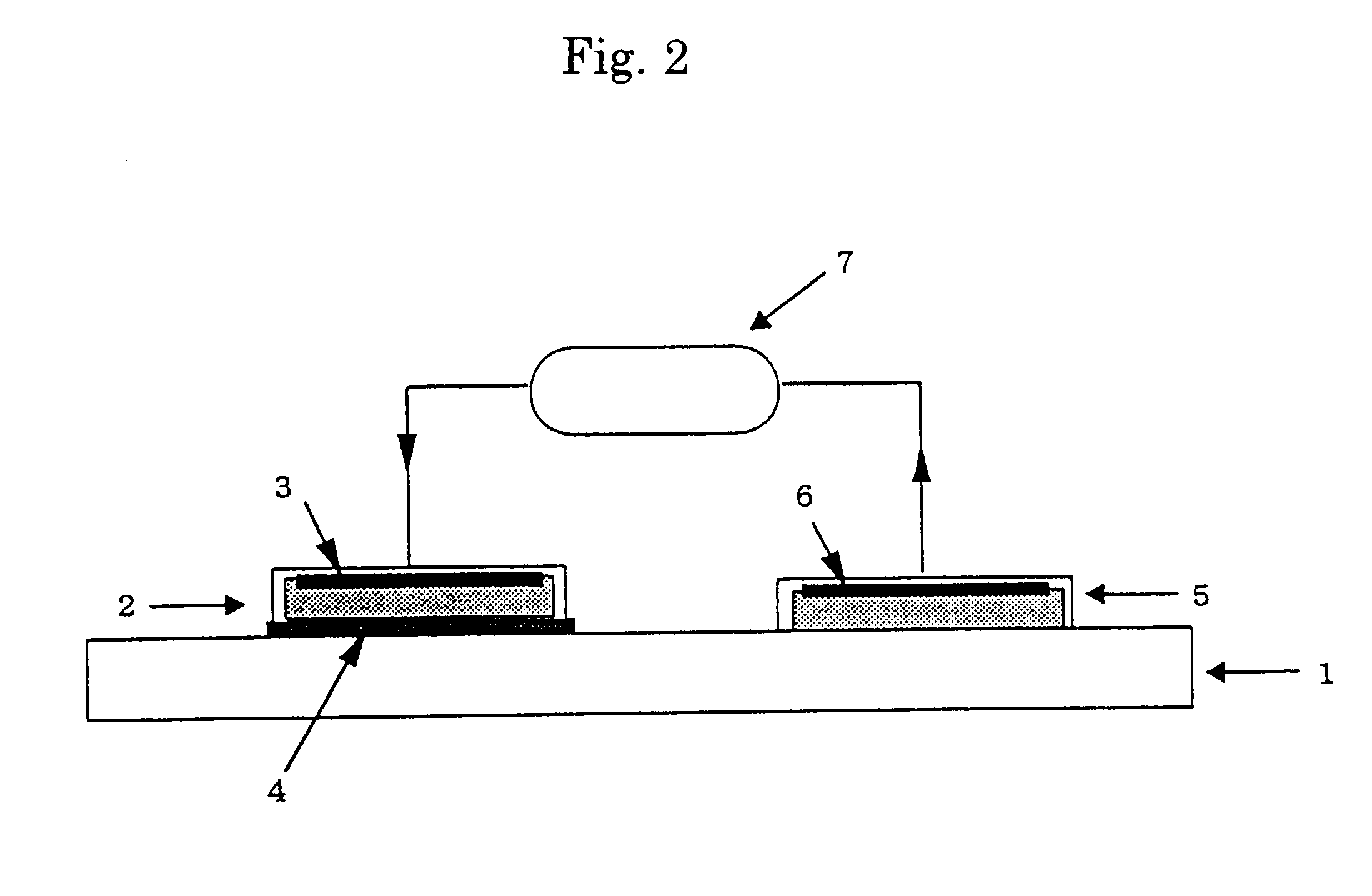
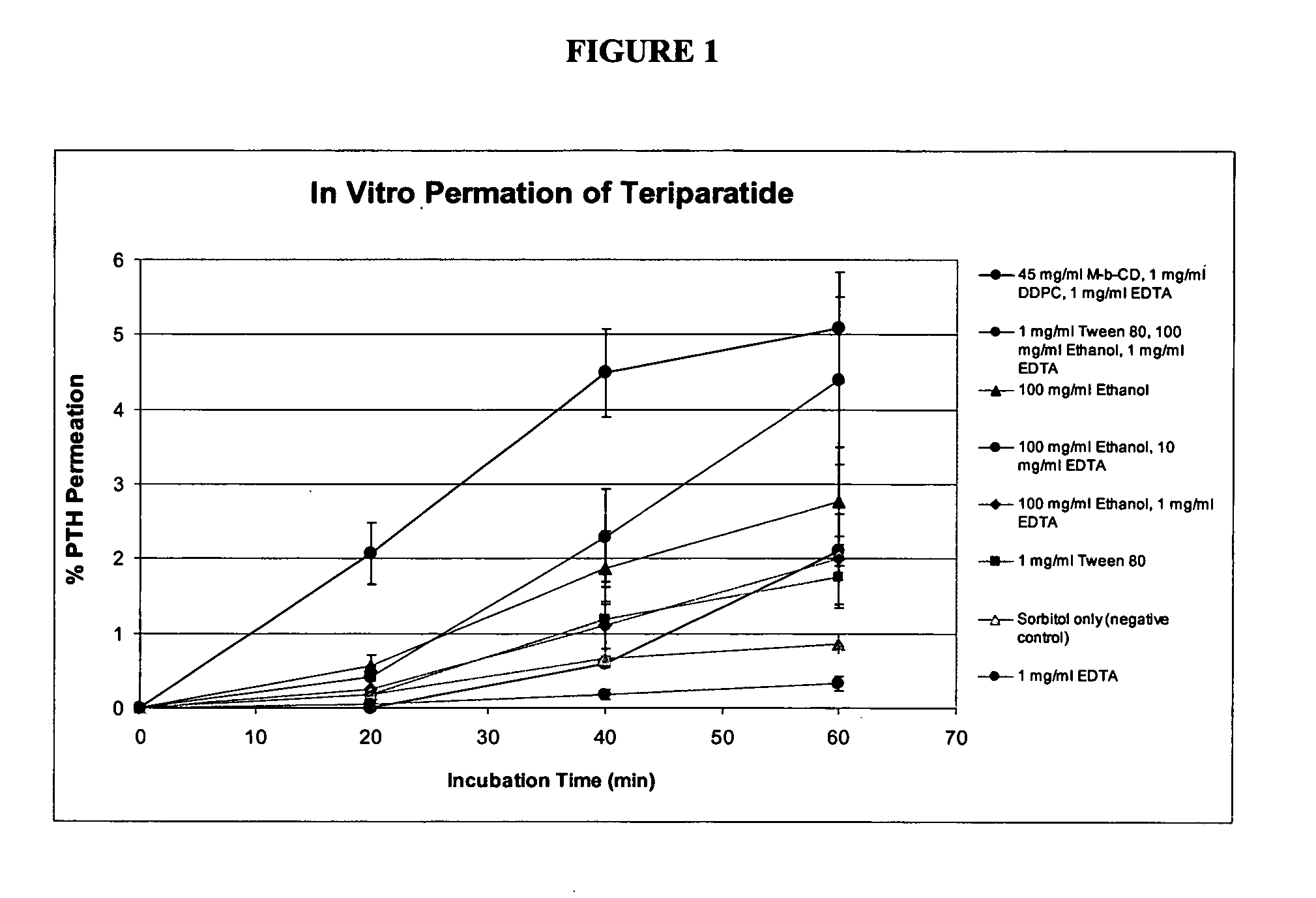
Iontophoresis method
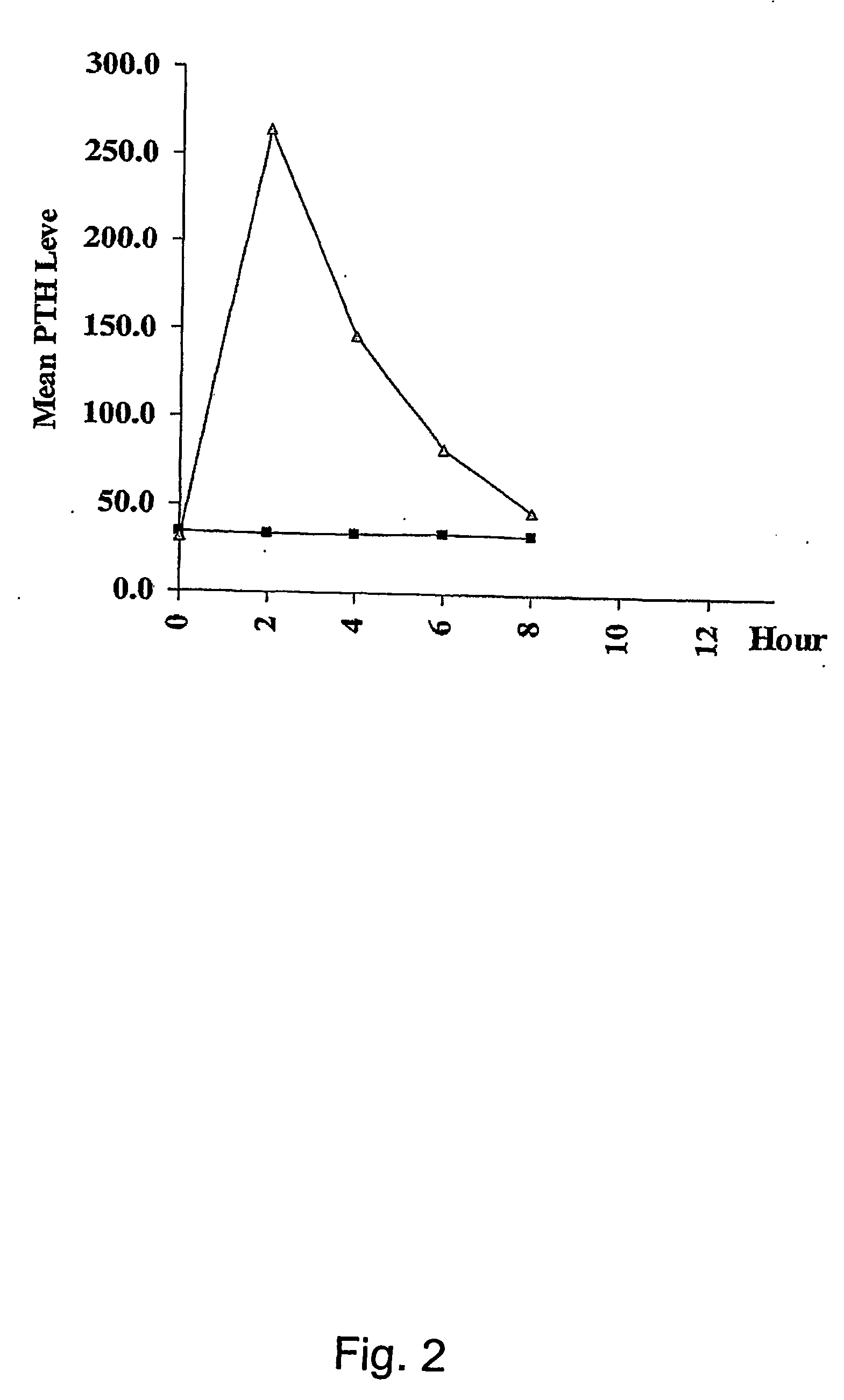
A method for transdermal administration of parathyroid hormone by iontophoresis, which comprises applying electric current at least 2 times a day, which method is repeated one to four times each week, or an apparatus for the iontophoresis can be widely applied for not only the prevention or treatment of osteoporosis but also for general bone diseases which require promotion of bone morphogenesis, for example, treatment of ordinary fractures.The method of the present invention produces excellent pharmacological effects such as few side effects and a high bioavailability in long term administration for the prevention and treatment of bone diseases.
Owner:HISAMITSU PHARM CO INC
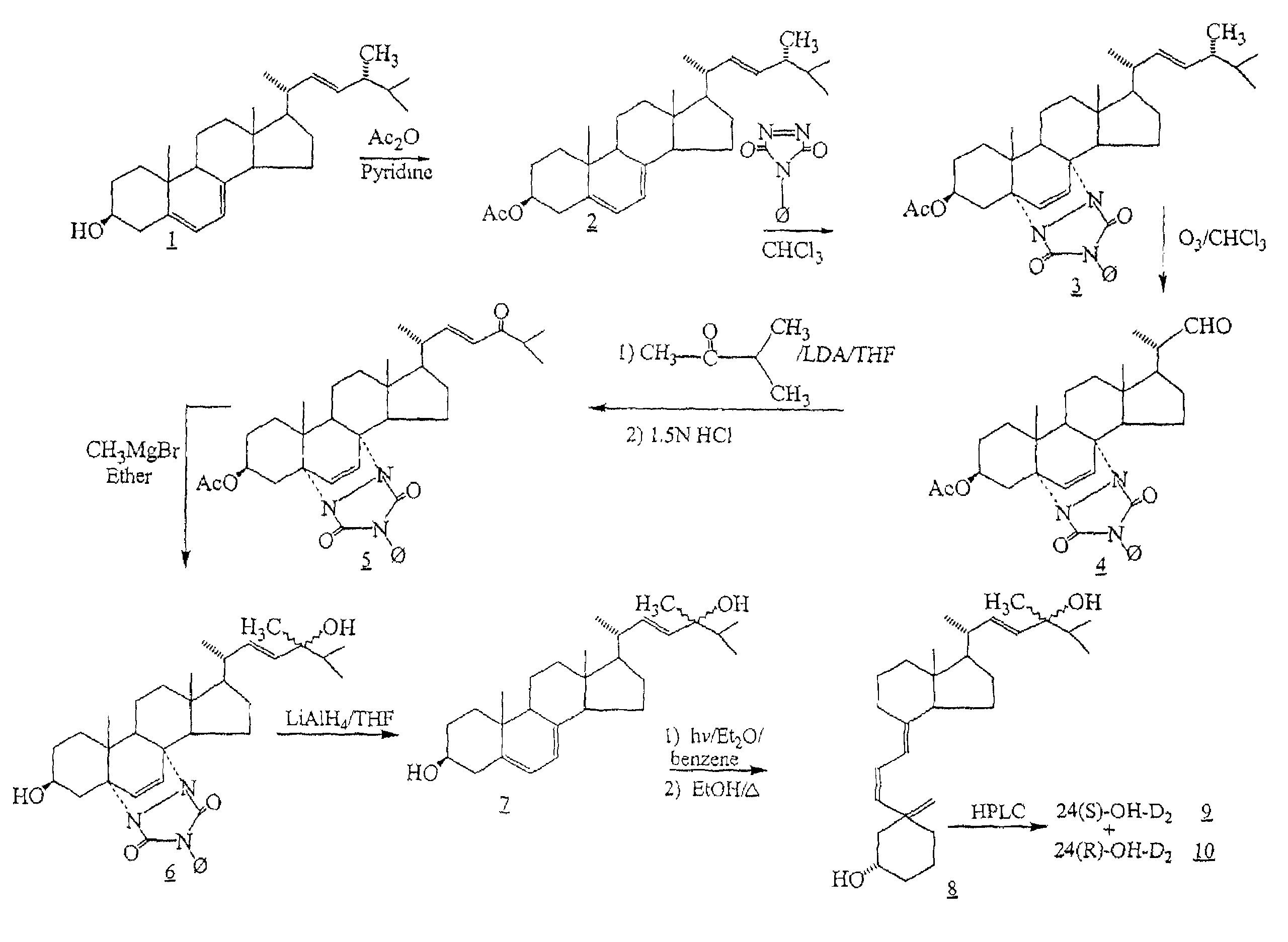
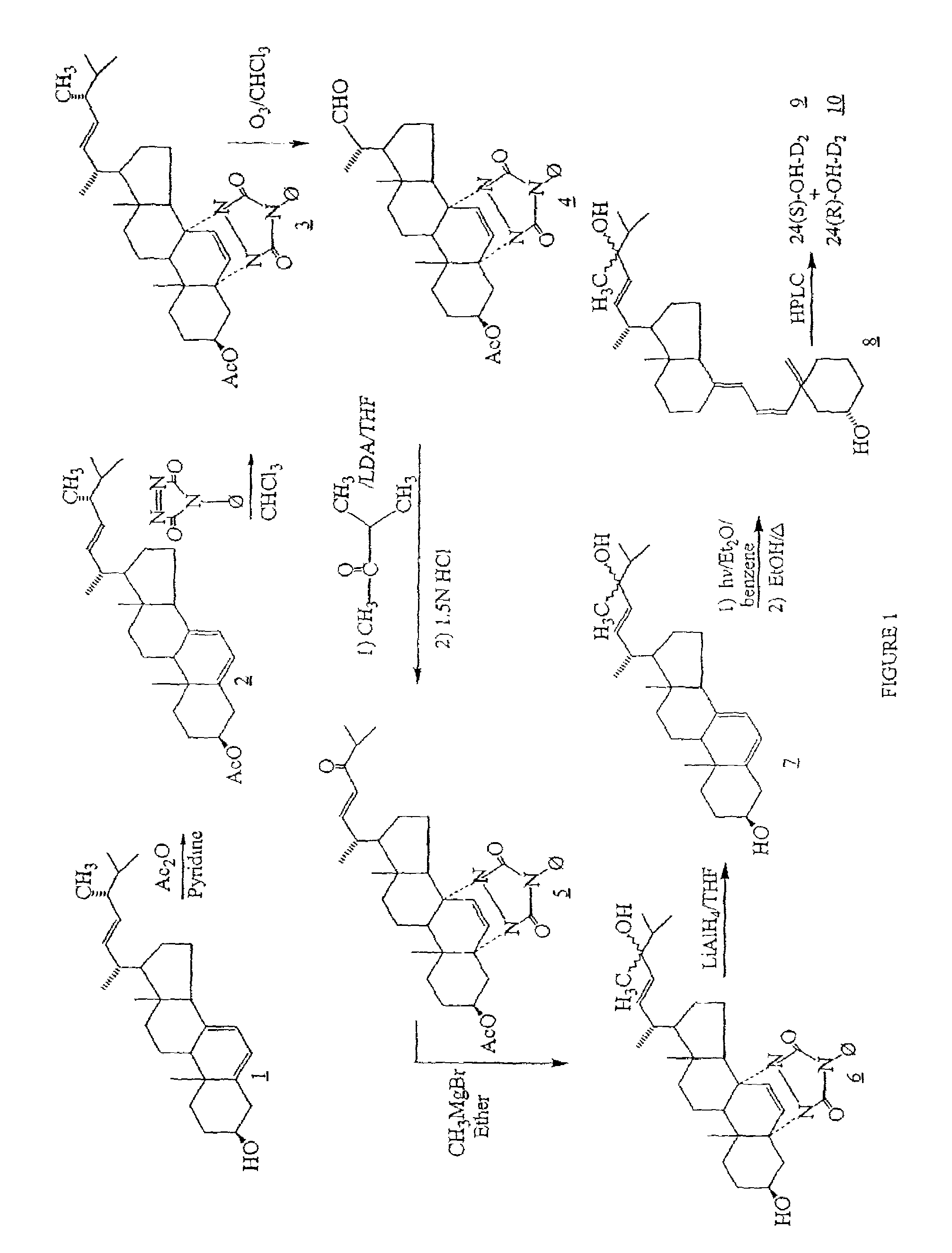
24-hydroxyvitamin D, analogs and uses thereof
The invention provides 24-hydroxyvitamin D compounds and methods for their use in the treatment and prophylaxis of hyperparathyroidism and hyperproliferative diseases, and in the modulation of the immune and inflammatory responses as well as the treatment of bone depletive disorders.
Owner:GENZYME CORP
Conformationally constrained parathyroid hormones with alpha-helix stabilizers
The present invention relates to conformationally constrained parathyroid hormone (PTH) analogs and derivatives of those analogs. The invention also provides methods of preparing and using the PTH analogs. Further, the invention encompasses compositions and methods for use in limiting undesired bone loss in a vertebrate at risk of such bone loss, in treating conditions that are characterized by undesired bone loss or by the need for bone growth, e.g. in treating fractures or cartilage disorders and for raising camp levels in cells where deemed necessary.
Owner:BRISTOL MYERS SQUIBB CO +1
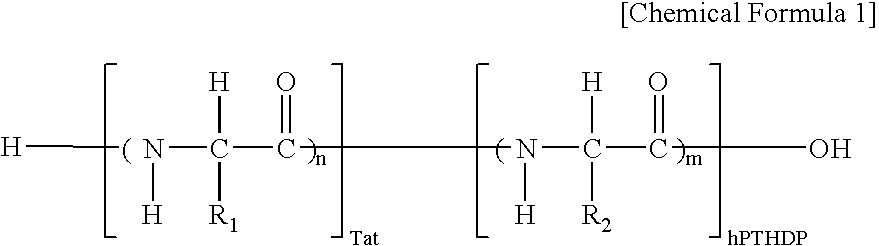
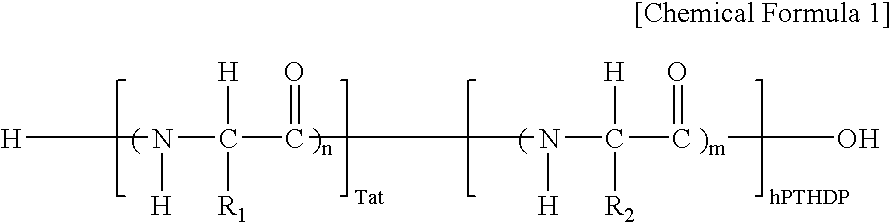
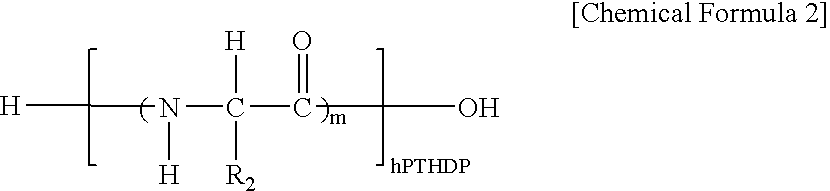
Fusion peptide of human parathyroid hormone derived peptide and tat peptide, preparation thereof, and skin slimming cosmetic composition comprising the same
InactiveUS20050048629A1EasilySafely penetrates into the integument and endotheliumCosmetic preparationsPeptide/protein ingredientsTat peptideIncreased Lipolysis
The present invention relates to a fusion peptide wherein a self cell-penetrating Tat peptide having a self penetrating signal is bound to a human parathyroid hormone-derived peptide, a preparation thereof, and a skin slimming cosmetic composition comprising the same. Since the fusion peptide wherein the Tat peptide is bound to the human parathyroid hormone-derived peptide has high stability and superior skin absorption, the present invention provides a skin slimming agent having superior lipolysis effects and improved durability of the effects.
Owner:LG HOUSEHOLD & HEALTH CARE LTD
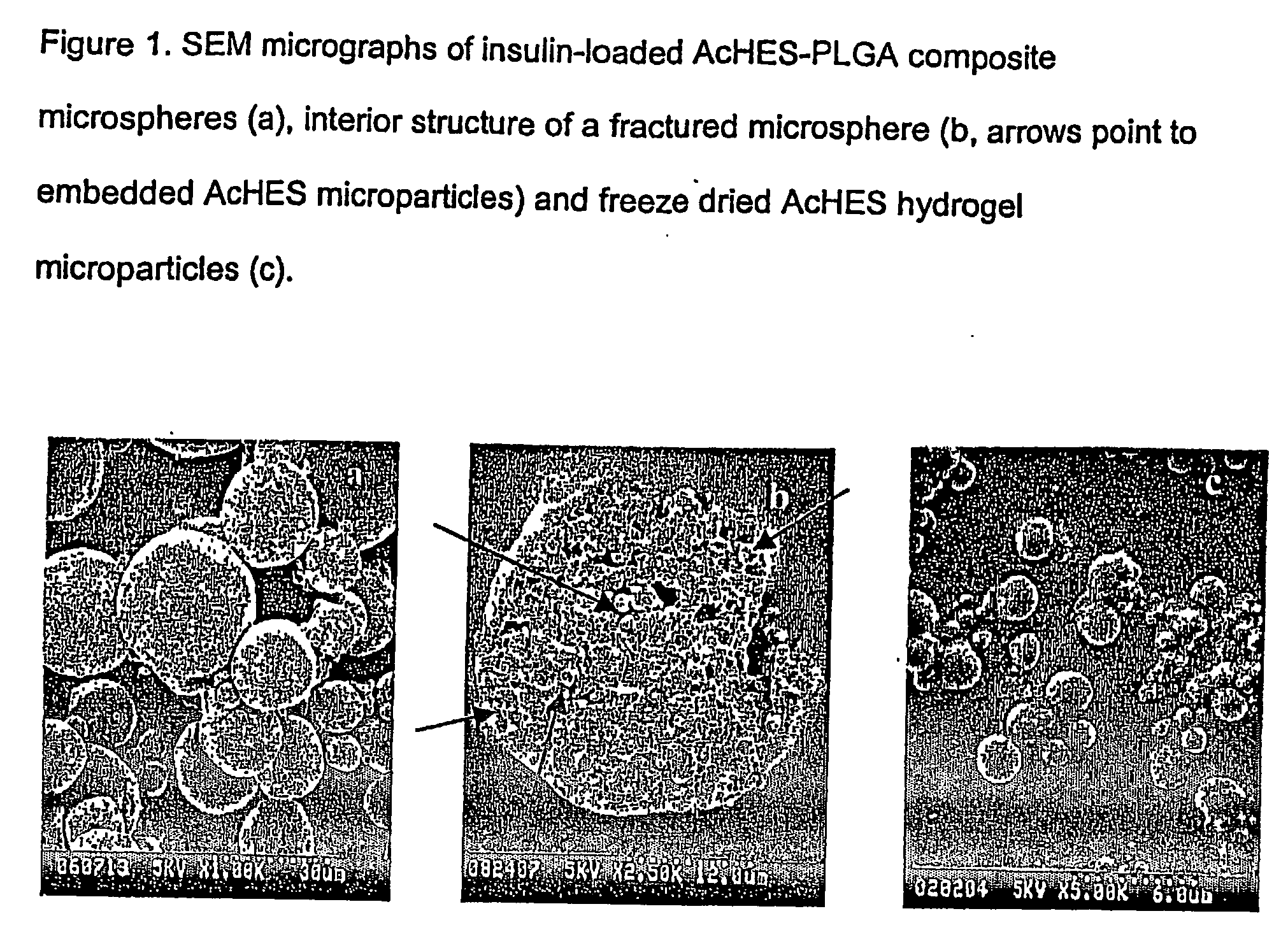
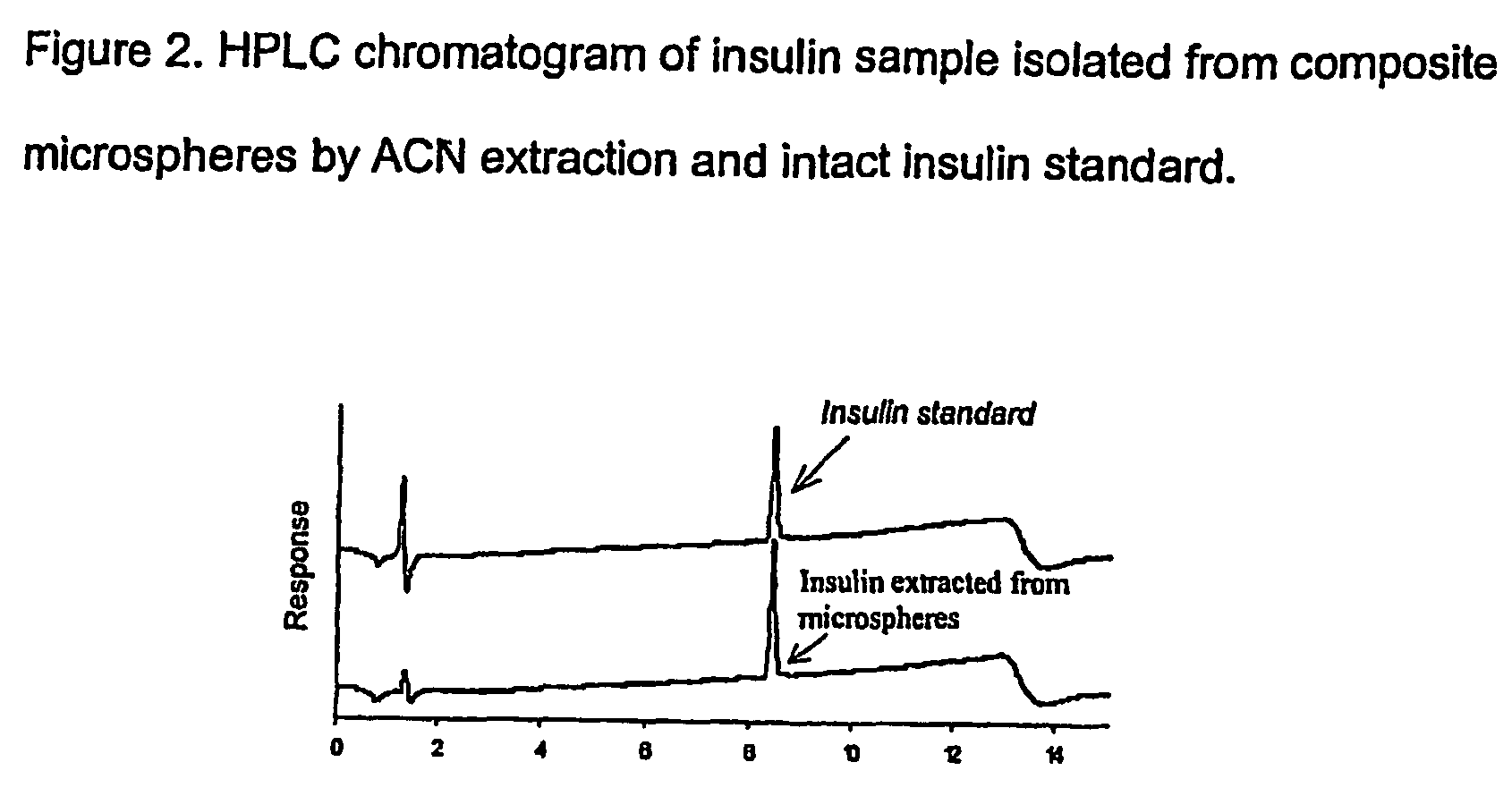
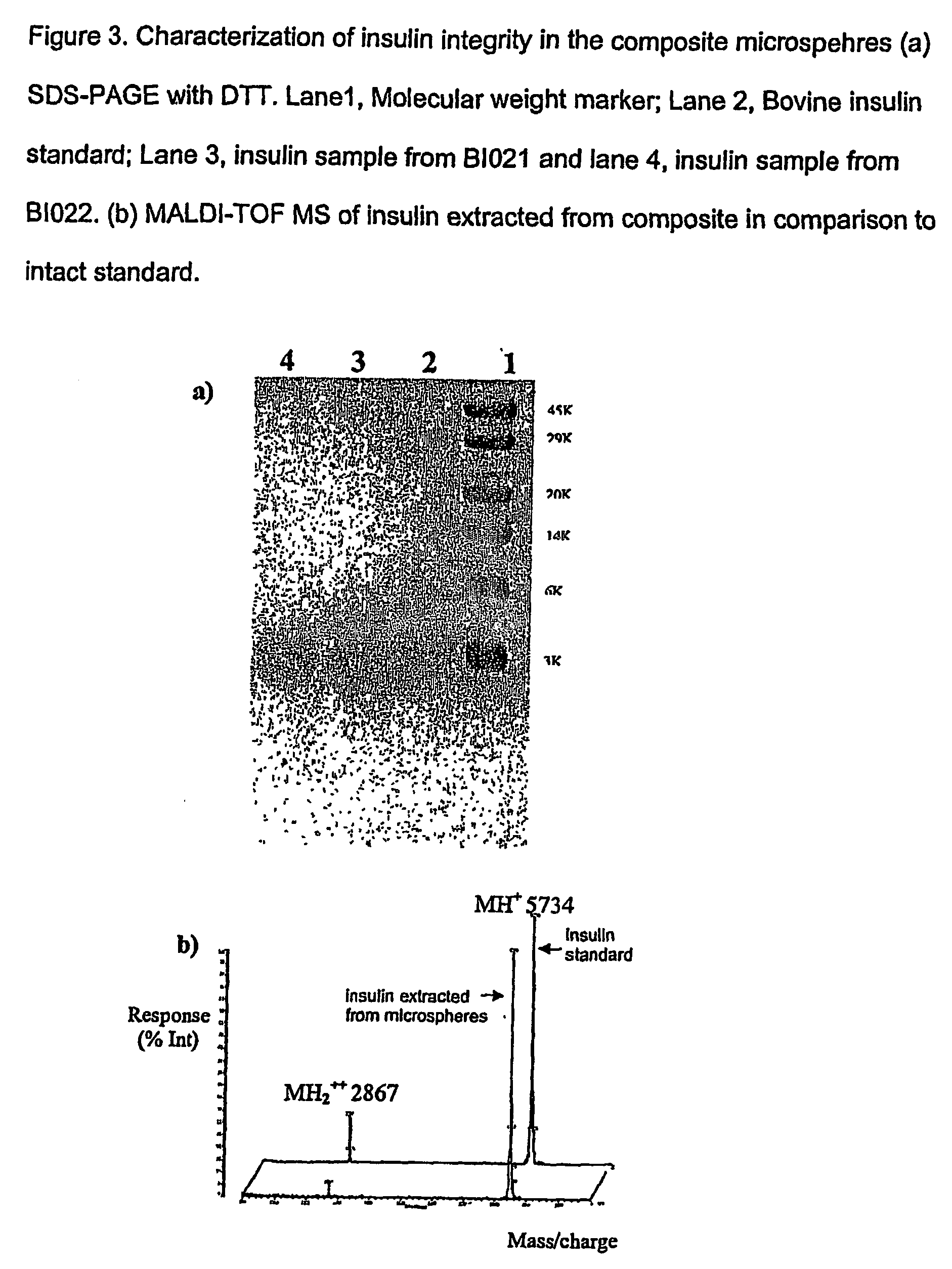
(Poly(acryloyl-hydroxyethyl starch)-plga composition microspheres
The present invention relates to a composite microsphere system comprising poly(D,L-lactide-co-glycolide) (PLGA), poly(acryloyl hydroxyethyl starch) (AcHES), and a pharmaceutically effective amount of a biologically active compound. The active compound may be, for example, an insulin, an interferon, a luteinizing hormone-releasing hormone (LHRH) analog, a somatostatin and / or derivatives thereof, a calicitonin, a parathyroid hormone (PTH), a bone morphogenic protein (BMP), an erythropoietin (EPO), an epidermal growth factor (EGF) or a growth hormone. This invention also relates to methods of using the composite microspheres, and methods of preparing same.
Owner:UNIV OF KENTUCKY RES FOUND
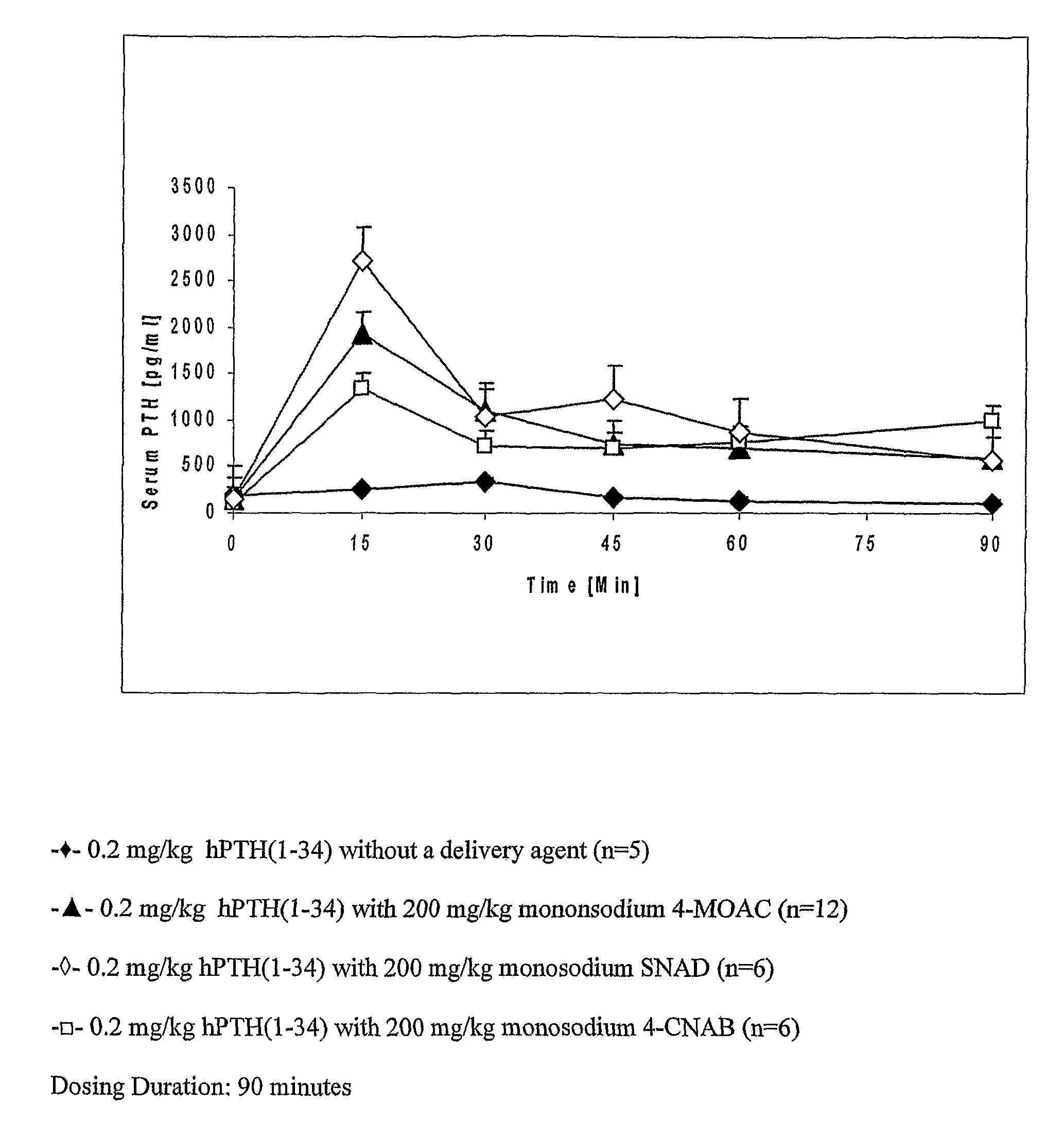
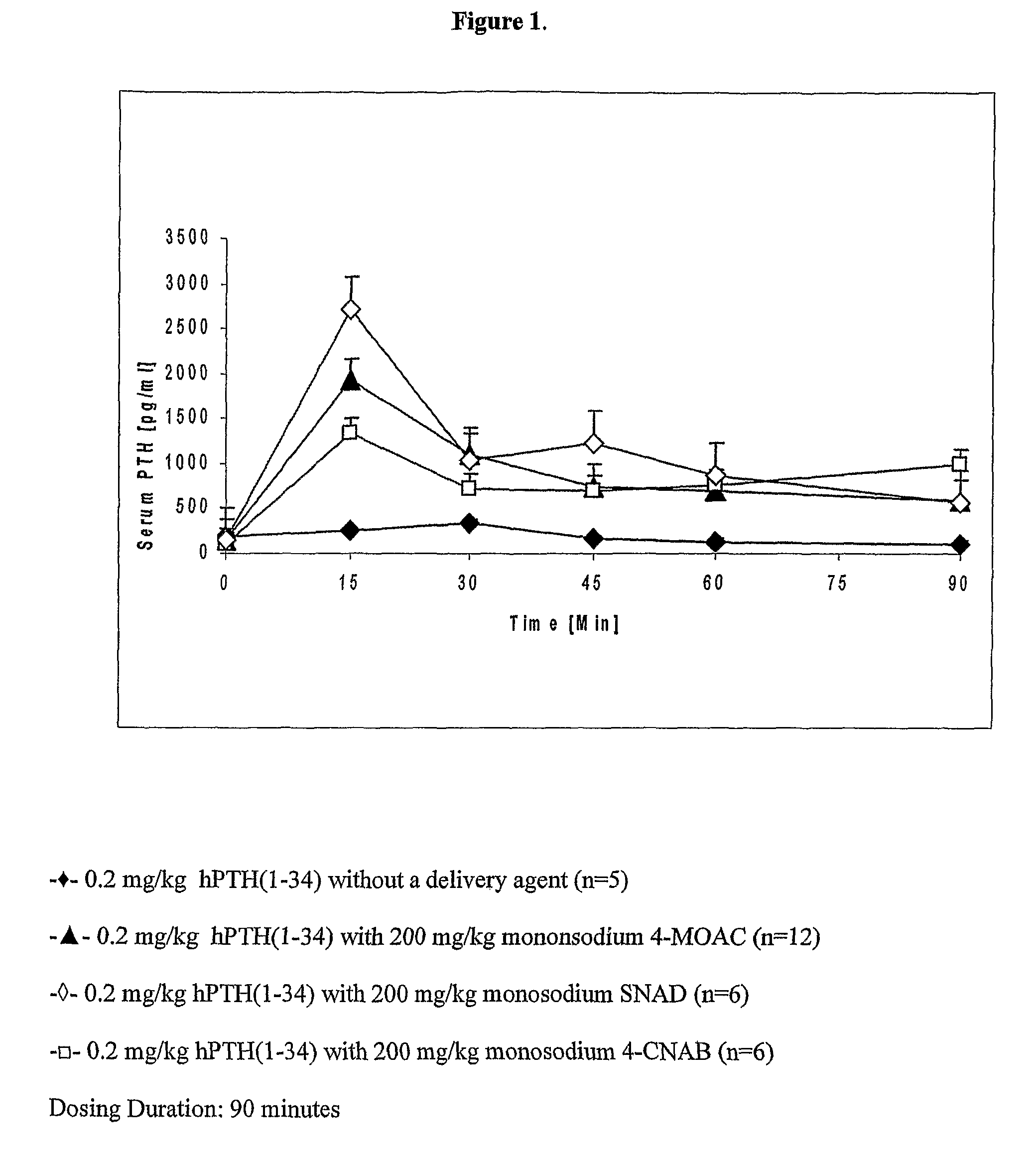
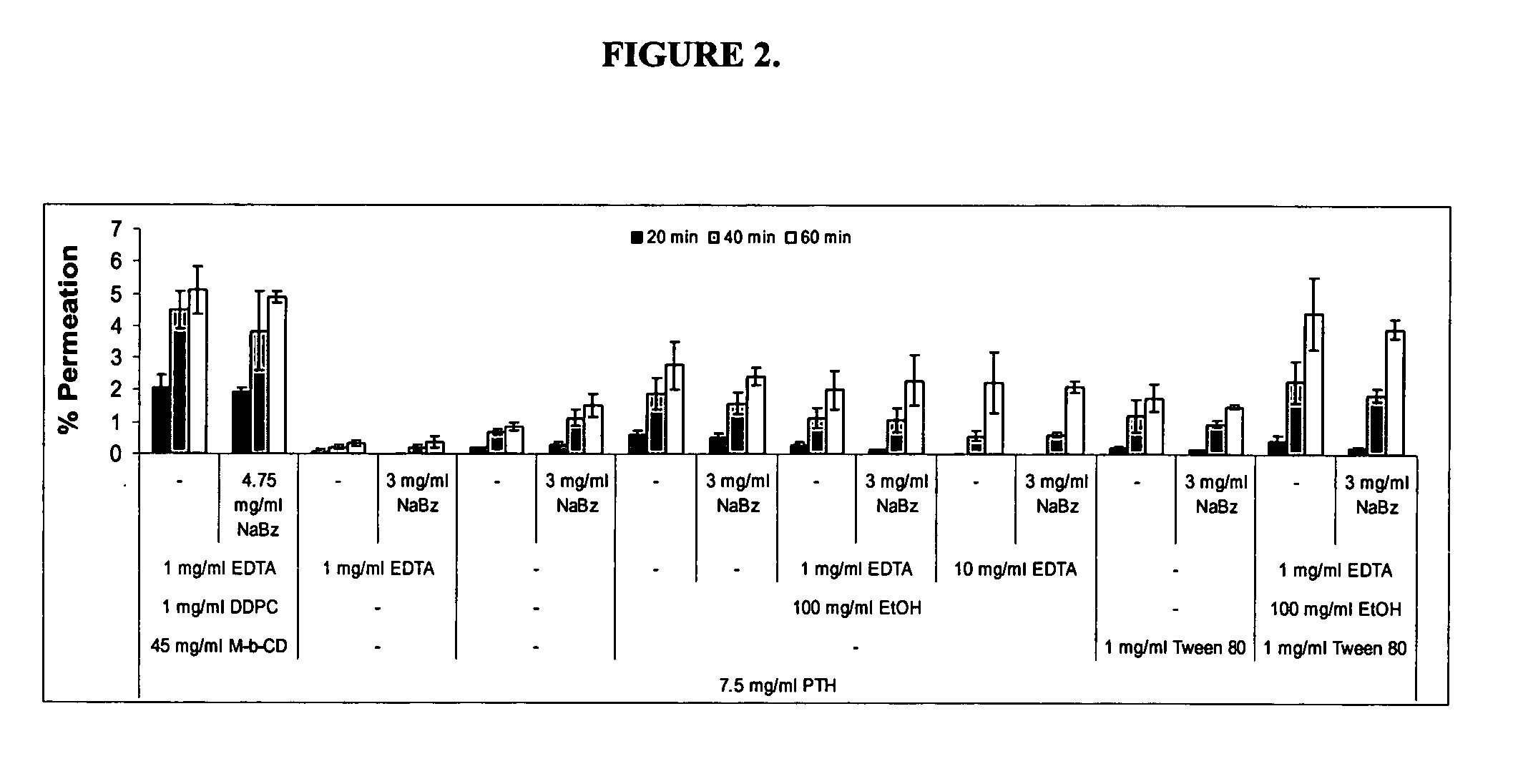
Compositions for buccal delivery of parathyroid hormone
ActiveUS8110547B2Peptide/protein ingredientsSkeletal disorderNormal parathyroid hormoneIntact parathyroid hormone
Compositions and pharmaceutical formulations for buccally delivering parathyroid hormone comprising a) a delivery agent, b) a PTH component and, optionally, c) an antiresorptive agent are provided.
Owner:NOVO NORDISK NORTH AMERICA OPERATIONS AS
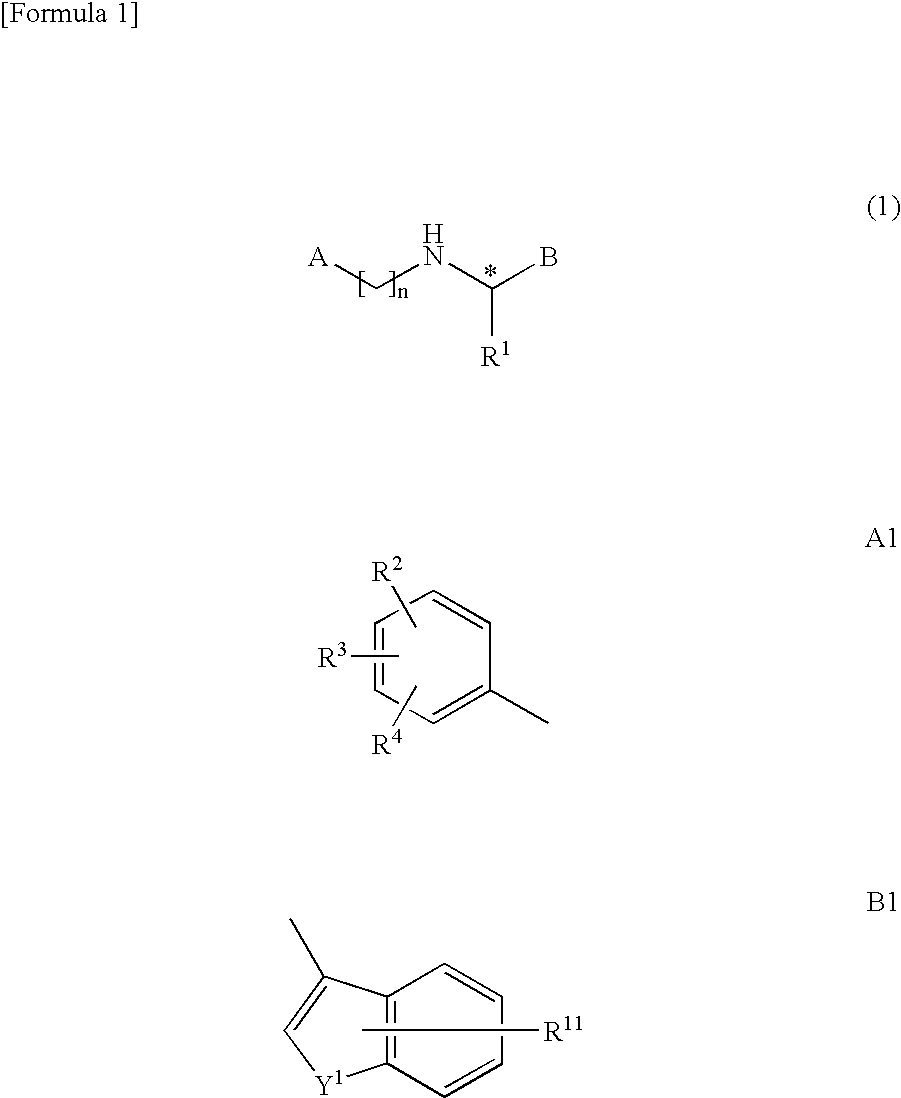
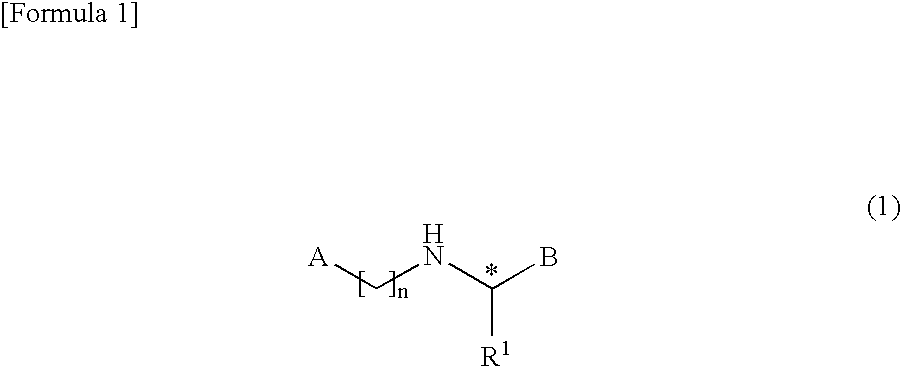
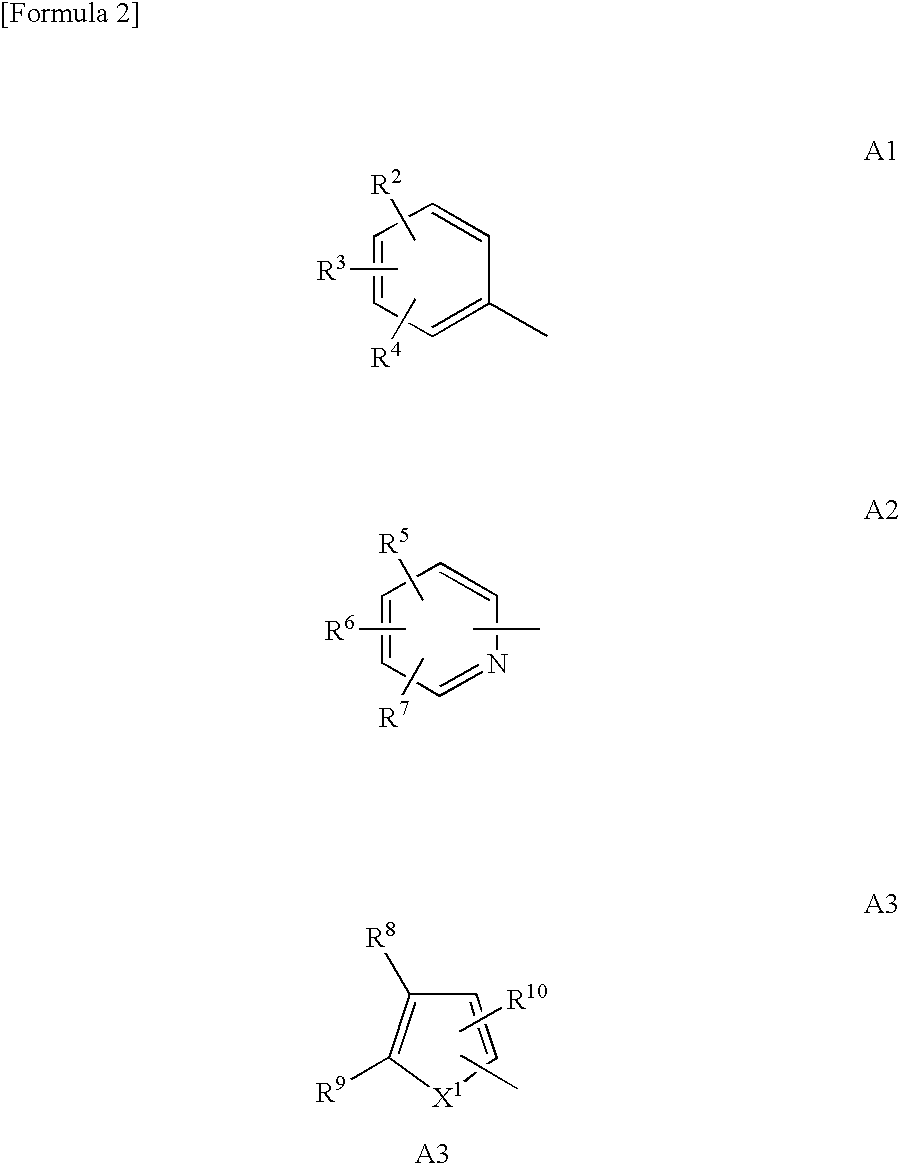
Bicyclic compound
A novel compound represented by the following formula (1) or a salt thereof: wherein symbol “A” represents a saturated heterocyclic group, a 5-membered heteroaromatic group having two heteroatoms in the ring, a group represented by the formula A1 (R2, R3, and R4 represent hydrogen atom, hydroxyl group, etc.), etc., B represents a group represented by the formula B1 (R11 represents hydrogen atom, hydroxyl group, etc.), etc., R1 represents an alkyl group, and symbol “n” represents an integer of 2 to 6, which has a parathyroid hormone depressing action and showing low toxicity, and a medicament containing the compound or salt thereof as an active ingredient.
Owner:ASAHI KASEI PHARMA
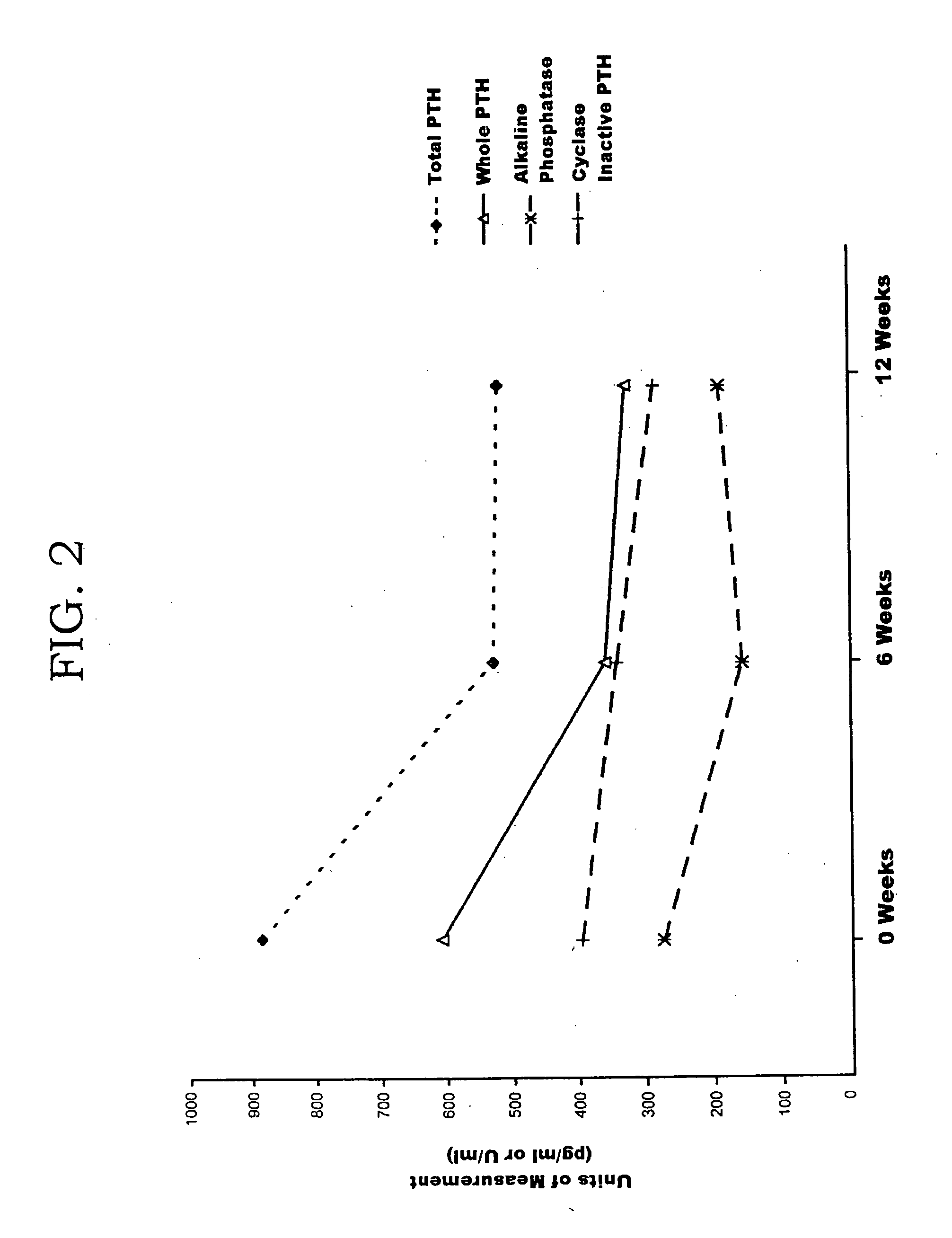
Human parathyroid hormone modifications, preparation and use
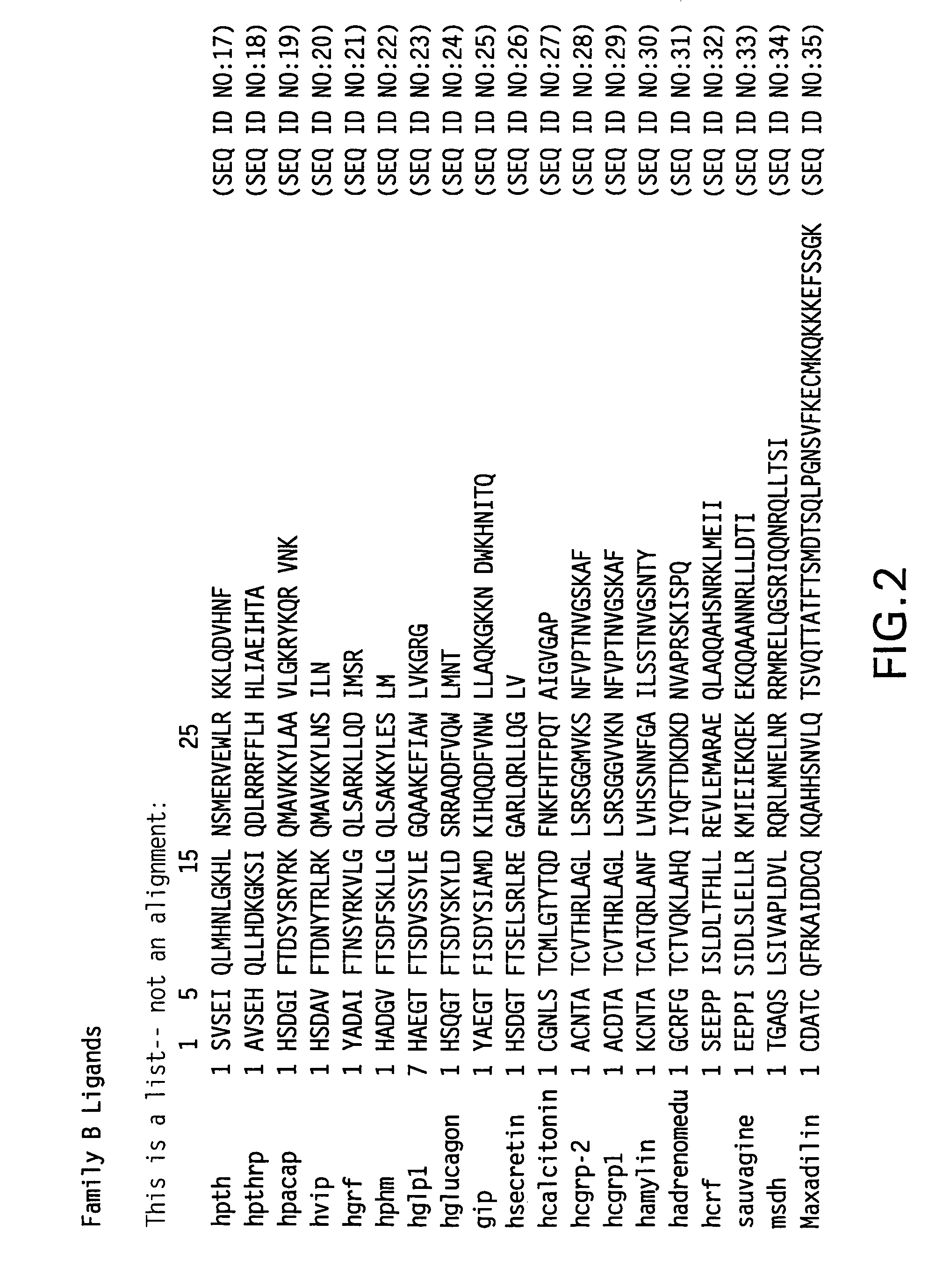
The present invention is related to synthetic and / or recombinant biologically active peptide derivatives of PTH(1-28). Some of the peptides of the invention are at least 90% identical to a peptide consisting essentially of the amino acid sequence X01ValSerGluIleGlnLeuMetHis AsnLeuGlyLysHisLeuAsnSer MetX02ArgValGluTrpLeuArgLysLysLeu (SEQ ID NO:1), wherein X01 is Ser, Ala or Gly; and X02 is Glu or Arg.
Owner:THE GENERAL HOSPITAL CORP
Method of delivering parathyroid hormone to a human
InactiveUS20060127320A1Good chemical stabilityImprove physical stabilityOrganic active ingredientsPeptide/protein ingredientsActuatorBottle
What is described is a method of delivering PTH to a human, comprising exposing a layer of mucosal cells to a mixture of PTH and an enhancer, wherein the enhancer is capable of modulating the barrier function of a cellular tight junction. Specifically, the method of delivering PTH to a human by intranasal administration comprises use of an aqueous solution of growth and excipients in a bottle and a droplet-generating actuator attached to the bottle and fluidly connected to the PTH solution in the container, wherein the actuator produces a spray of the PTH solution through a tip of the actuator when the actuator is engaged, wherein the spray of PTH solution has a spray pattern ellipticity ratio of from about 1.0 to about 1.4 when measured at a height of 3.0 cm from the actuator tip.
Owner:NASTECH PHARMA
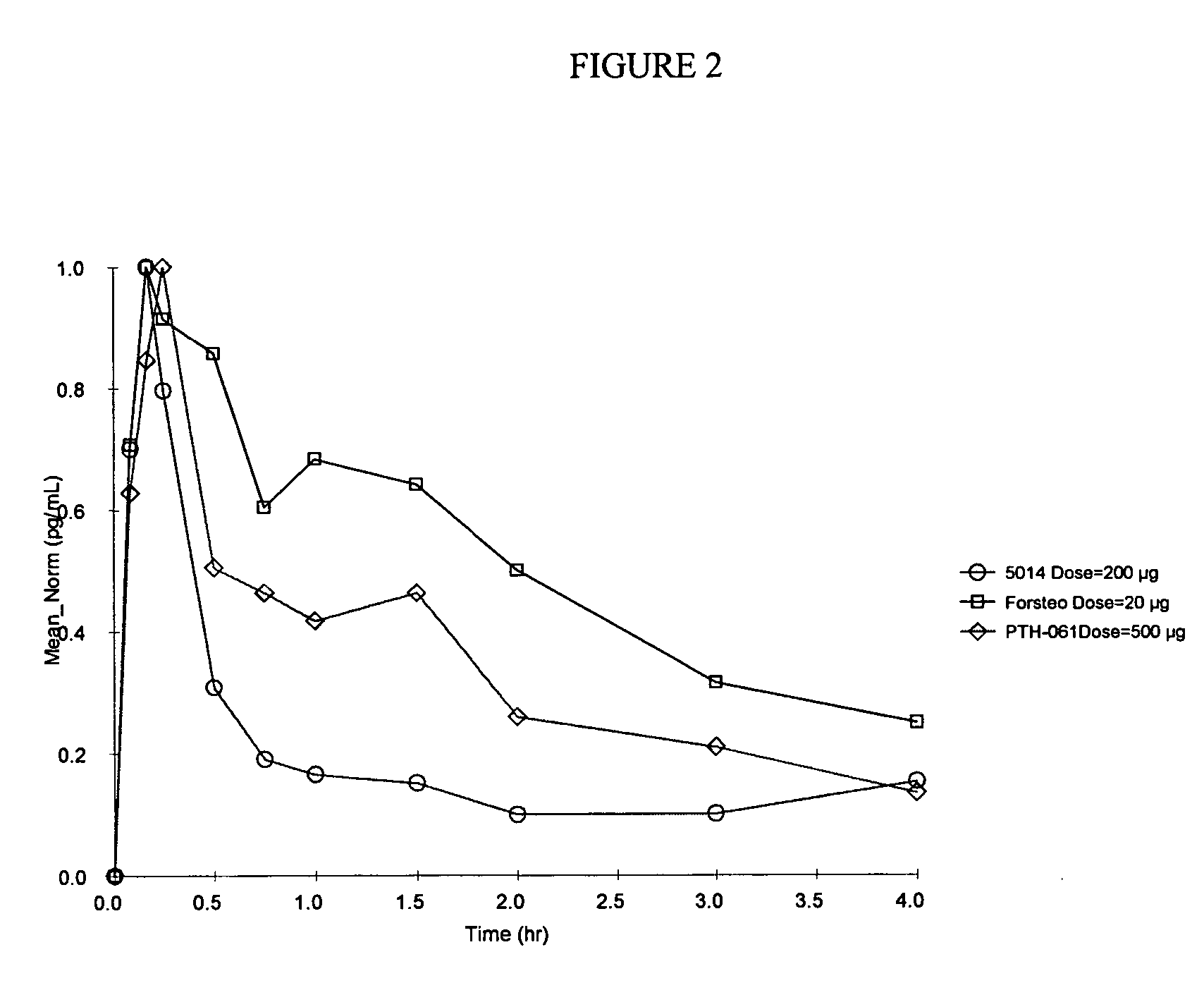
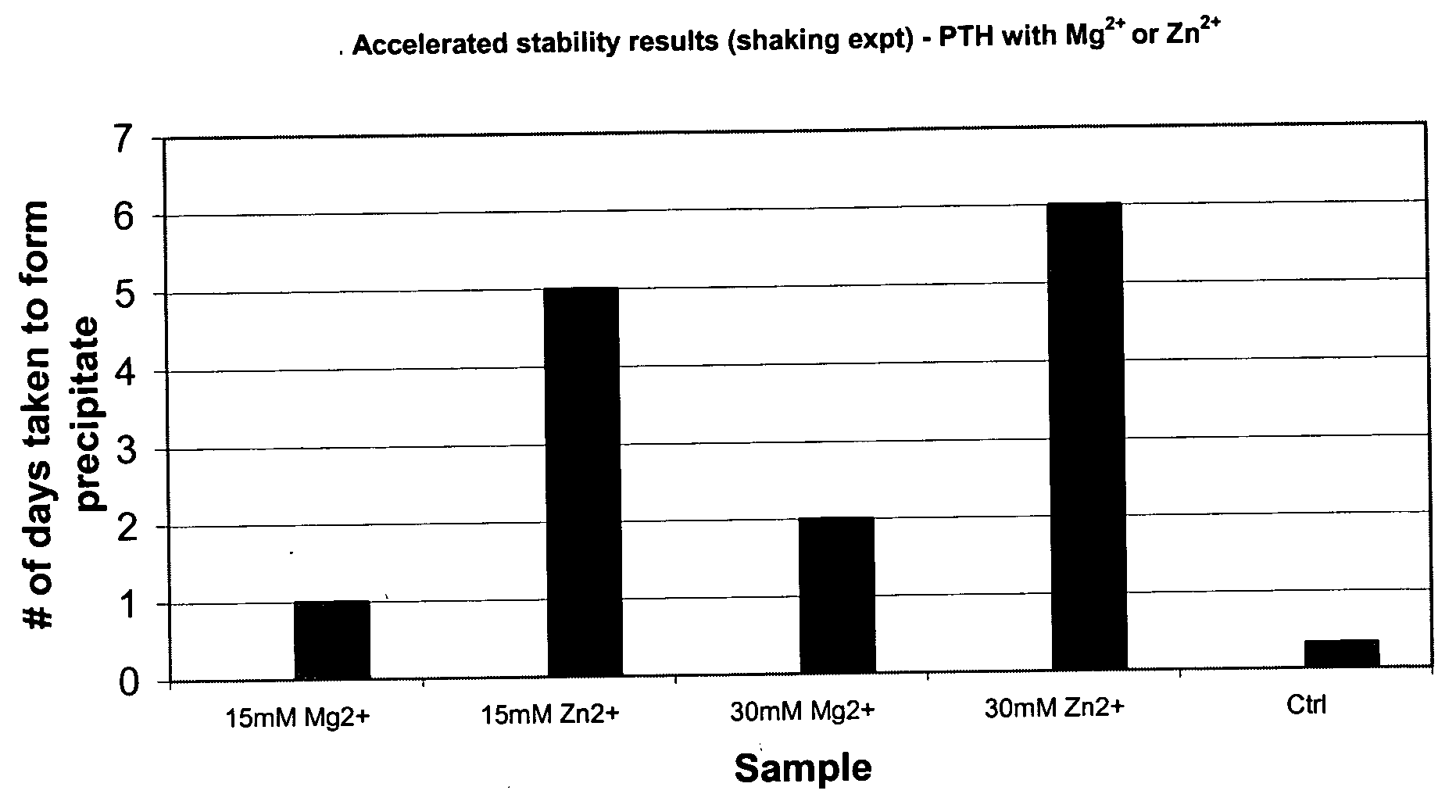
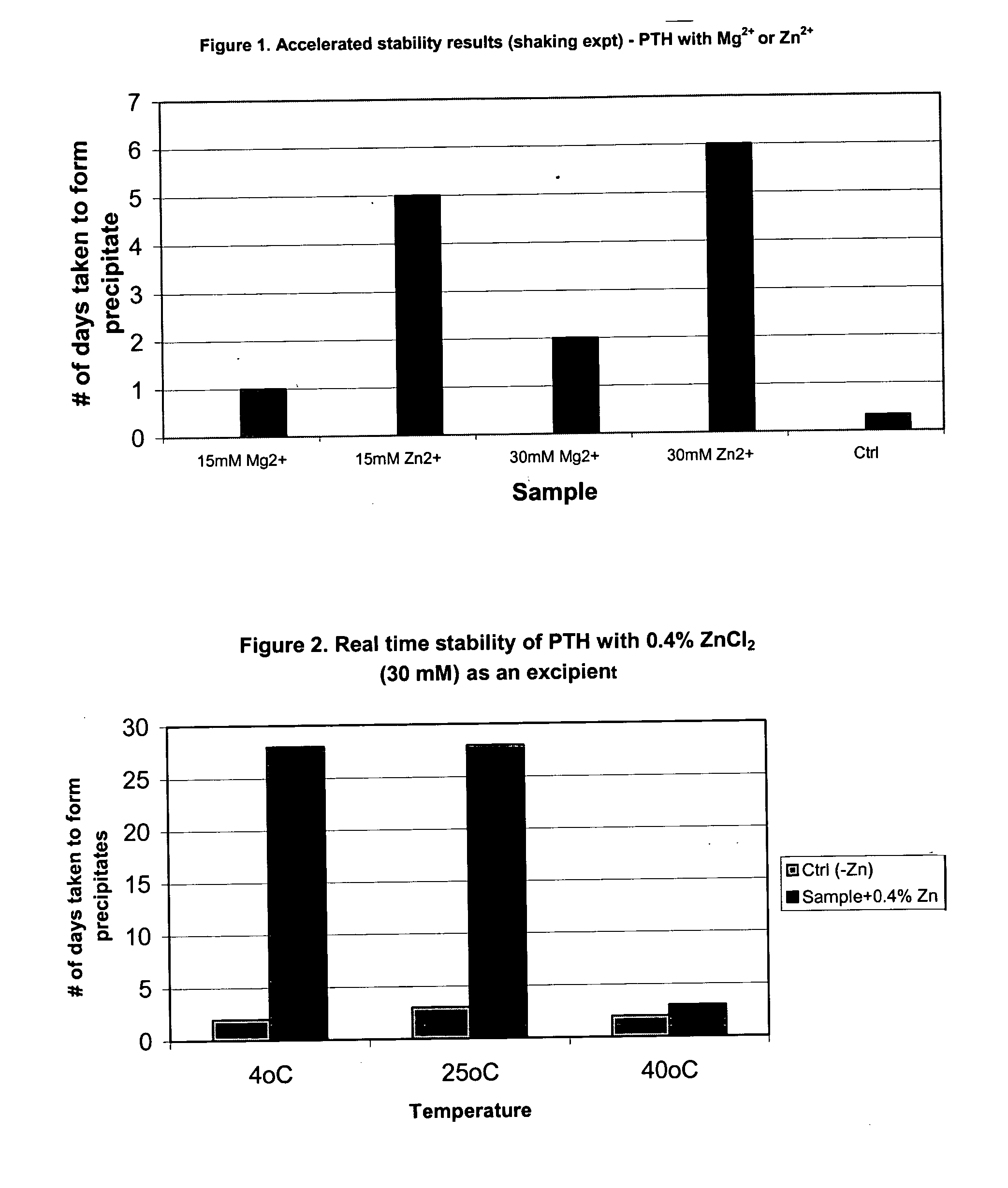
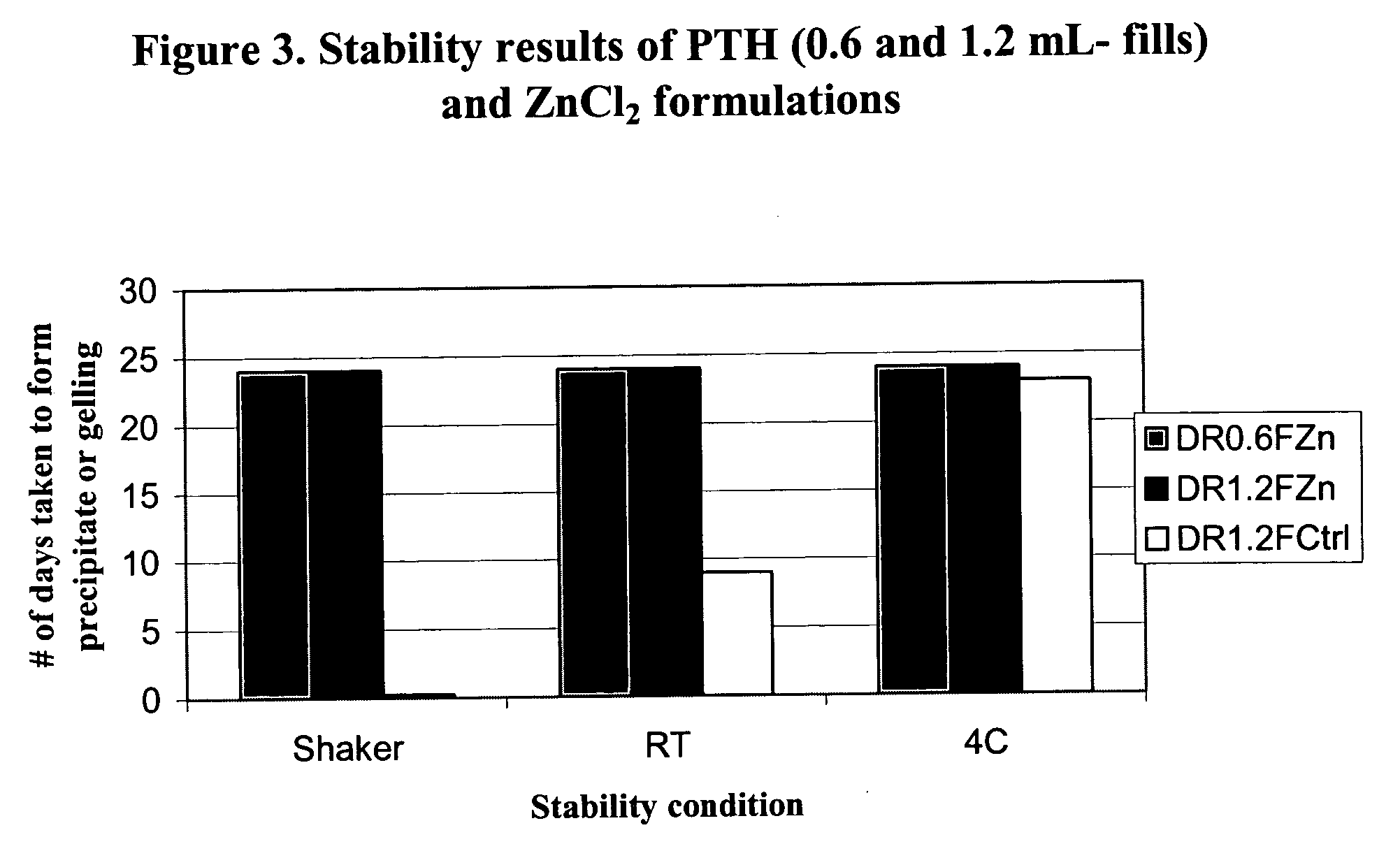
Stabilized formulation of parathyroid hormone
InactiveUS20050032698A1Improve stabilityStabilize the parathyroid hormonePeptide/protein ingredientsInorganic active ingredientsChemistryPARATHYROID HORMONE PREPARATIONS
The invention relates to PTH formulations in which the PTH is present with at least one divalent cation effective to stabilize the PTH.
Owner:NPS PHARM INC
GRAS composition for enhanced mucosal delivery of parathyroid hormone
What is described is an aqueous pharmaceutical composition for intranasal delivery of PTH, comprising a PTH molecule, and one or more excipients selected from the group consisting of a chelating agent, an alcohol, and a surface active agent, wherein the PTH molecule selected from the group consisting of SEQ NO: 1, SEQ NO: 2, and SEQ NO: 3.
Owner:MARINA BIOTECH INC
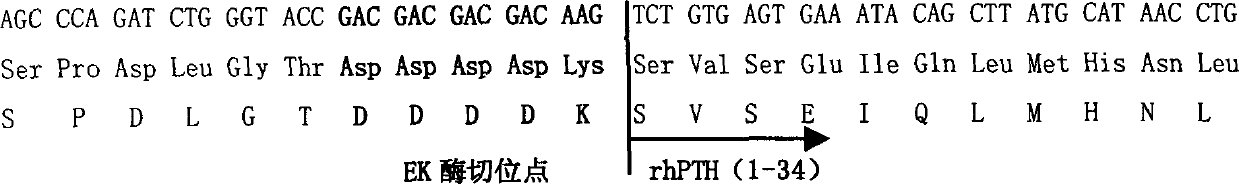
Recombinant human parathormone PTH1-34 preparation method
ActiveCN1807456AHigh expressionEasy to purifyParathyroid hormonesHybrid peptidesGenetic engineeringDNA
The invention provides the method of merged protein which contains recombined human parathormone, coding the merged protein's DNA sequence, the carrier which contains the DNA sequence, the host cell which contains the carrier, using genetic engineering to prepare the merged protein and then produce PTH1-34. Enzyme cutting the merged protein by enterokinase can produce the recombined human parathormone PTH (1-34) has high physiologically active. The expression product is high, purity technology is simple and the cost is depressed, it can be used to the production of recombined human parathormone in force.
Owner:SHANGHAI CELGEN BIO PHARMA CO LTD
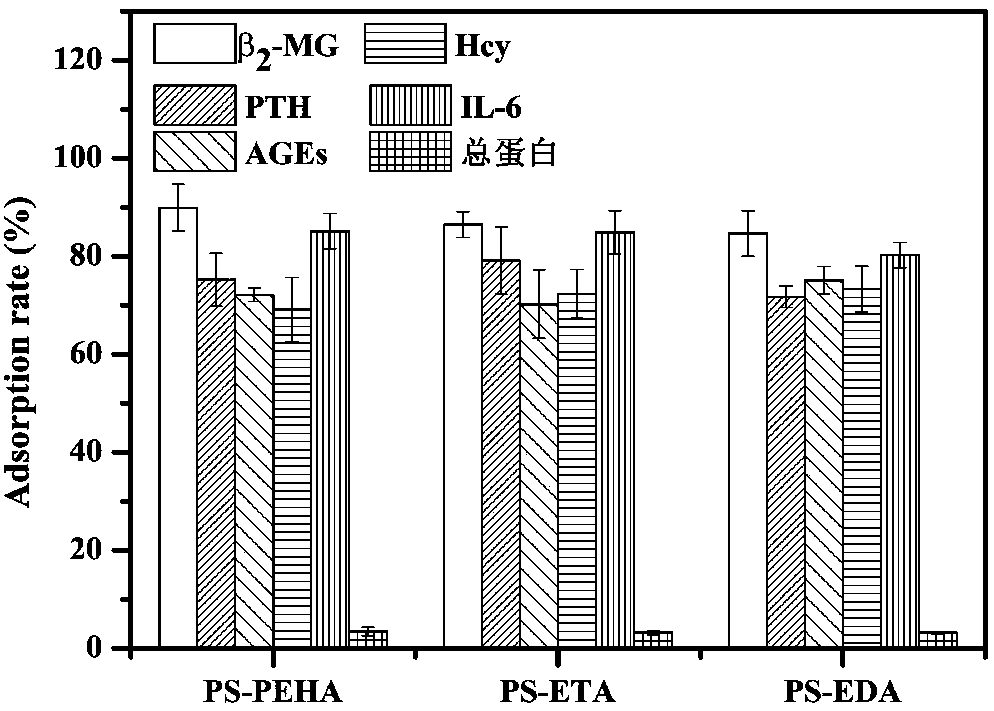
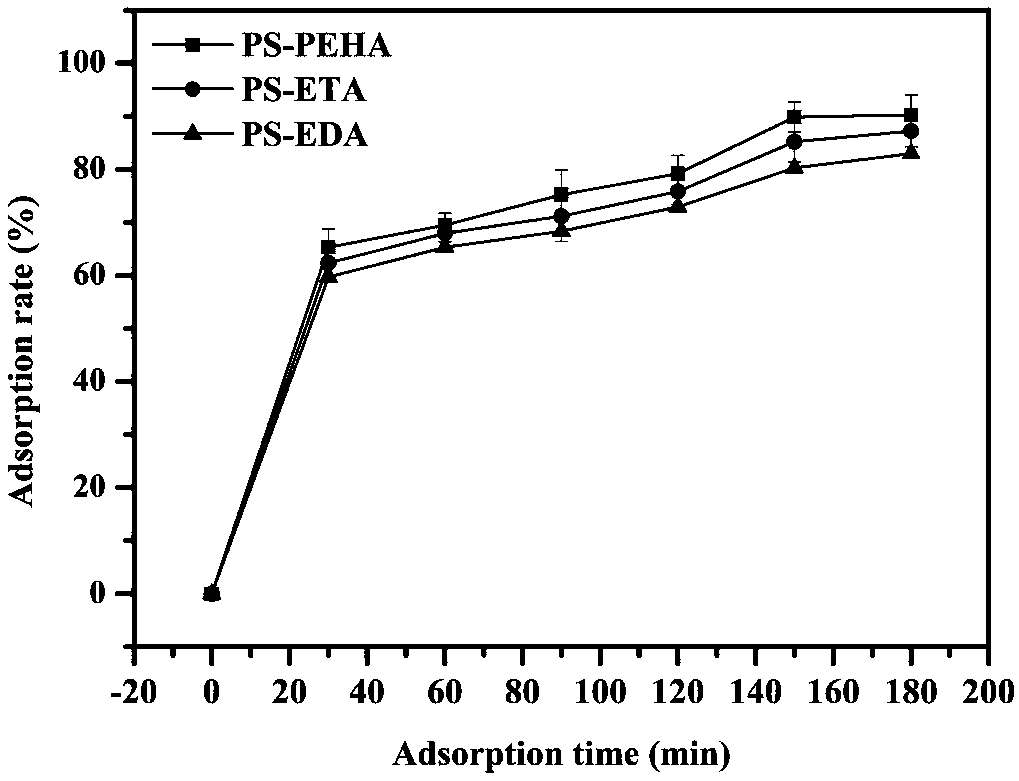
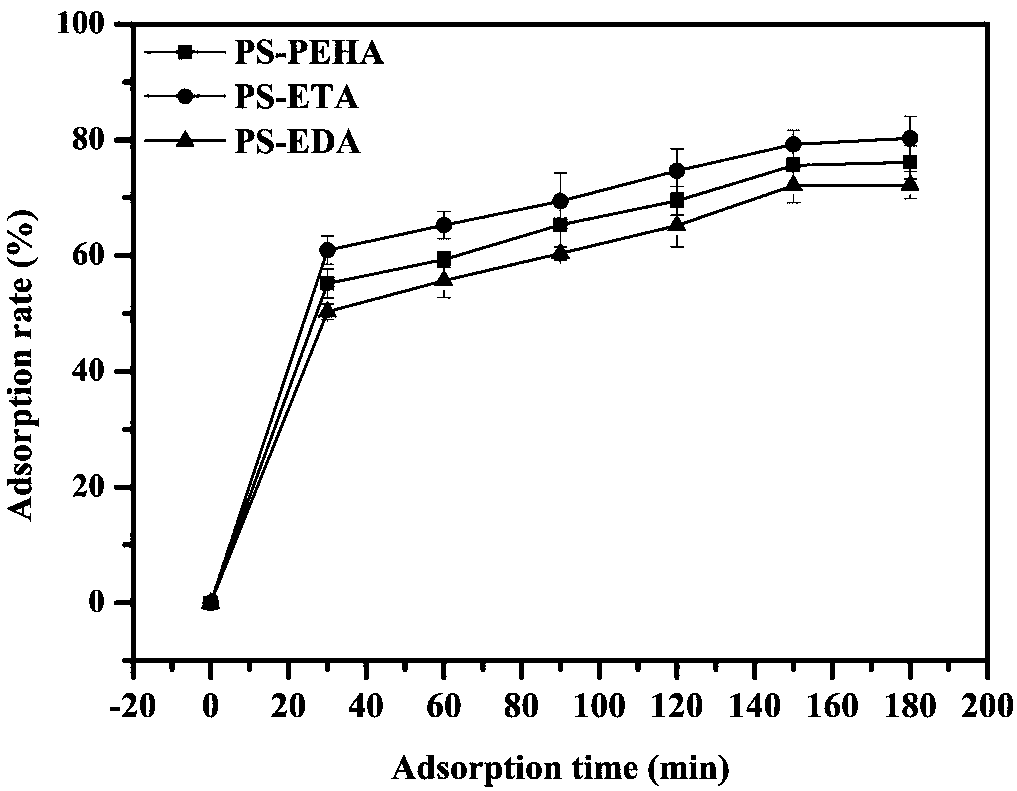
Adsorbent used for clearing middle and macro molecule toxin in body of uremia patients and preparation method thereof
ActiveCN108371945AEfficient removalHigh adsorption selectivityOther chemical processesOther blood circulation devicesSorbentPolystyrene
The invention relates to an adsorbent used for clearing middle and macro molecule toxin in body of uremia patients and a preparation method thereof. According to the molecule size of middle and macromolecules such as beta2-MG, PTH, AGEs, Hcy and IL-6 and performance characteristics thereof, a functional adsorbent which can efficiently clear main middle and macro molecule toxins such as beta2-MG,PTH, AGEs, Hcy and IL-6 in the blood of the uremia patients is prepared by taking a mesoporous polystyrene hydrophobic resin as a carrier, which undergoes chloromethylation activation, and then performing chemical crosslinking grafting of vinylamine; and the adsorbent has large adsorption capacity, good mechanical strength, good biocompatibility and high adsorption selectivity. The adsorbent is used for treating uremia by blood perfusion, can take the advantages of blood filtering mainly for clearing micromolecular toxin, and provides a new method for treating uremia by combination of hematodialysis and blood perfusion.
Owner:山东卓逸医疗科技股份有限公司
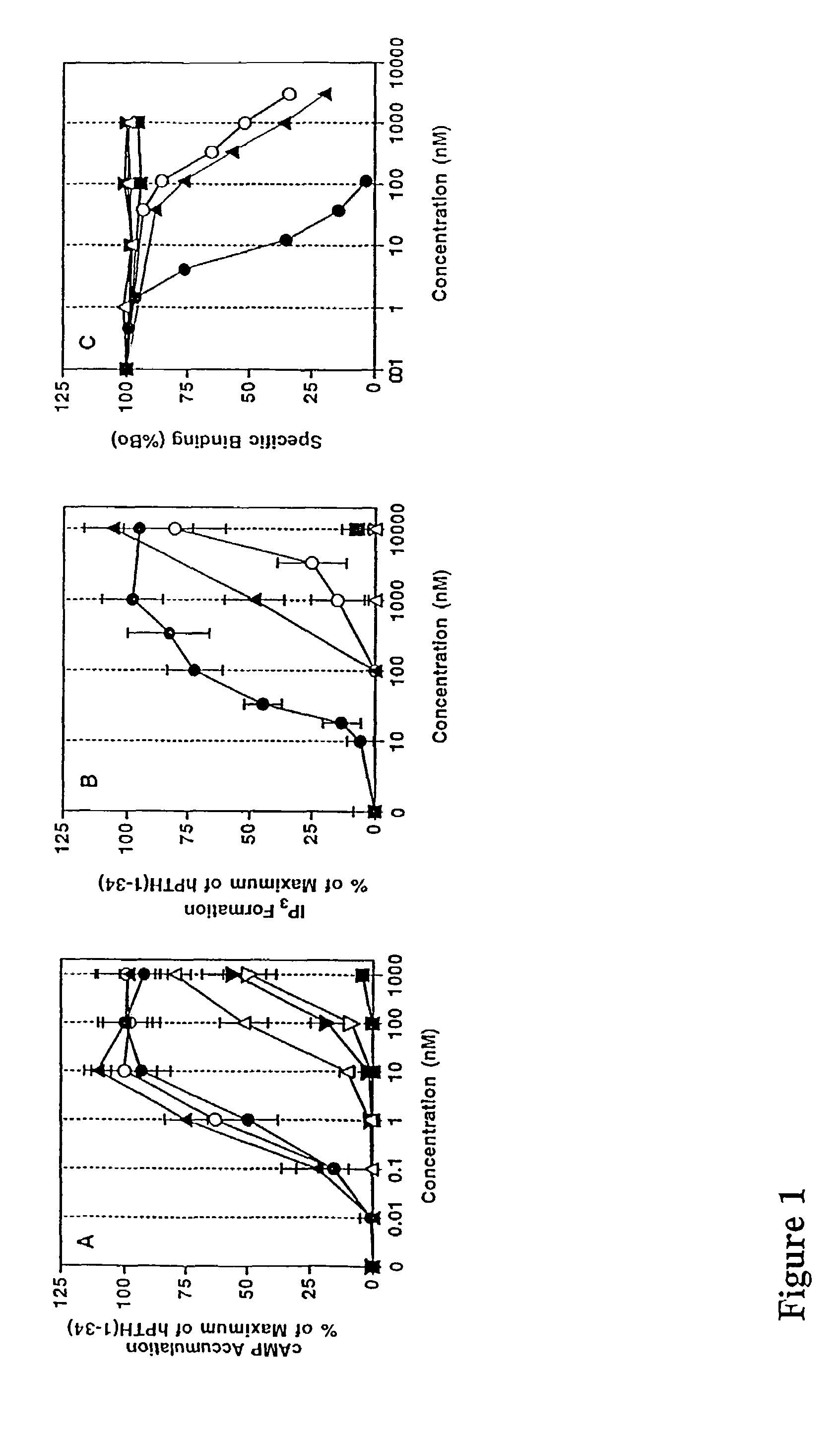
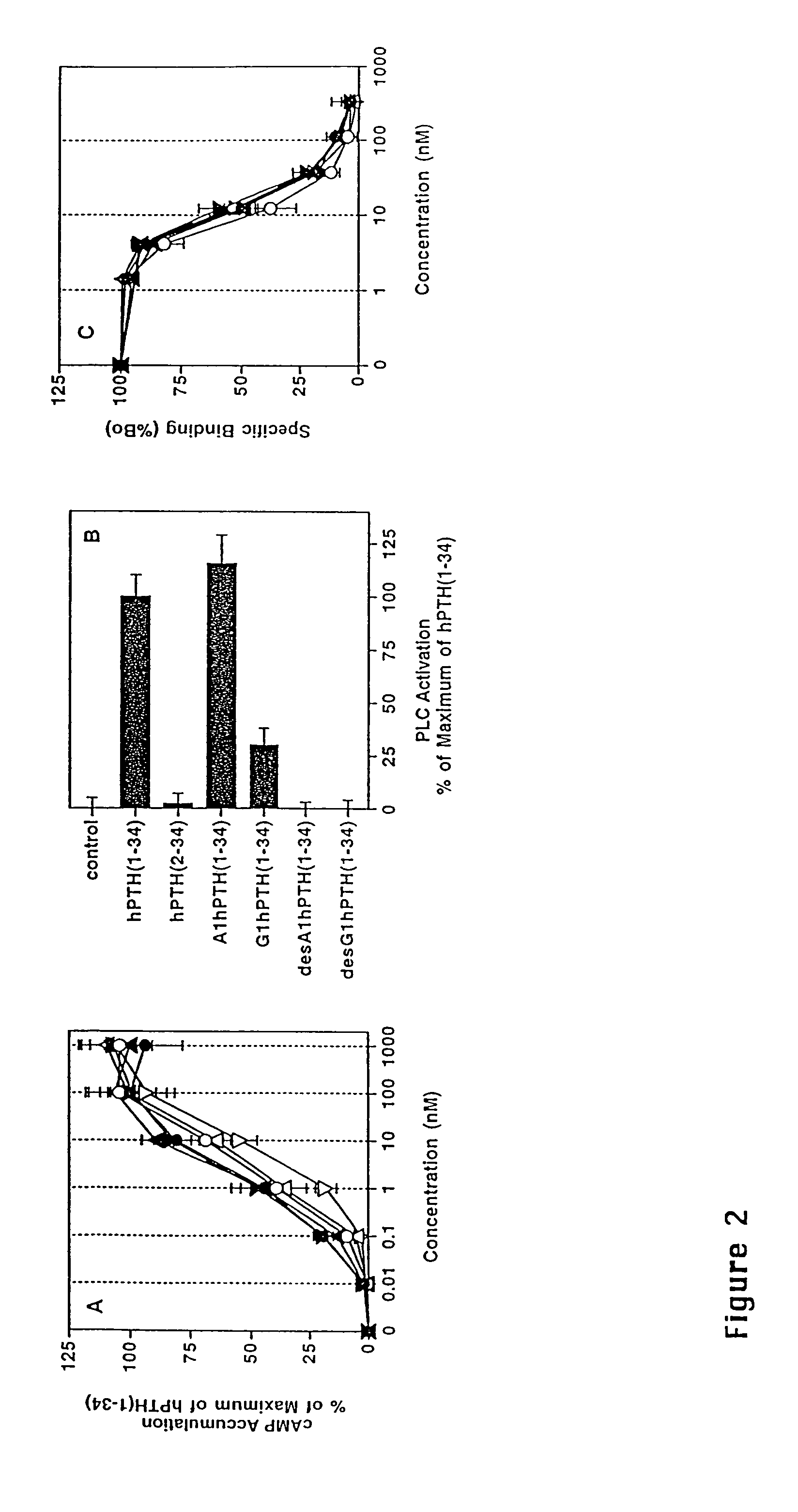
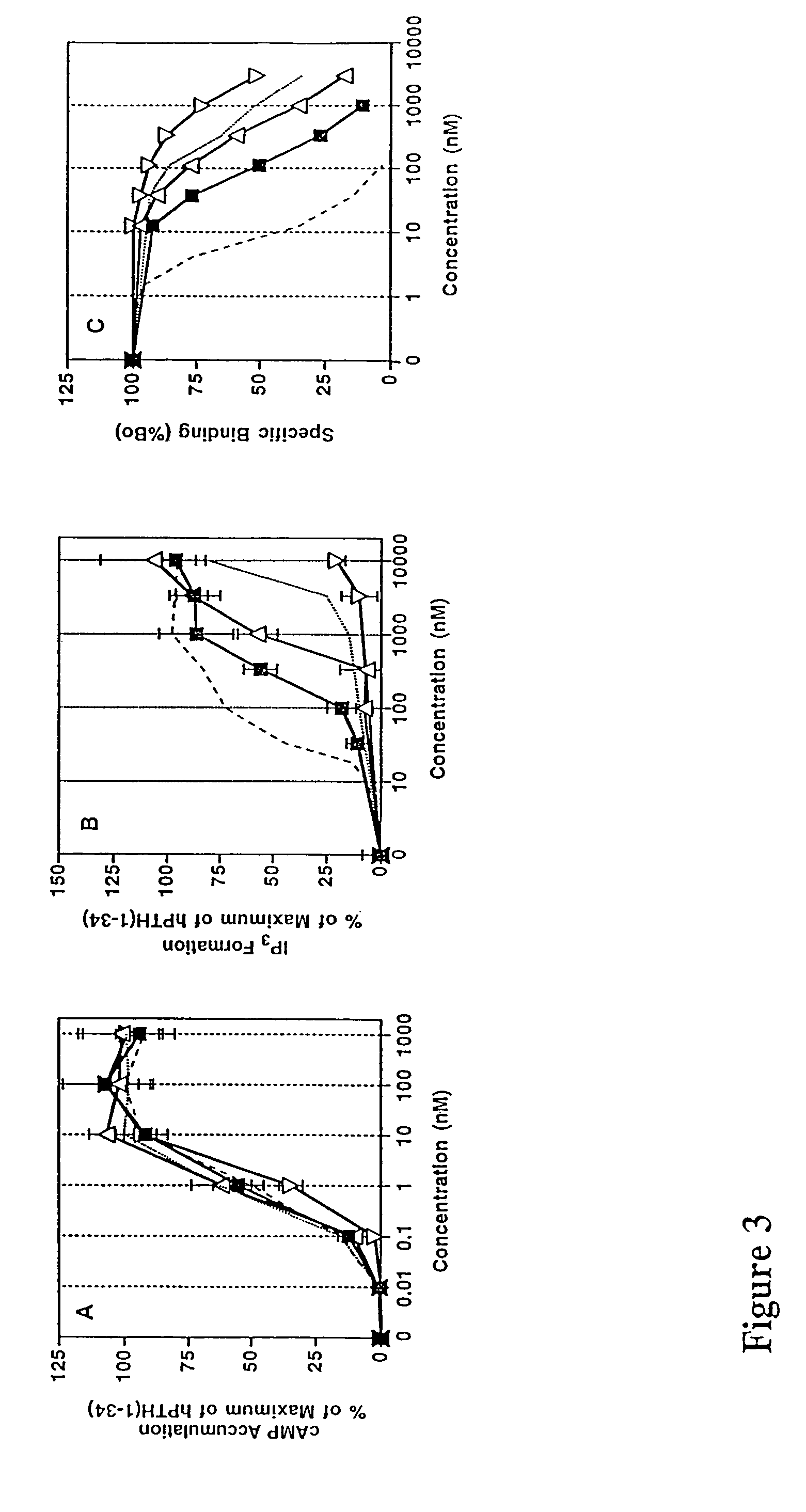
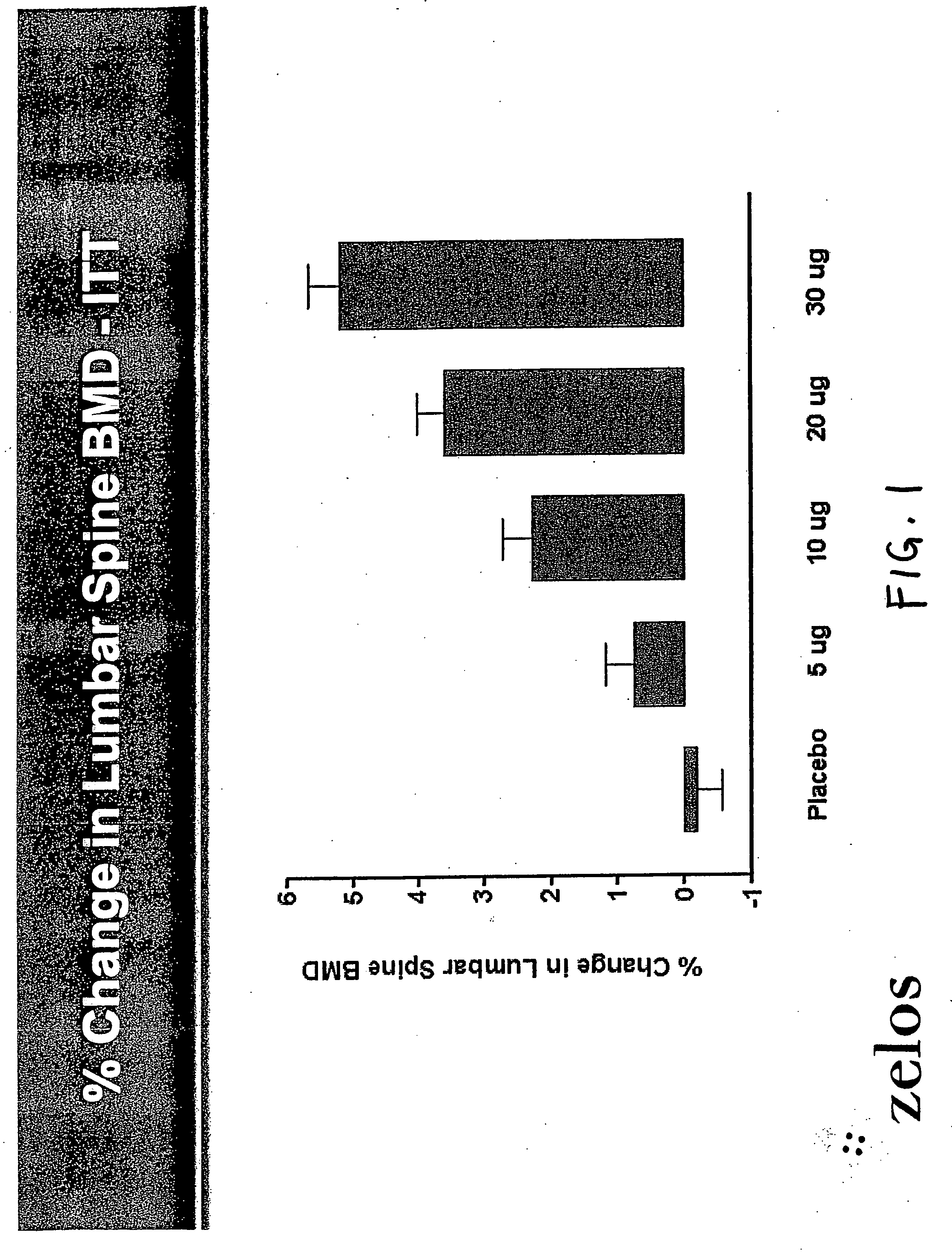
Parathyroid hormone analogues and methods of use
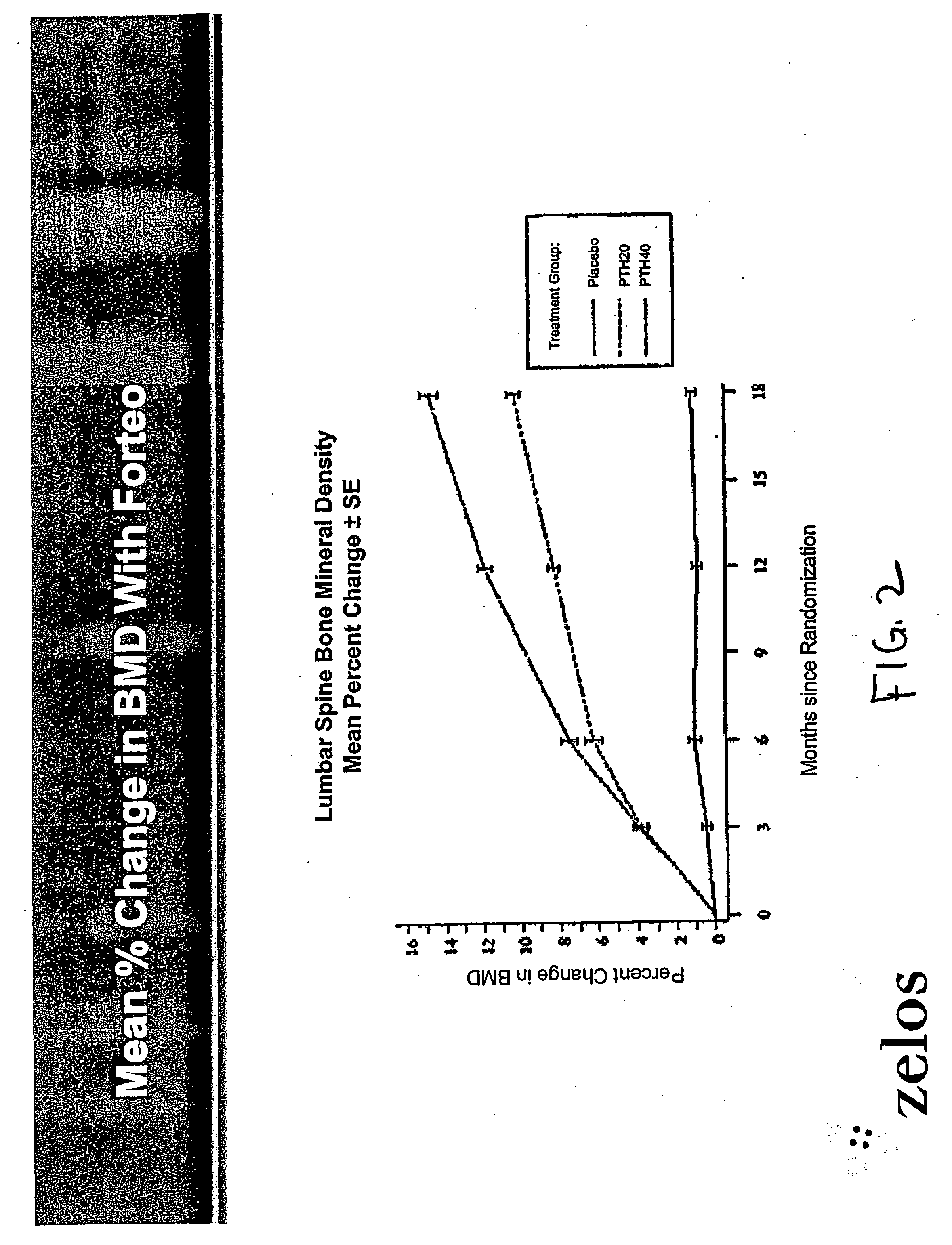
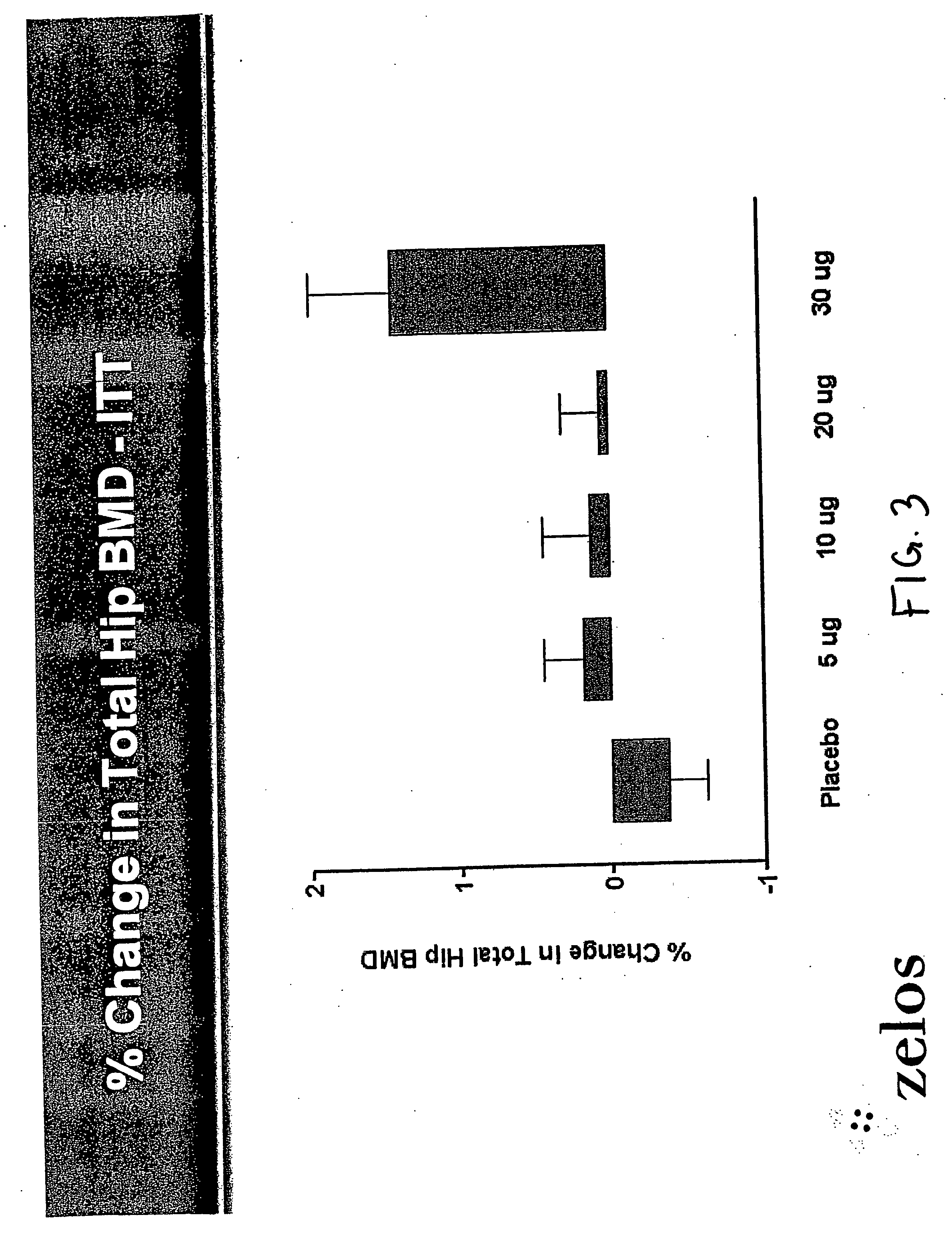
InactiveUS20070099831A1Increasing bone mineral densityRestore boneBiocideOrganic active ingredientsPhospholipaseParathyroid Hormone Analogue
The present invention is directed to novel methods of treating a subject with a bone deficit disorder. The methods generally include administering to a subject in need thereof a pharmaceutically acceptable formulation comprising a parathyroid hormone (PTH) peptide analogue in a daily dose of 2 μg to 60 μg, wherein said PTH peptide analogue has a reduced phospholipase-C activity and maintains adenylate cyclase activity.
Owner:ZELOS THERAPEUTICS
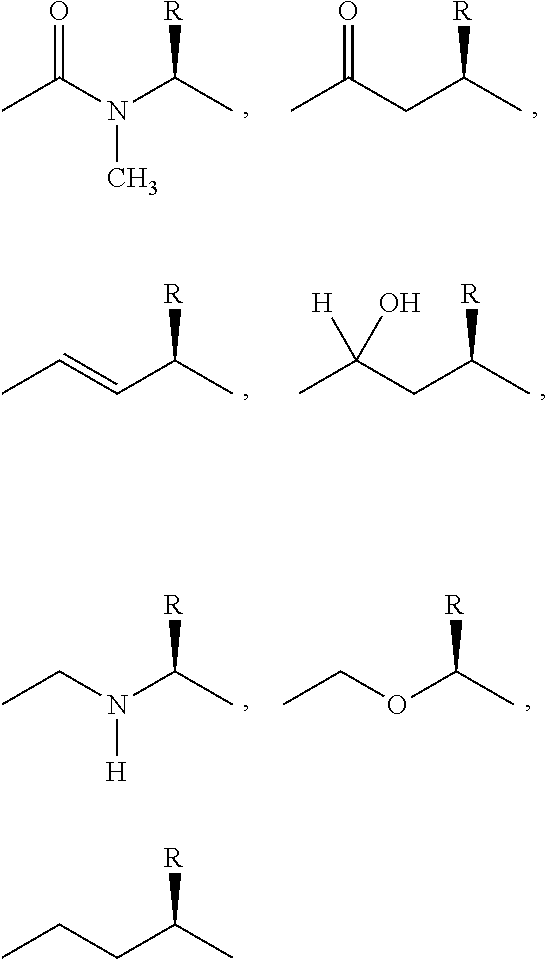
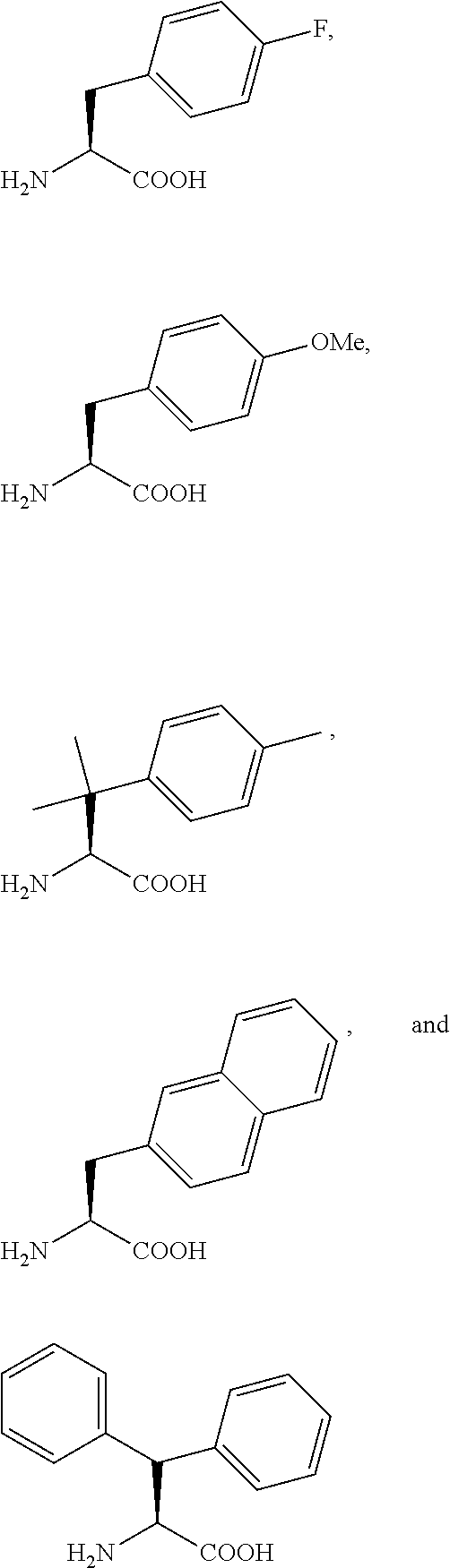
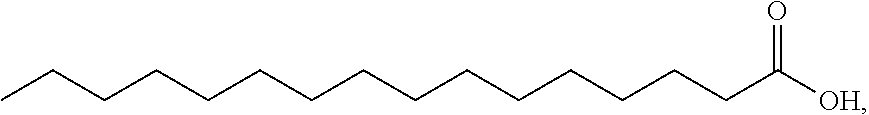
PYRIDO [4,3-d] PYRIMIDIN-4 (3H) -ONE DERIVATIVES AS CALCIUM RECEPTOR ANTAGONISTS
The present invention is directed to novel pyrido[4,3-d]pyrimidin-4(3H)-one derivatives and pharmaceutically acceptable salts thereof of structural formula I wherein the variables R1, R2, R3, R4 and R5 are as described herein. Also provided are pharmaceutical compositions comprising the compounds of formula I as well as methods of treatment employing compounds of formula I to treat a disease or disorder characterized by abnormal bone or mineral homeostasis such as hypoparathyroidism, osteoporosis, osteopenia, periodontal disease, Paget's disease, bone fracture, osteoarthritis, rheumatoid arthritis, and humoral hypercalcemia of malignancy.
Owner:PFIZER INC
Nucleic acids encoding parathyroid hormone (PTH) derivatives
Owner:THE GENERAL HOSPITAL CORP
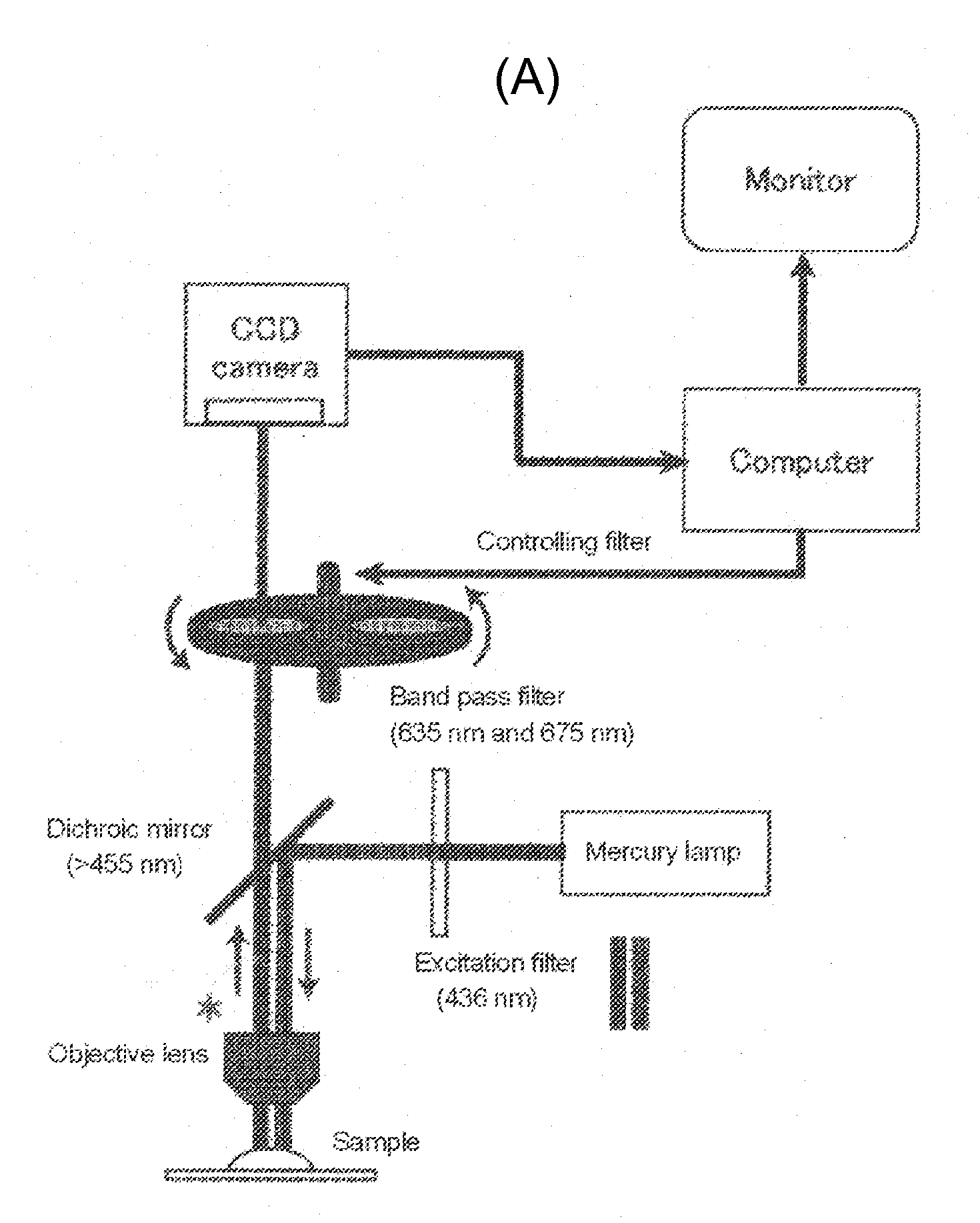
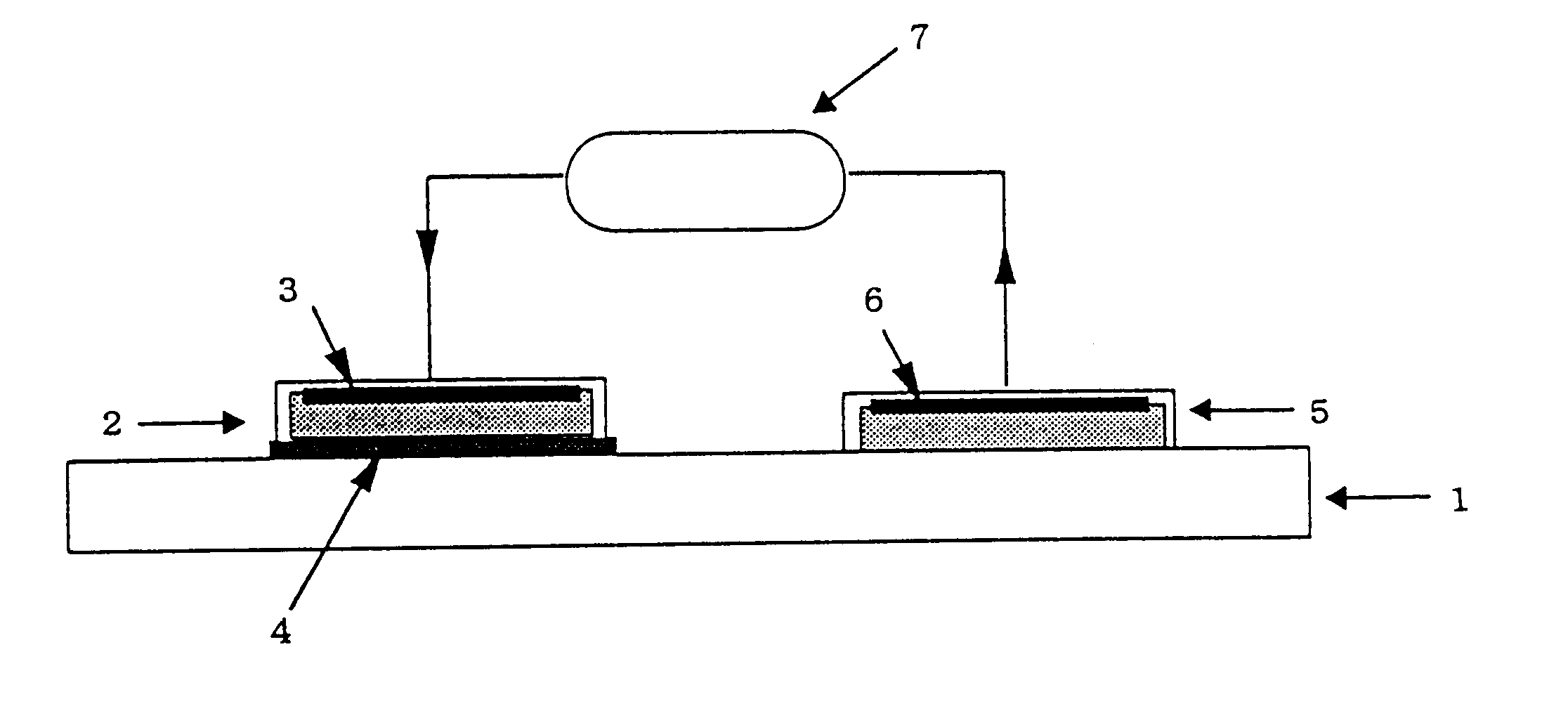
Tumor site or parathyroid gland identification device and method
InactiveUS20140163391A1Easy diagnosisImproved surgical outcomeMaterial analysis by optical meansEndoscopesLight irradiationMedicine
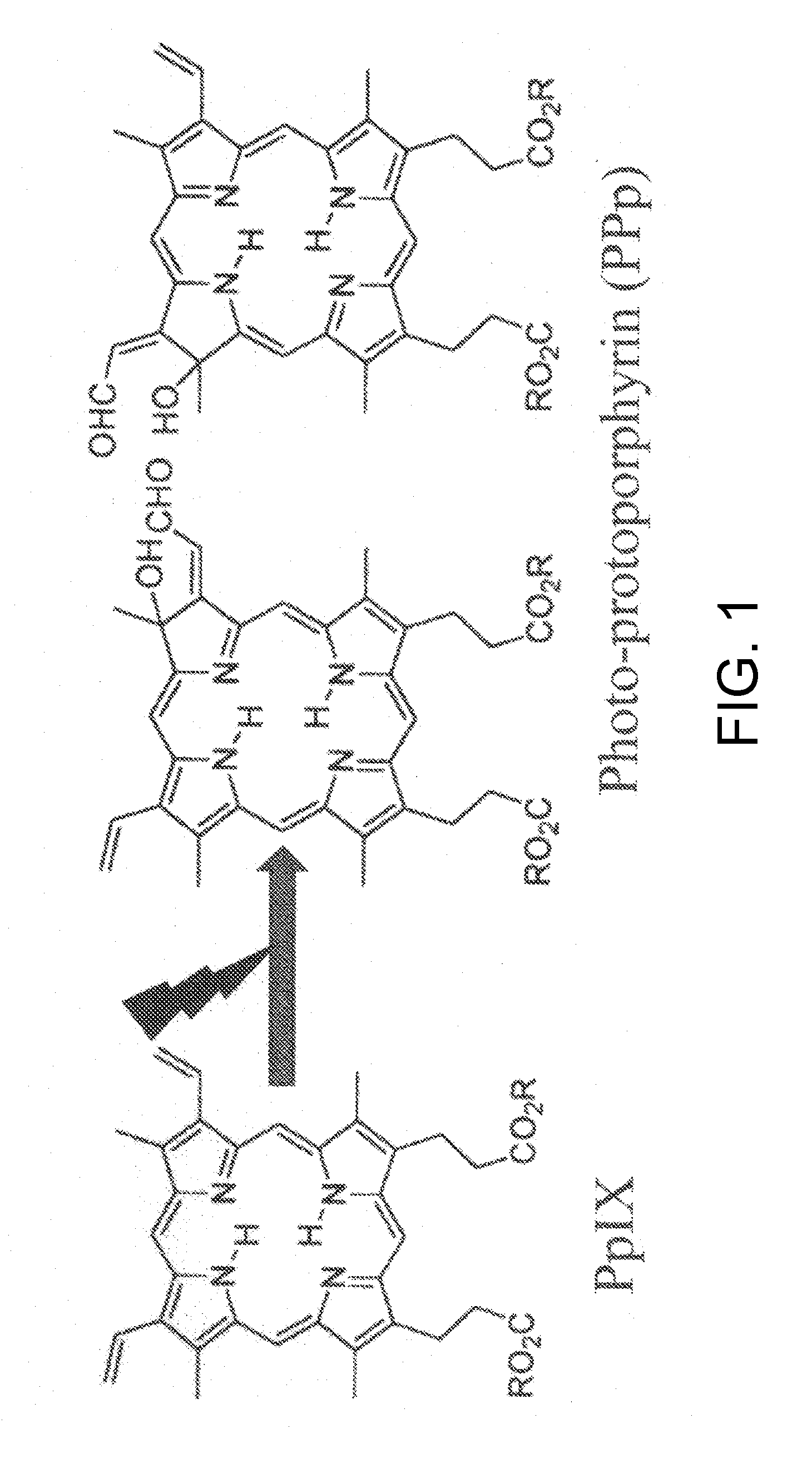
The present invention provides a device for identifying a tumor site in a subject, the device spectroscopically detecting fluorescence of protoporphyrins present in the tumor site,the protoporphyrins being protoporphyrin IX (PpIX) and photo-protoporphyrin (PPp), andthe device comprising:a light irradiation unit that converts part of PpIX into PPp;a spectroscopy unit that separates PpIX fluorescence and PPp fluorescence:a spectroscopy detection unit that detects the relative fluorescence intensity of the PpIX fluorescence and the PPp fluorescence; anda tumor discrimination unit that discriminates between the tumor site and a non-tumor site based on the relative fluorescence intensity of PpIX and PPp.
Owner:KYOTO PREFECTURAL PUBLIC UNIV CORP
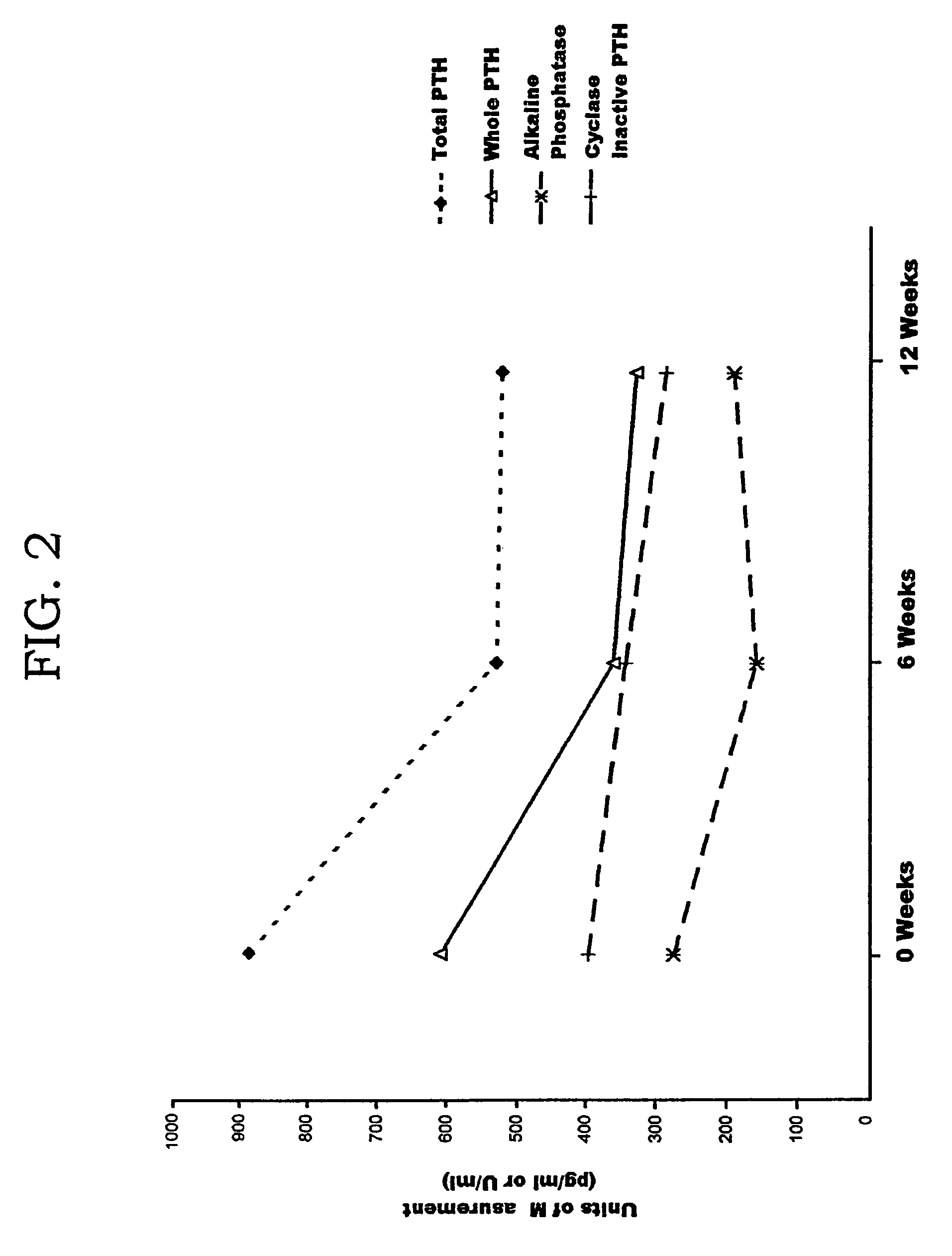
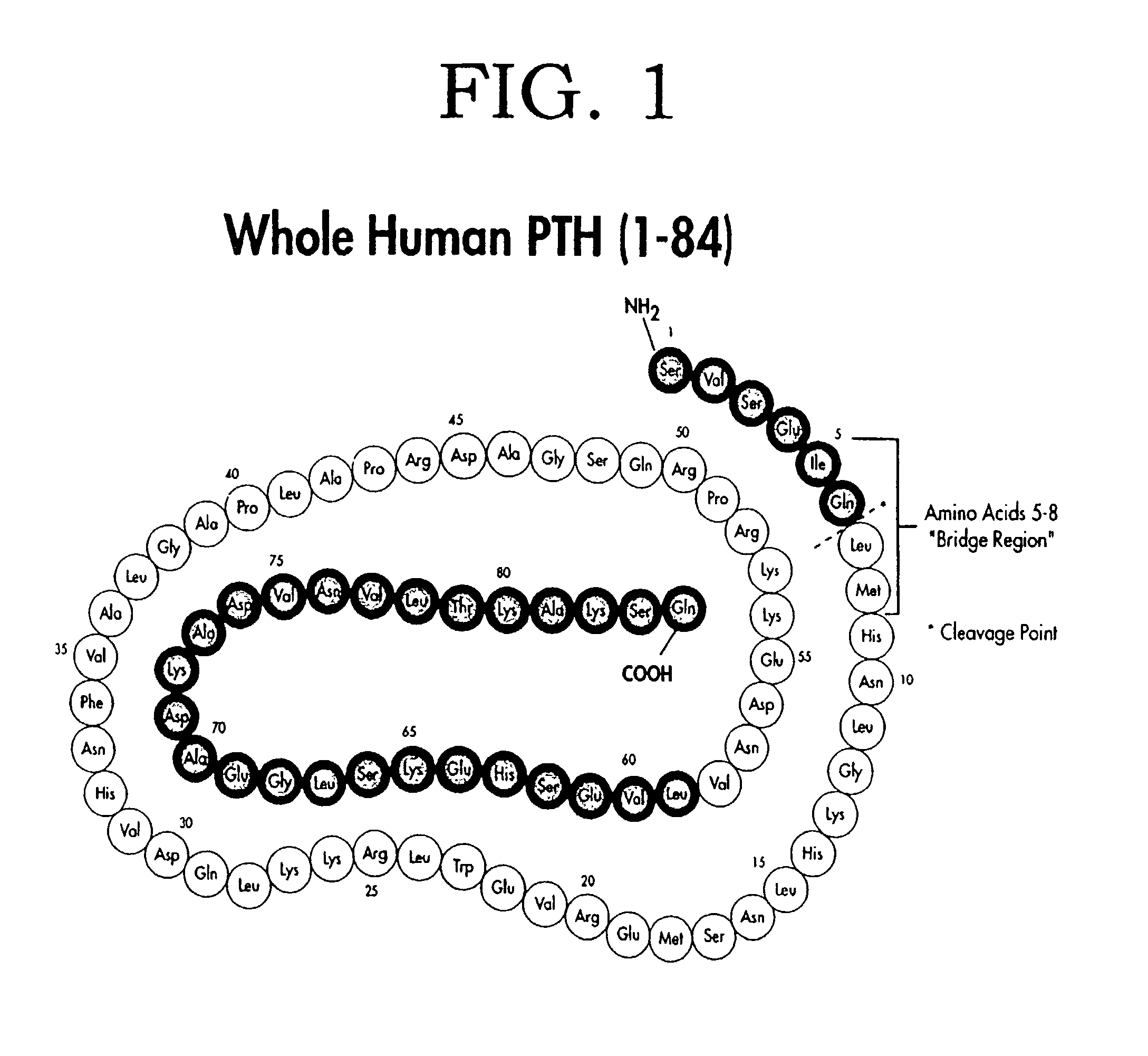
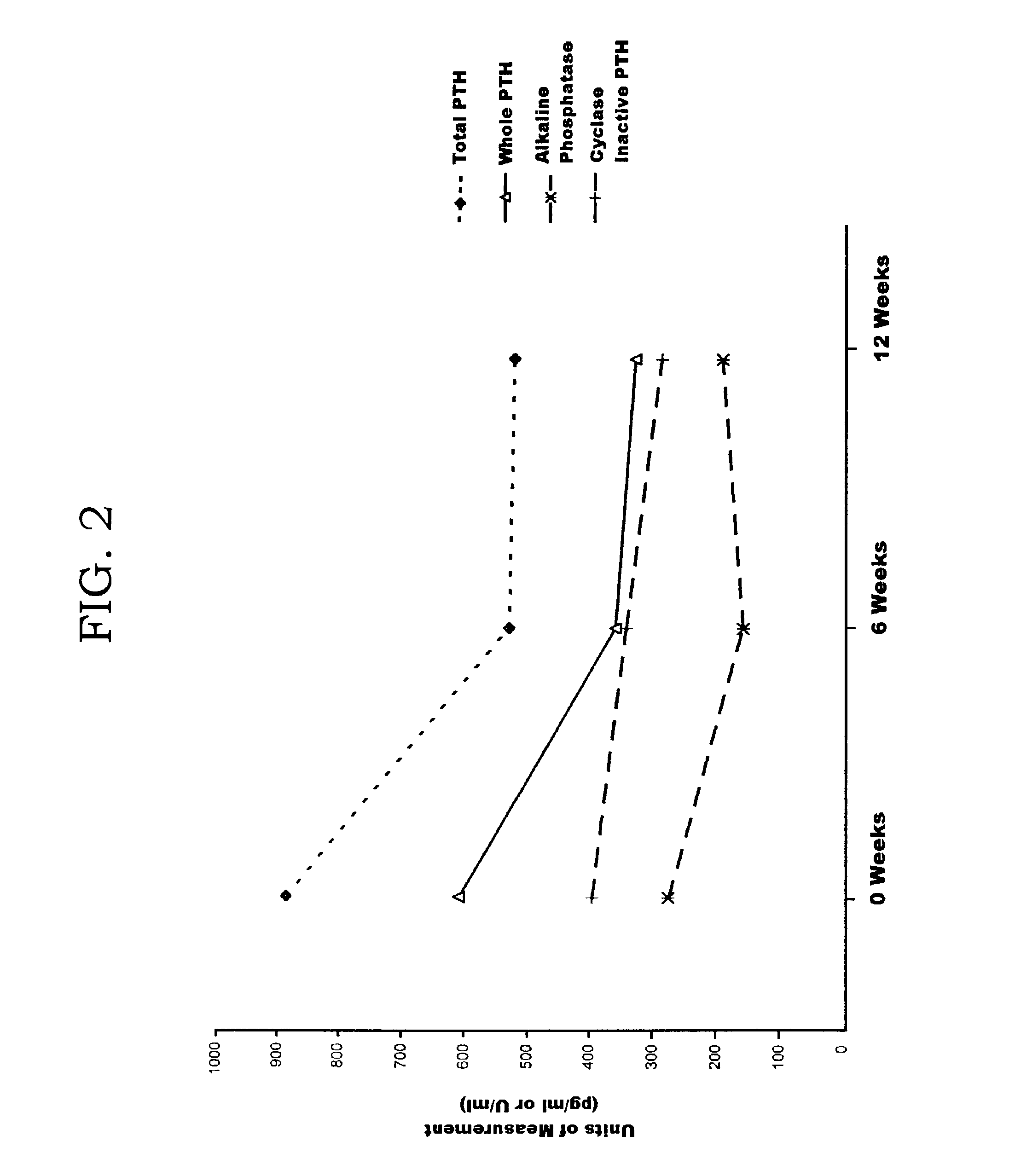
Methods and controls for monitoring assay quality and accuracy in parathyroid hormone measurement
InactiveUS7459276B2Extended shelf/storageImprove accuracyBiological material analysisBiological testingPhysiologyParathyroid hormone measurement
The present invention relates to the use of control compositions and kits comprising such to evaluate and monitor the consistency of assays utilized to determine parathyroid hormone levels.
Owner:SCANTIBODIES LAB
Methods for differentiating and monitoring parathyroid and bone status related diseases
InactiveUS20050095631A1Inhibition releaseMicrobiological testing/measurementDisease diagnosisPeptide fragmentParathyroid disease
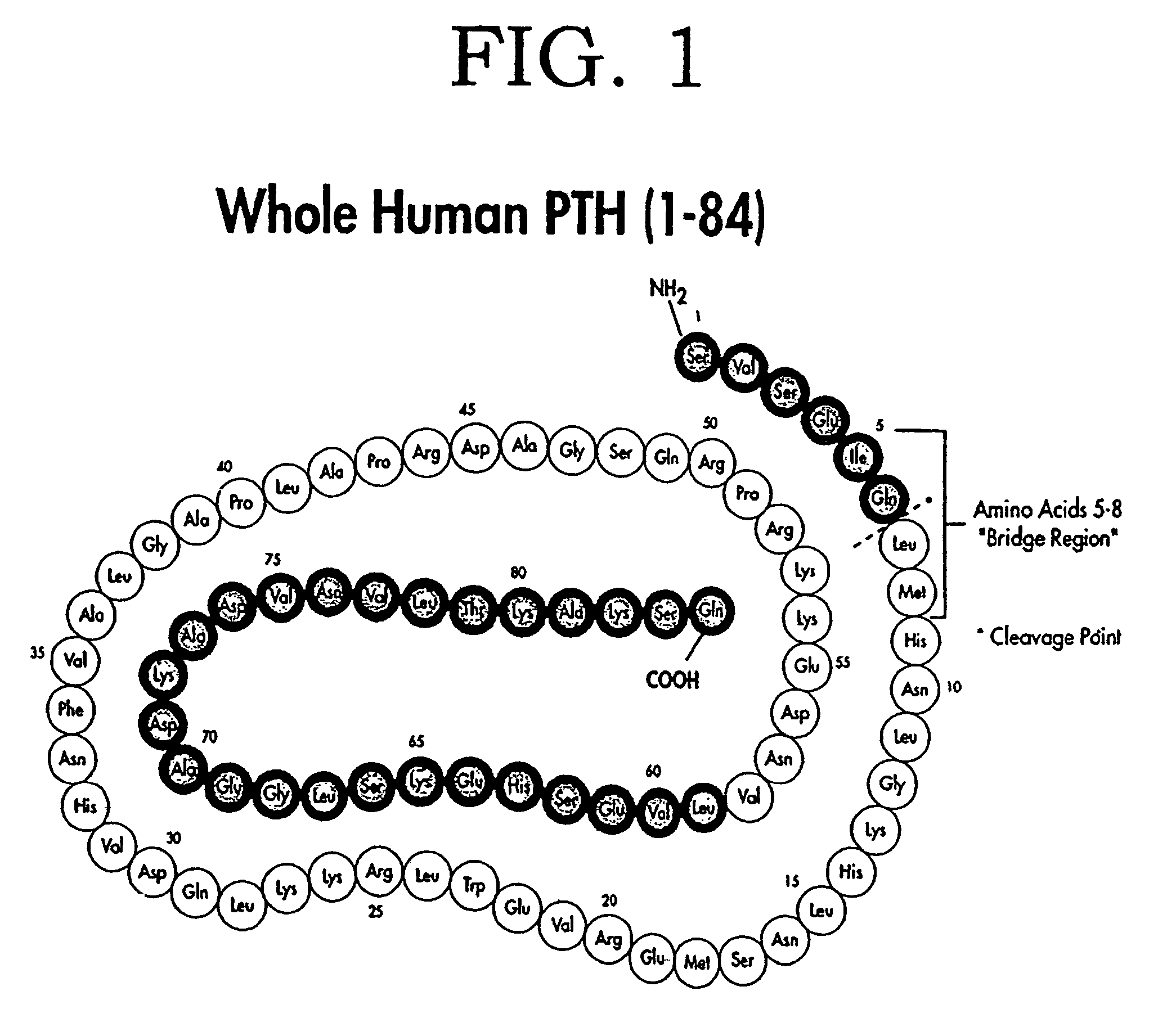
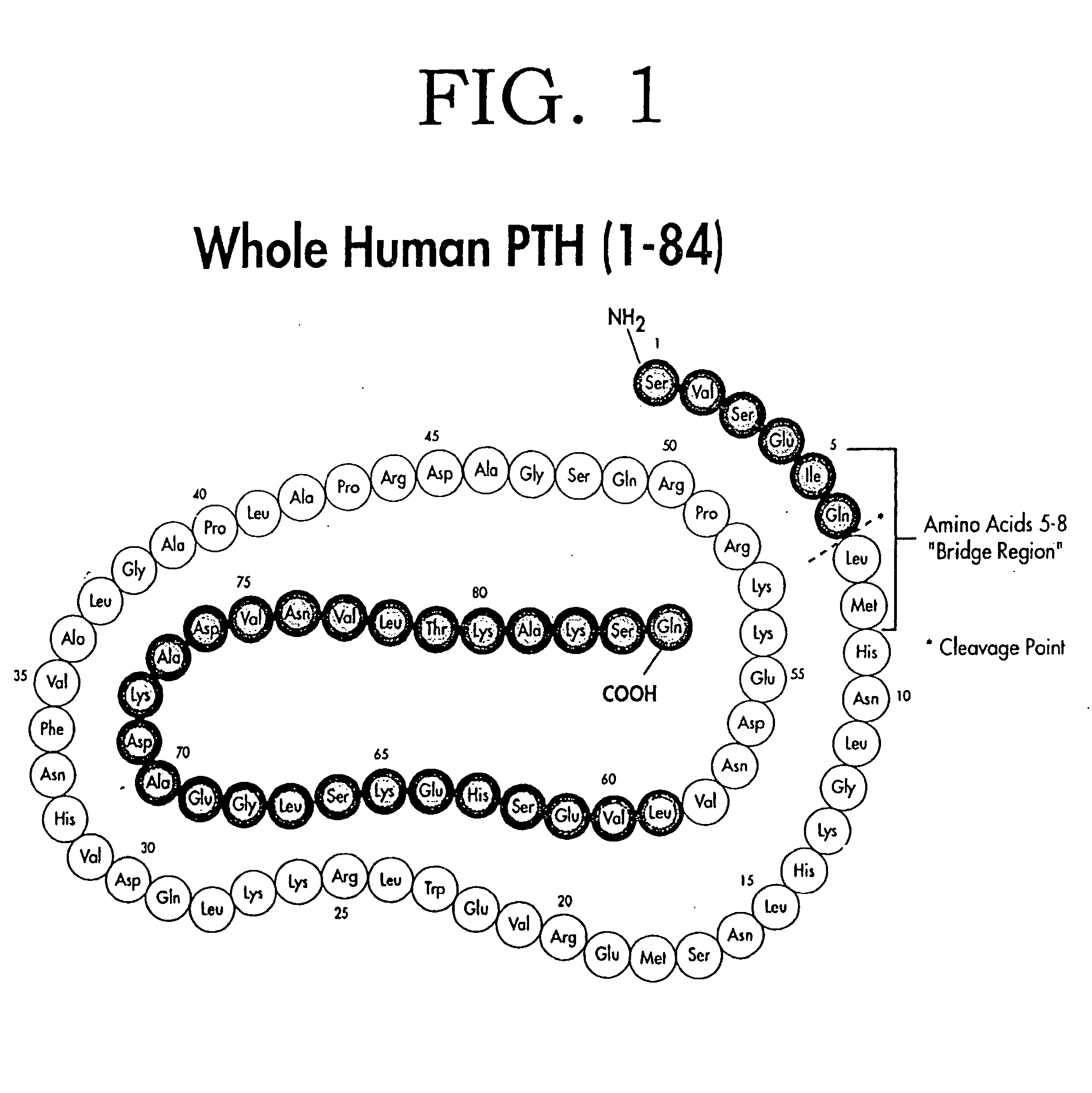
The present invention relates to novel methods and devices for differentiating in a patient parathyroid diseases, such as hyperparathyroidism and related bone diseases, from normal or non-disease states. One detects whole or non-fragmented (1 to 84) parathyroid hormone in a biological sample and also a large non-whole parathyroid hormone peptide fragment that can function as a parathyroid hormone antagonist. By either comparing values or using independently the value of either the large non-whole parathyroid hormone peptide fragment, the whole parathyroid hormone, or the combination of these values one is able to differentiate parathyroid and bone related disease states, as well as differentiate such states from normal states.
Owner:SCANTIBODIES LAB
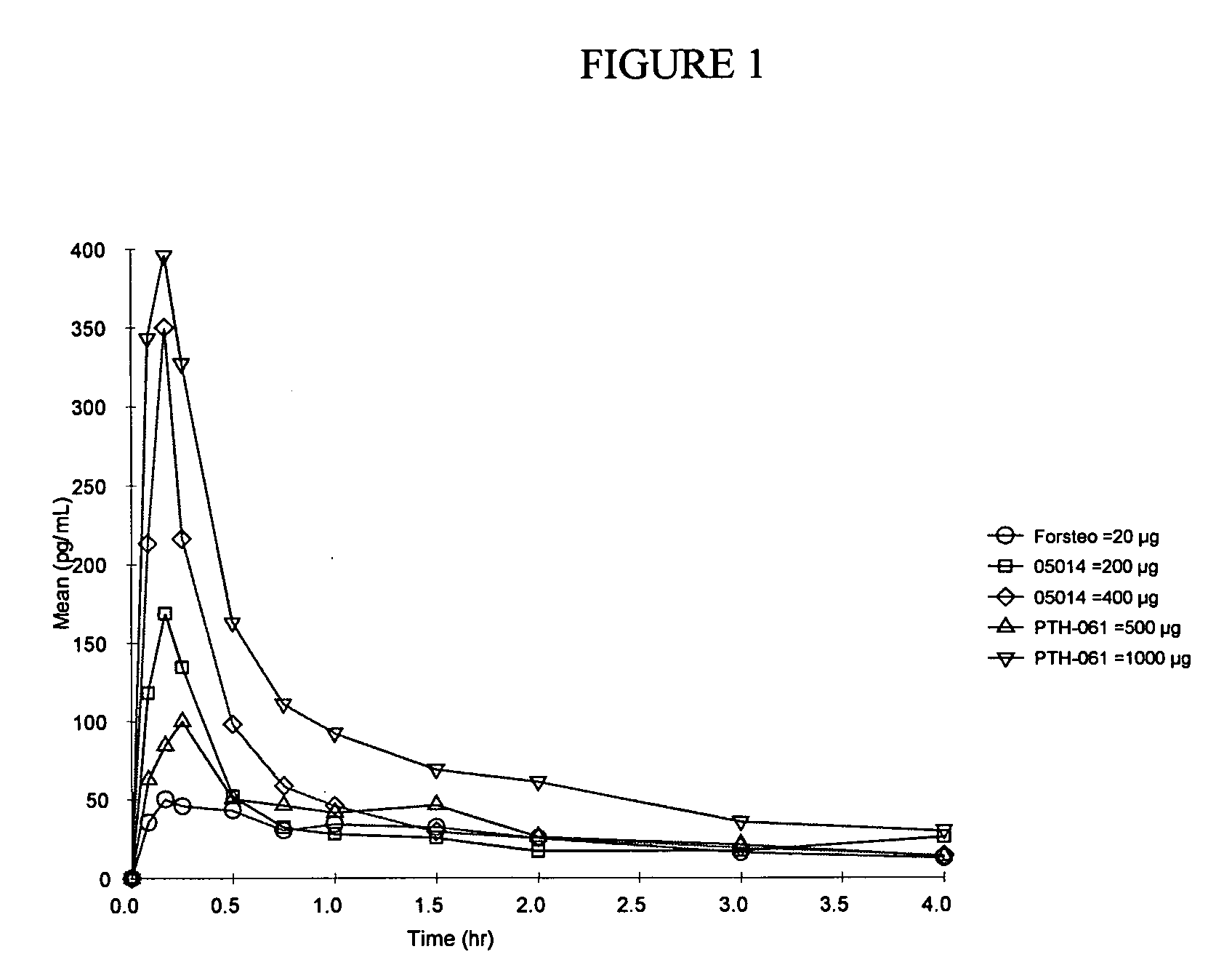
Parathyroid Hormone Analogues and Methods of Use
InactiveUS20090010940A1Low incidenceImprove benefitAntibacterial agentsBiocideSide effectParathyroid Hormone Analogue
The present invention is directed to novel methods of treating a subject with a bone deficit disorder. The methods generally include administering to a subject in need thereof a pharmaceutically acceptable formulation comprising a parathyroid hormone (PTH) peptide analogue in a daily dose sufficient to result in an effective pharmacokinetic profile and maintained adenylate cyclase activity, while simultaneously reducing undesirable side effects.
Owner:MORLEY PAUL +4
Prepn process of slow release parathyroid hormone microballoon
InactiveCN1739795AHigh drug loadingImprove stabilityPeptide/protein ingredientsGranular deliveryFreeze-dryingMicrosphere
The slow release parathyroid hormone microballoon contains parathyroid hormone 2-20 wt%, high molecular weight polysaccharide 2-20 wt%, small molecular weight saccharide 0-10 wt%, and slow release polymer 75-95 wt%. The preparation process includes the following steps: A. dissolving parathyroid hormone in water solution of high molecular weight polysaccharide to form inner water phase; B. adding the inner water phase into the dichloromethane solution of polylactic acid-hydroxy acetic acid copolymer to form initial W / O emulsion; C. emulsifying the initial W / O emulsion into compound W / O / W emulsion via saturating outer water phase of PVA-NaCl aqua with dichloromethane and adding the initial W / O emulsion; D. adding compound W / O / W emulsion to NaCl aqua via stirring to solidify microballoon; and E. water washing the solidified microballoon and freeze drying. The present invention has improved release curve of parathyroid hormone microballoon, high stability of parathyroid hormone and increased medicine carrying amount of the microballoon.
Owner:SHANGHAI JIAO TONG UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com






![PYRIDO [4,3-d] PYRIMIDIN-4 (3H) -ONE DERIVATIVES AS CALCIUM RECEPTOR ANTAGONISTS PYRIDO [4,3-d] PYRIMIDIN-4 (3H) -ONE DERIVATIVES AS CALCIUM RECEPTOR ANTAGONISTS](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/34f9b00a-7f53-4816-814c-6dbb659ca913/US20080085887A1-20080410-D00000.png)
![PYRIDO [4,3-d] PYRIMIDIN-4 (3H) -ONE DERIVATIVES AS CALCIUM RECEPTOR ANTAGONISTS PYRIDO [4,3-d] PYRIMIDIN-4 (3H) -ONE DERIVATIVES AS CALCIUM RECEPTOR ANTAGONISTS](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/34f9b00a-7f53-4816-814c-6dbb659ca913/US20080085887A1-20080410-D00001.png)
![PYRIDO [4,3-d] PYRIMIDIN-4 (3H) -ONE DERIVATIVES AS CALCIUM RECEPTOR ANTAGONISTS PYRIDO [4,3-d] PYRIMIDIN-4 (3H) -ONE DERIVATIVES AS CALCIUM RECEPTOR ANTAGONISTS](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/34f9b00a-7f53-4816-814c-6dbb659ca913/US20080085887A1-20080410-C00001.png)