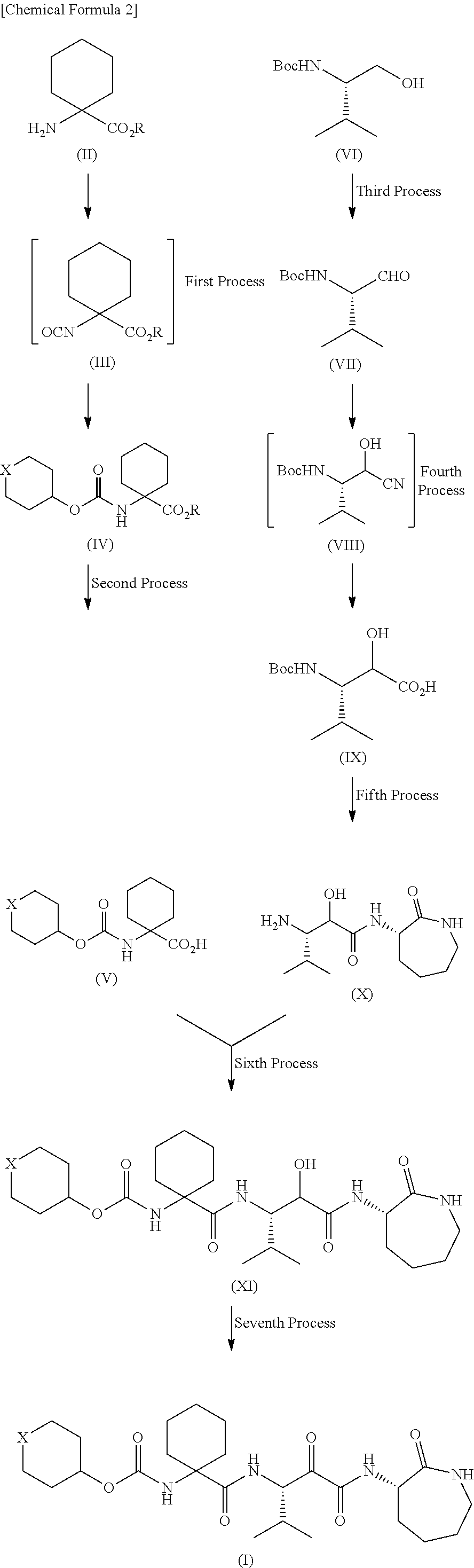
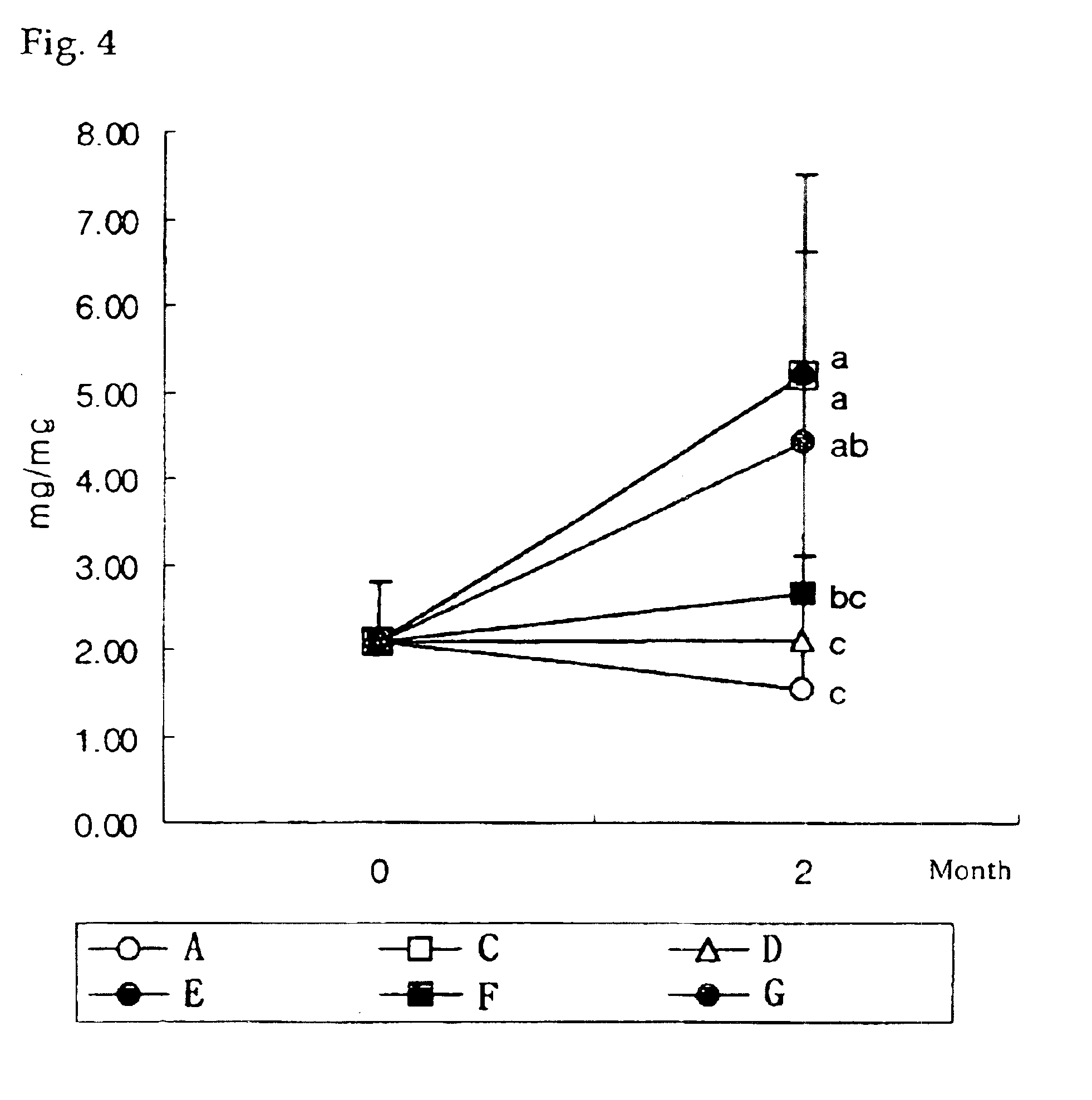Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
38 results about "BONE RESORPTION INHIBITORS" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Bone resorption inhibitors are drugs that inhibit mineralization or resorption of the bone by blocking the action of osteoclasts. They are used to treat postmenopausal and glucocorticoid induced osteoporosis, Paget's disease of the bone and malignant hypercalcemia. Types of Bone resorption inhibitors.
Method and device for the controlled delivery of parathyroid hormone
InactiveUS7497855B2Efficiently providePeptide/protein ingredientsMicromachined deliveryOsteopoikilosisMedical device
Method and devices are provided for extended and controlled delivery of parathyroid hormone to a patient. The method includes implanting a medical device into the patient, the medical device comprising a substrate, a plurality of reservoirs in the substrate, a release system contained in each of the reservoirs, wherein the release system comprises parathyroid hormone; and controllably releasing a pharmaceutically effective amount of the parathyroid hormone from the reservoirs. The parathyroid hormone can be released intermittently, such as once daily over an extended period (e.g., two months, ten months, or more.). The device can further include reservoirs containing a bone resorption inhibitor or other drug for release. The devices are useful in delivering PTH for the treatment and prevention of bone loss, such as associated with osteoporosis.
Owner:MICROCHIPS BIOTECH INC
Surface modifying method of orthopedic implanted medical instrument
ActiveCN105327396AAvoid absorptionPromote generationImpression capsDentistry preparationsBone tissueProsthesis
The invention discloses a surface modifying method of an orthopedic implanted medical instrument. An active strontium salt and a bisphosphonate are loaded on the surface of the implanted instrument. In-situ long-time controllable release of the active strontium salt (osteogenic component and bone resorption inhibitor) and bisphosphonate (bone resorption inhibitor) around the implanted instrument, synergistic effect of the active strontium salt and the bisphosphonate is utilized, and inhibiting of absorption of original bones and promoting of generation of new bones are realized so as to achieve the objective of quick-sound integration of an orthopedic transplanted device (prosthesis) with surrounding bone tissue. The orthopedic implanted instrument realizing surface modification through the method is especially suitable for treatment of pathologically induced bone absorption and fracture patients.
Owner:PEKING UNIV
Injectable calcium phosphate solid rods and pastes for delivery of osteogenic proteins
Osteogenic proteins are delivered via an injectable solid rod or hardenable paste. The formulation comprises a calcium phosphate material, an osteogenic protein, and optional additives and active ingredients such as a bone resorption inhibitor. Methods of making injectable pharmaceutical compositions and methods of using the osteogenic compositions to treat bone defects are also disclosed.
Owner:ETEX +1
Softgel formulations of bisphosphonates bone resorption inhibitors
InactiveUS20050142185A1Improve bioavailabilityInhibiting bone resorptionBiocidePhosphorous compound active ingredientsSoftgelGlycerol
The present invention provides a pharmaceutical formulation suitable for filling softgel capsules comprising: (a) from about 1% to about 90% by weight of a bisphosphonic acid or a pharmaceutically acceptable salt; and (b) from about 40% to about 80% by weight of a liquid carrier comprising 50% to 80% by weight polyethylene glycol; 5% to 15% by weight of glycerin; and 5% to 20% by weight water. The invention also describes a method for preparing alendronate or its pharmaceutical acceptable salts in encapsulated therapeutic dosage form, comprising the steps of reducing the size of alendronate particles to an average size no larger than about 80 microns, then mixing the micronized particles of alendronate with a solvent essentially consisting predominantly of polyethylene glycol of a molecular weight no greater than about 1000, and heating the mixture at a temperature of from about 40° C. to about 50° C. until the alendronate is dissolved in the solvent, and then encapsulating therapeutic doses of the dissolved alendronate in gelatin capsules soluble in water but insoluble in said solvent.
Owner:BELENO ALFREDO BERTHEL +1
Pharmaceutical compositions and preparations for treatment of metabolic bone disease
The present invention relates to a pharmaceutical composition for the treatment of metabolic bone disease and the method of preparation thereof, and more particularly, to an improved pharmaceutical composition for the therapeutic treatment of metabolic disease and the method of preparation thereof, wherein said composition is prepared as a composite pharmaceutical agent which comprises calcitriol; which reduces the rate of spine fractures and increases bone density; alendronate, a bone resorption inhibitor, as two main active ingredients in an optimal mixing ratio to exert the greatest synergistic therapeutic effect; and adequate amount of other additives such as a resorption fortifier of alendronate. Thereof, the pharmaceutical composition according to the present invention can inhibit hypercalcemia caused when administered by calcitriol alone, compensate the inhibitory activity of bone remodeling caused by alendronate due to the presence of calcitriol, and improve drug compliance associated with the usual difficulty in administration as well as a side effect in esophagus, thus effectively preventing the occurence of osteoporosis.
Owner:YUYU IND
Injectable solid hyaluronic acid carriers for delivery of osteogenic proteins
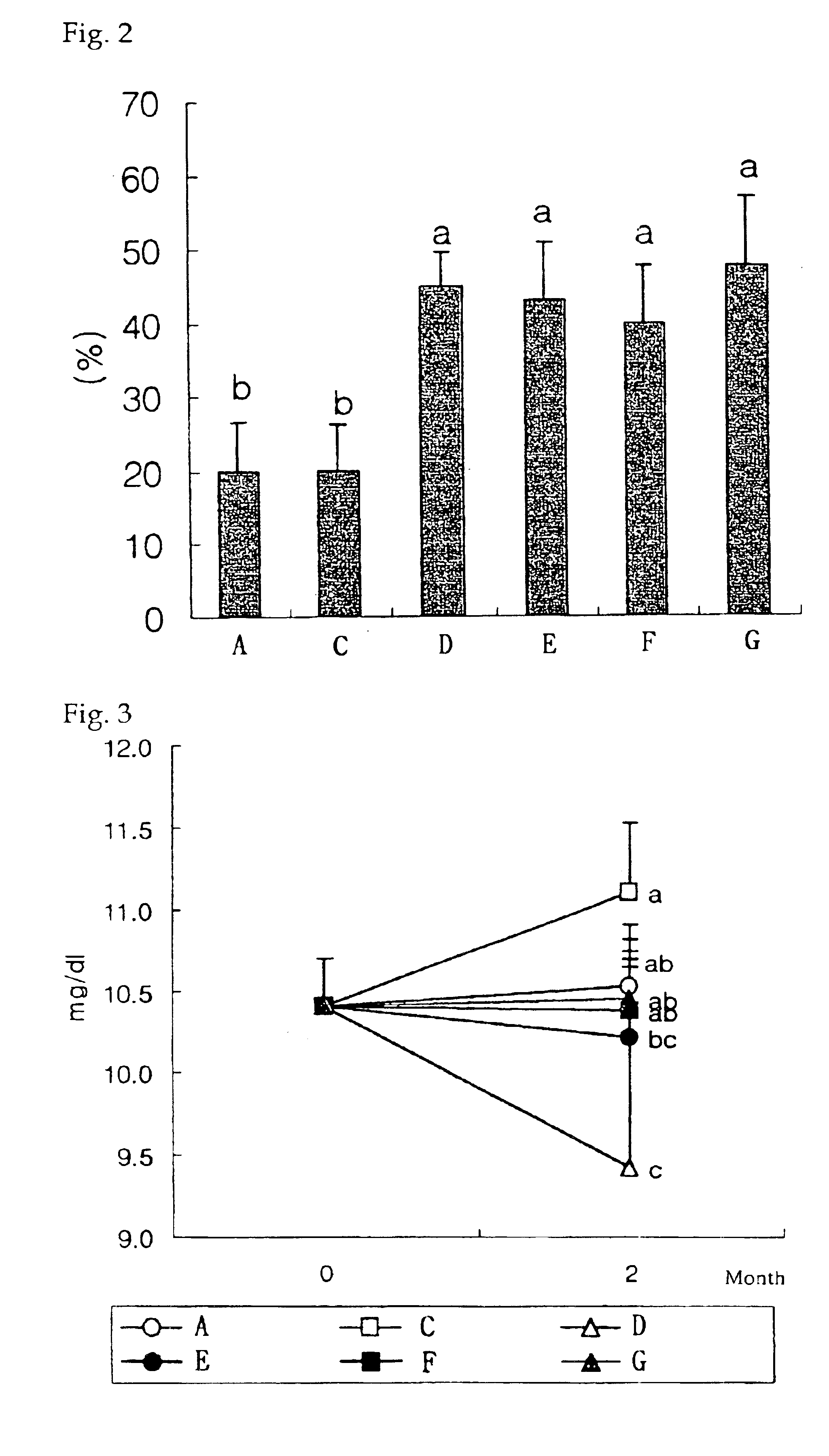
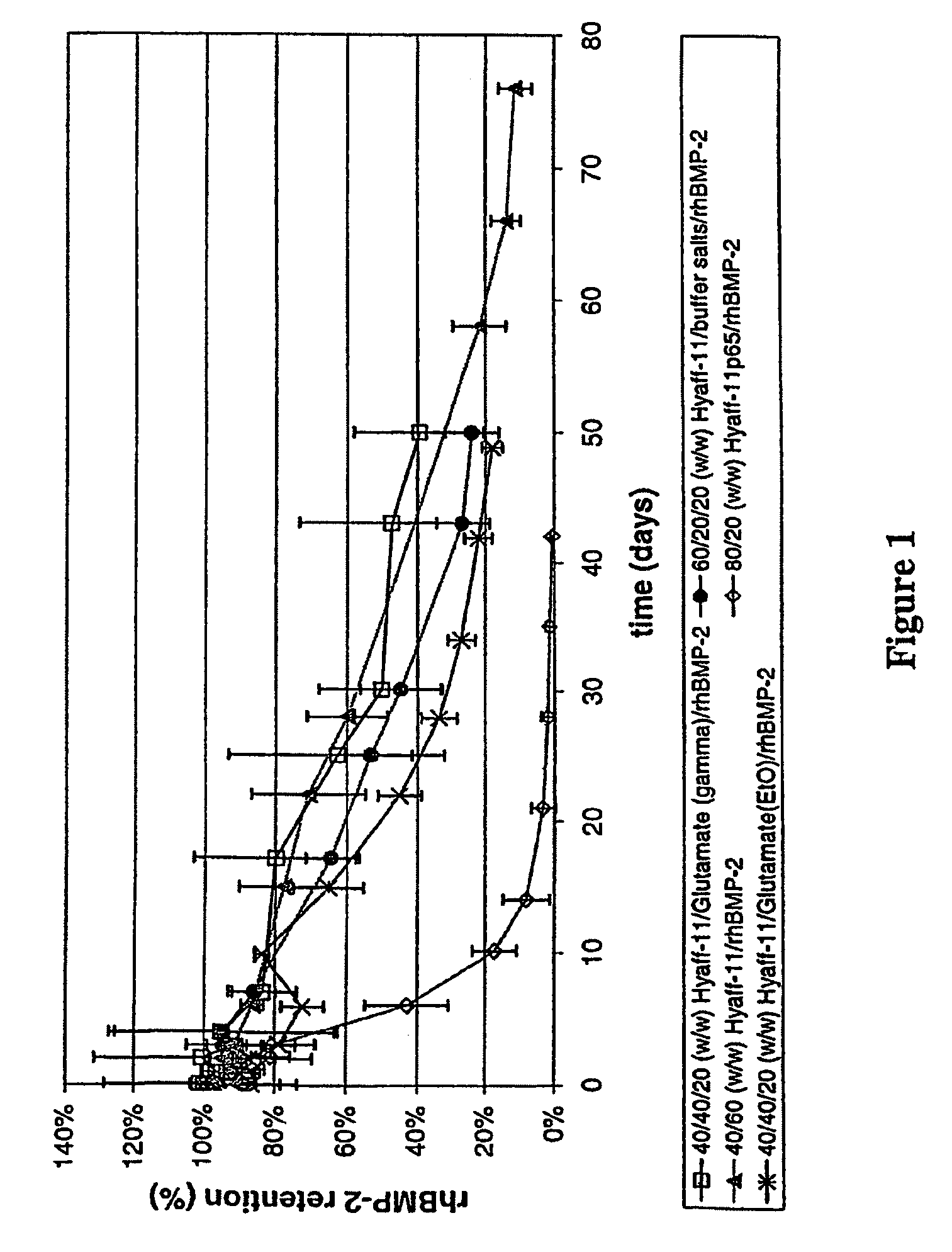
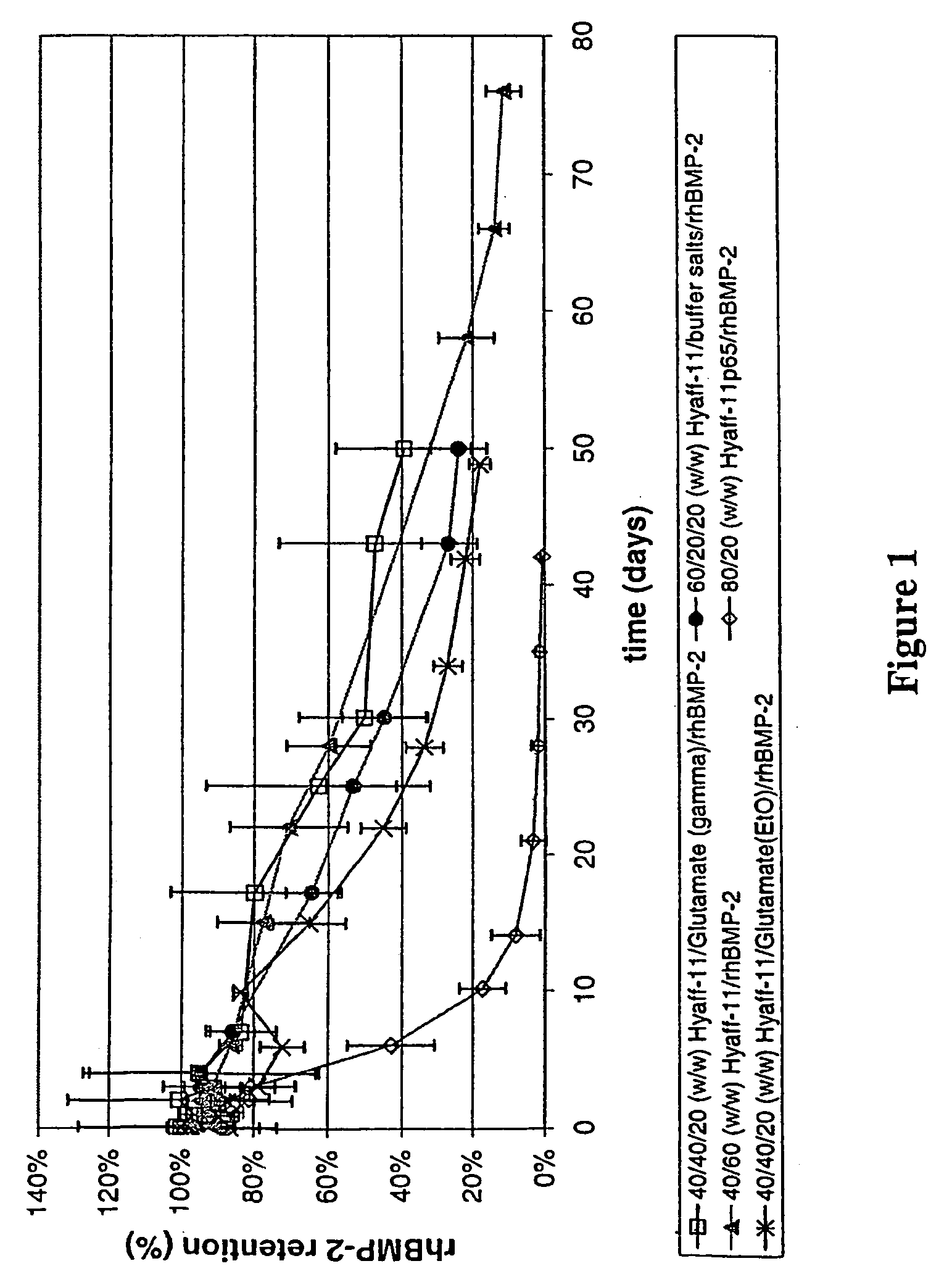
InactiveUS20090181058A1High retention rateMinimizing and reducing incidence and severityOrganic active ingredientsPeptide/protein ingredientsImplantable rodOsteogenic proteins
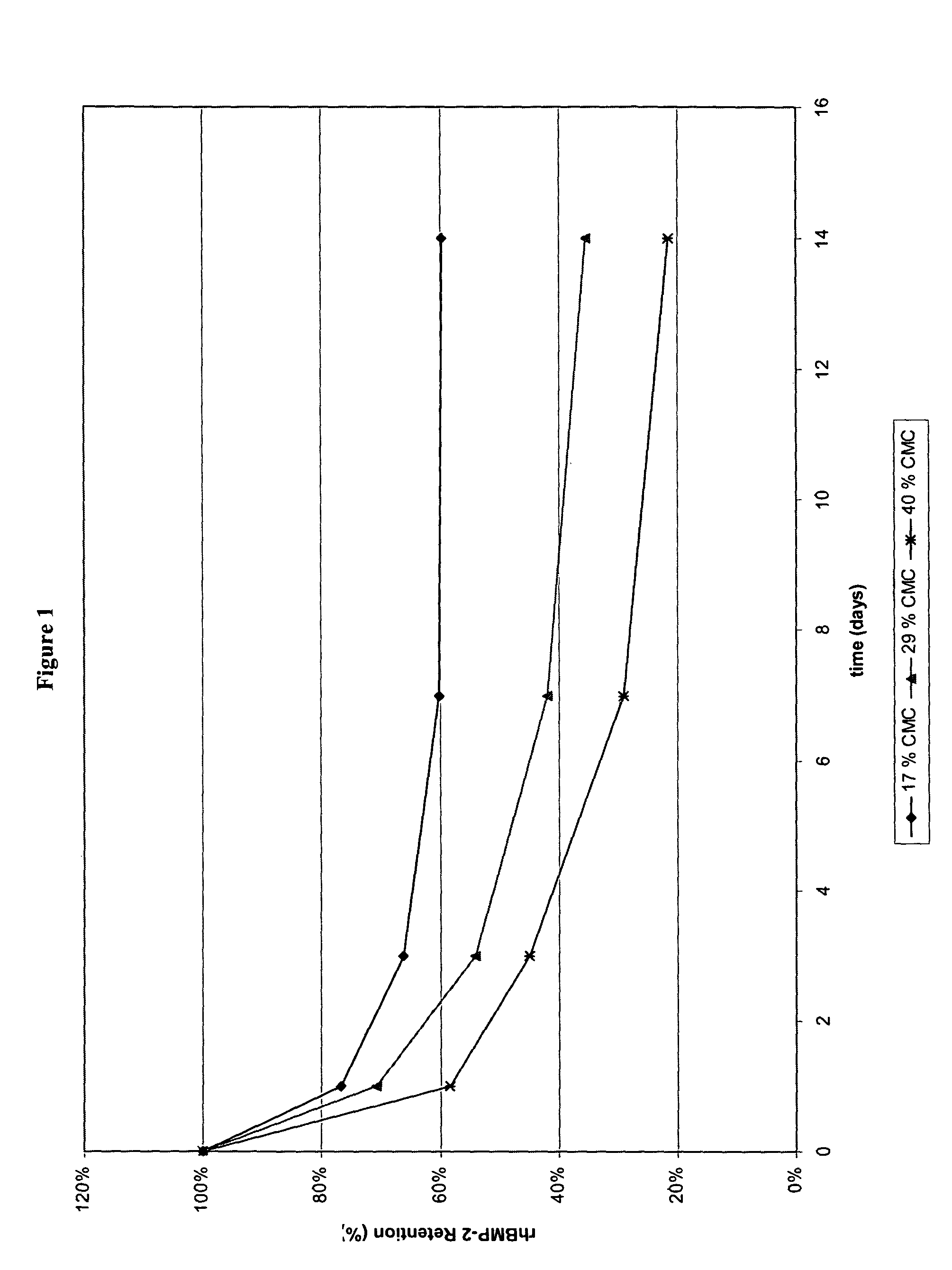
Methods of using an injectable or implantable rod-shaped formulation for delivery of osteogenic proteins to treat osteoporotic and / or osteopenic bone are disclosed. The formulation comprises hyaluronic acid derivatives and osteogenic proteins, and optional excipients and active ingredients such as a bone resorption inhibitor.
Owner:WYETH LLC +1
Injectable solid hyaluronic acid carriers for delivery of osteogenic proteins
InactiveUS20050287135A1High retention rateMinimizing and reducing incidence and severityBiocidePeptide/protein ingredientsImplantable rodOsteogenic proteins
An injectable or implantable rod-shaped formulation is disclosed for delivery of osteogenic proteins. The formulation comprises hyaluronic acid derivatives and osteogenic proteins, and optional excipients and active ingredients such as a bone resorption inhibitor. Methods of making injectable rod-shaped pharmaceutical compositions and methods of using the osteogenic compositions to treat osteoporotic and / or osteopenic bone are also disclosed.
Owner:WYETH +1
Bone Densifying Agent Characterized By Use Of Cathepsin K Inhibitor With Pth
InactiveUS20070238769A1Increased more efficientlyIncrease bone densityBiocideOrganic chemistryCathepsin KBone formation
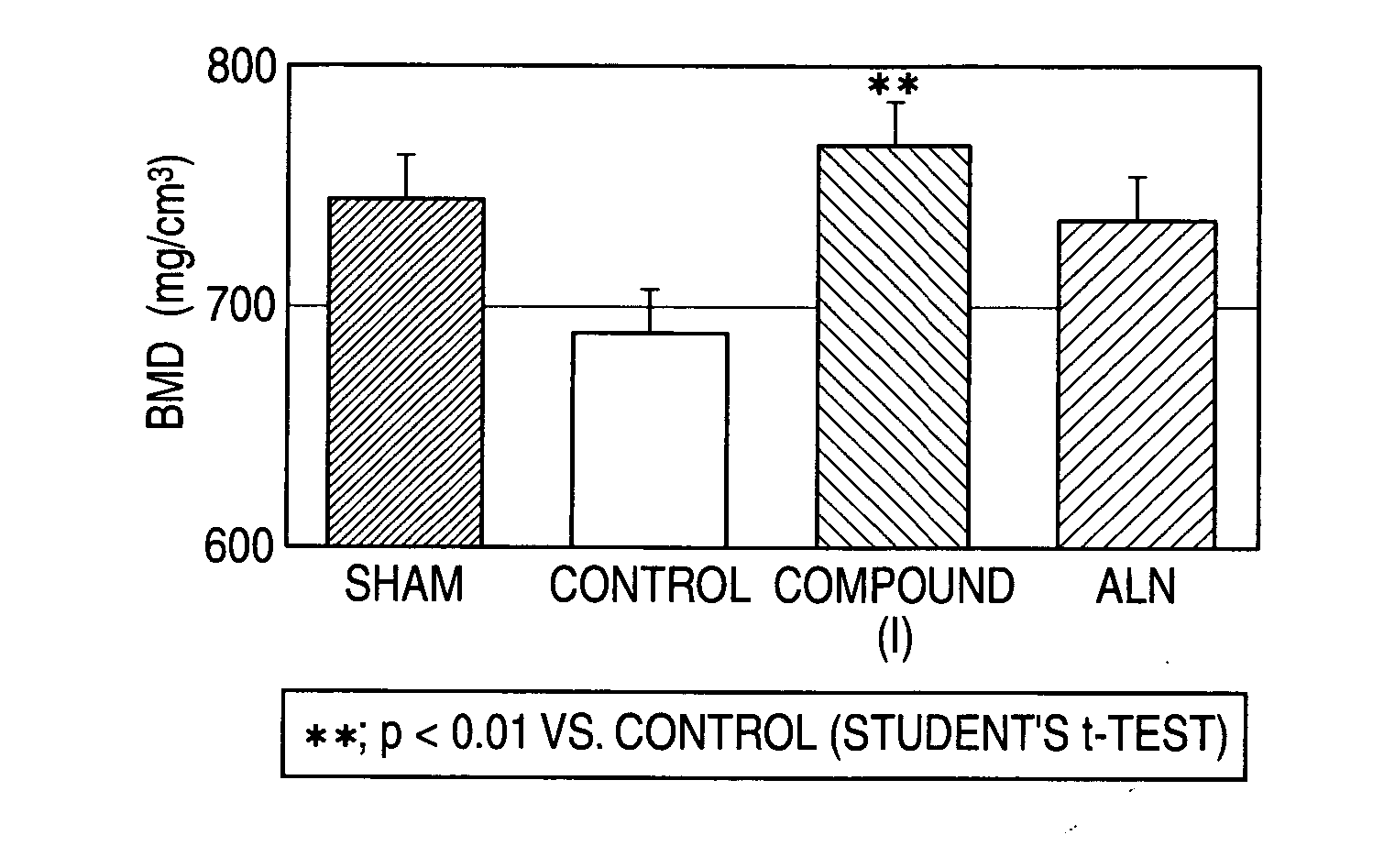
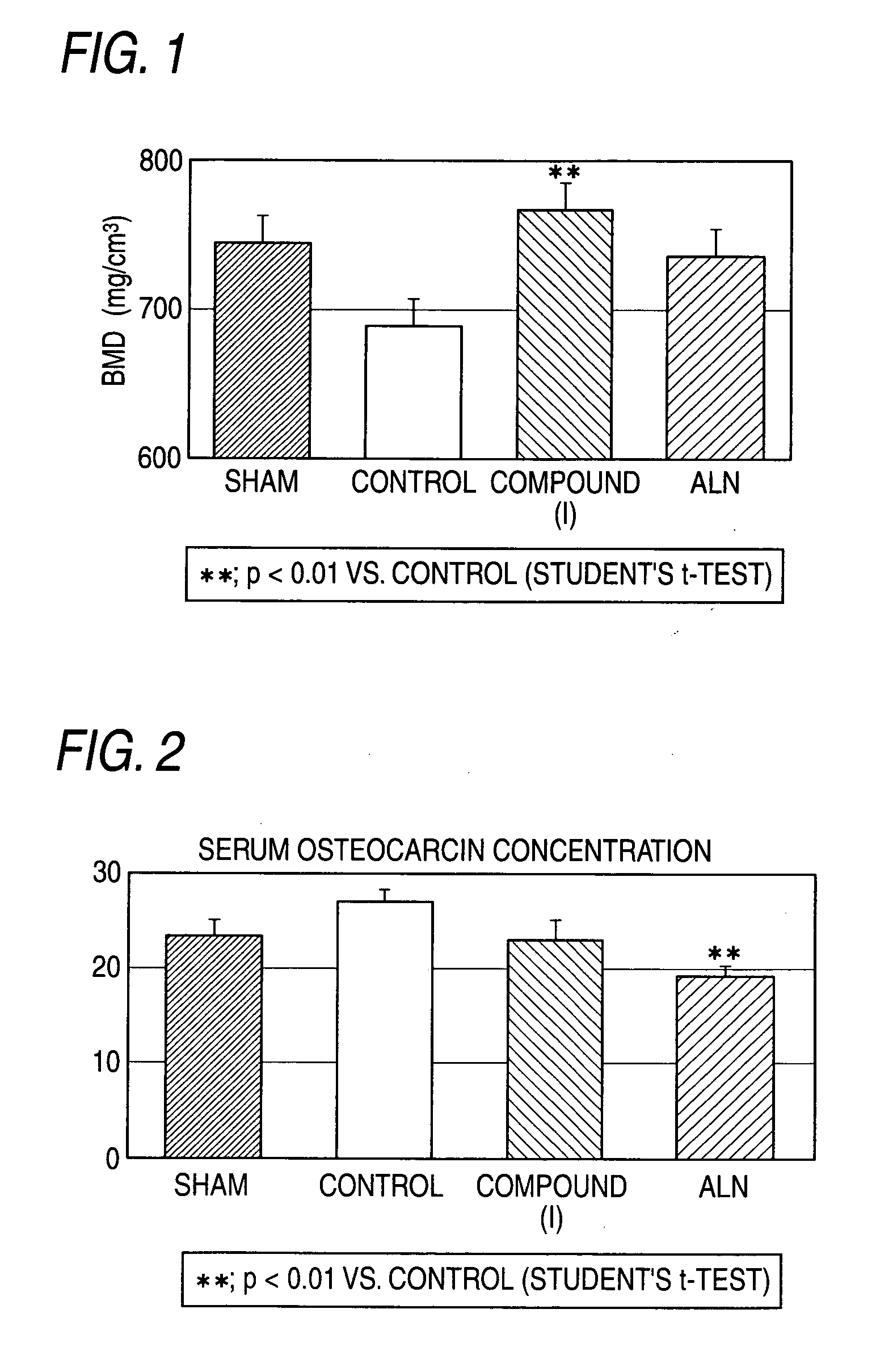
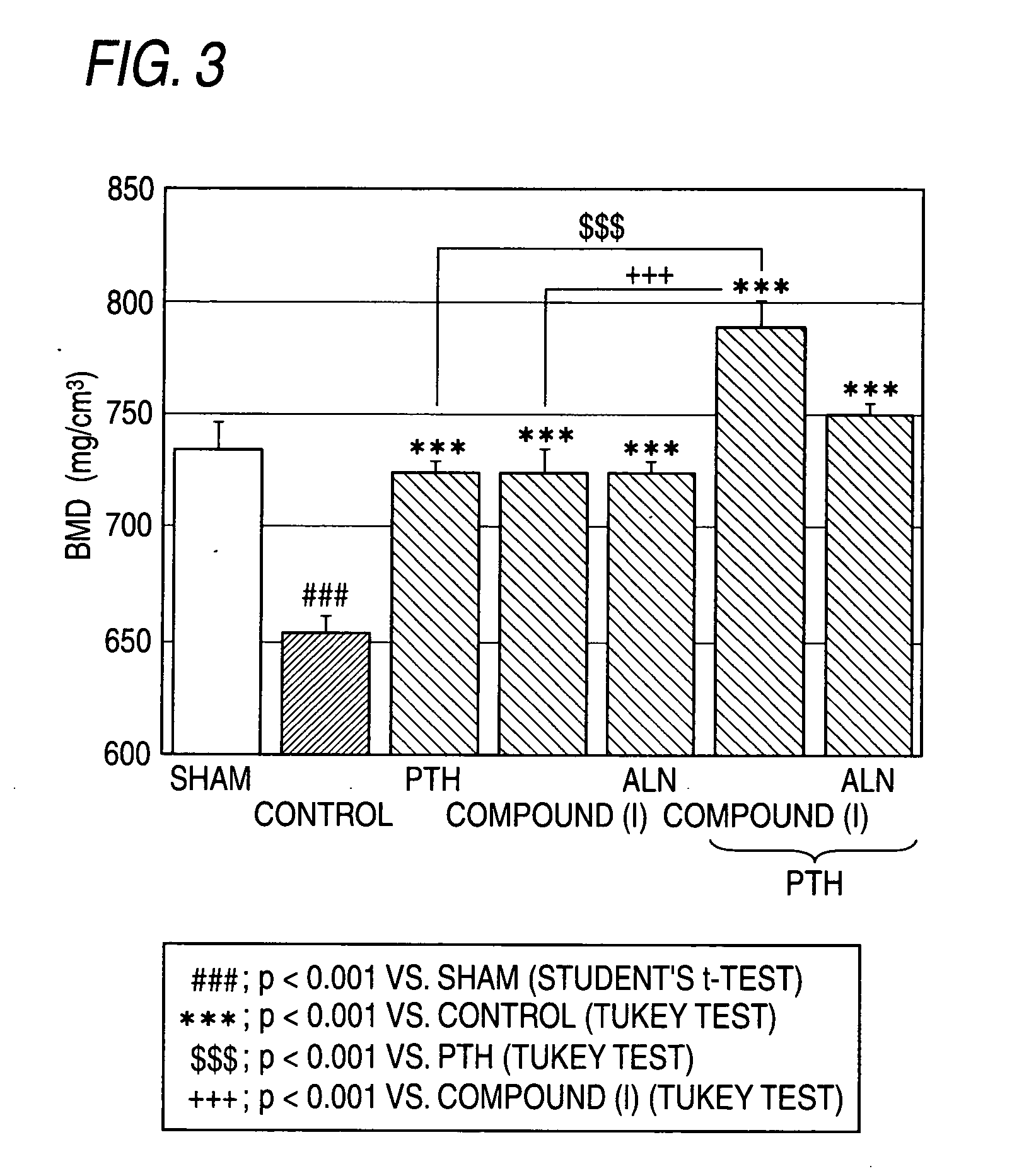
The present invention relates to an agent for increasing BMD characterized by use of a cathepsin K inhibitor as a bone resorption inhibitor in combination with a type of PTH as a bone formation stimulator. This agent for increasing BMD is useful for the treatment of osteoporosis, bone fracture, arthritis, rheumatoid arthritis, osteoarthritis, hypercalcemia, osteometastasis of carcinoma, periodontal disease, bone Paget's disease and other bone metabolic diseases. For example, as the cathepsin K inhibitor, there can be mentioned a compound of formula (W) and a salt thereof, etc.
Owner:ONO PHARMA CO LTD
Methods useful in the treatment of bone resorption diseases
The invention relates to a combined pharmaceutical preparation comprising parathyroid hormone and a bone resorption inhibitor, said preparation being adapted for (a) the administration of parathyroid hormone during a period of approximately 6 to 24 months; (b) after the administration of parathyroid hormone has been terminated, the administration of a bone resorption inhibitor during a period of approximately 12 to 36 months.
Owner:NPS PHARM INC
Implantable device for controlled, extended delivery of parathyroid hormone
InactiveUS20090163895A1Efficiently providePeptide/protein ingredientsMedical devicesPharmaceutical drugBone quality
Method and devices are provided for extended and controlled delivery of parathyroid hormone to a patient. The method includes implanting a medical device into the patient, the medical device comprising a substrate, a plurality of reservoirs in the substrate, a release system contained in each of the reservoirs, wherein the release system comprises parathyroid hormone; and controllably releasing a pharmaceutically effective amount of the parathyroid hormone from the reservoirs. The parathyroid hormone can be released intermittently, such as once daily over an extended period (e.g., two months, ten months, or more.). The device can further include reservoirs containing a bone resorption inhibitor or other drug for release. The devices are useful in delivering PTH for the treatment and prevention of bone loss, such as associated with osteoporosis.
Owner:MICROCHIPS BIOTECH INC
Injectable calcium phosphate solid rods and pastes for delivery of osteogenic proteins
Osteogenic proteins are delivered via an injectable solid rod or hardenable paste. The formulation comprises a calcium phosphate material, an osteogenic protein, and optional additives and active ingredients such as a bone resorption inhibitor. Methods of making injectable pharmaceutical compositions and methods of using the osteogenic compositions to treat bone defects are also disclosed.
Owner:ETEX +1
Remedies for periodontosis
The invention relates to an alveolar bone resorption inhibitor for use in topical injection by an alveolar mucosa injection method, which comprises a bisphosphonic acid derivative or a salt thereof as an active ingredient and to an alveolar bone resorption inhibitor for human periodontal tissue topical injection, which comprises a bisphosphonic acid derivative or a salt thereof in a concentration small enough so that local irritation to human periodontal tissues as the administrating site is acceptable but sufficient enough for expressing alveolar bone resorption inhibitory action by topical injection.
Owner:ASTELLAS PHARMA INC
Formulations of lycopene in combination with bisphosphonates bone resorption inhibitors
InactiveUS20140356423A1Improve bioavailabilityInhibiting bone resorptionBiocideHydrocarbon active ingredientsLycoperseneSoftgel
The present invention provides a pharmaceutical formulation suitable for filling softgel capsules comprising: (a) from about 1% to about 90% by weight of a bisphosphonic acid or a pharmaceutically acceptable salt; (b) from 1% to about 99% by weight of lycopene and (c) from about 40% to about 80% by weight of a liquid carrier comprising 50% to 80% by weight polyethylene glycol; 5% to 15% by weight of glycerin; and 5% to 20% by weight water. The invention also describes a method for preparing alendronate or its pharmaceutical acceptable salts in encapsulated therapeutic dosage form in combination with lycopene.
Owner:ANGRES ISAAC A
Process for preparing (cycloheptylamino) methylene biphosphonic acid
InactiveCN1363556ASimple and fast operationMild reaction conditionsGroup 5/15 element organic compoundsBONE RESORPTION INHIBITORSHydrochloric acid
A process for preparing (cycloheptylamino methylene biphosphonic acid as bone absorption inhibitor is prepared through reacting trialkyl phosphite on cycloheptylamine and trialkyl orthoformate, adding hydrochloric acid and hydrolyzing. Its advantages include gentle reaction condition and simple operation.
Owner:深圳市资福药业有限公司
Bone densifying agent characterized by use of cathepsin K inhibitor with PTH
InactiveUS7632813B2Increased more efficientlyIncrease bone densityBiocideOrganic chemistryCathepsin KArthritis
The present invention relates to an agent for increasing BMD characterized by use of a cathepsin K inhibitor as a bone resorption inhibitor in combination with a type of PTH as a bone formation stimulator. This agent for increasing BMD is useful for the treatment of osteoporosis, bone fracture, arthritis, rheumatoid arthritis, osteoarthritis, hypercalcemia, osteometastasis of carcinoma, periodontal disease, bone Paget's disease and other bone metabolic diseases. For example, as the cathepsin K inhibitor, there can be mentioned a compound of formula (W) and a salt thereof, etc.
Owner:ONO PHARMA CO LTD
Inhibitor of cancer bone metastasis
InactiveUS20070155693A1Enhanced inhibitory effectBiocidePhosphorous compound active ingredientsSignalling moleculesTyrosine-kinase inhibitor
The subject of the present invention is to provide means to fully achieve the inhibition of cancer bone metastasis, which was accomplished through the repeated selection of agents with aiming at obtaining more beneficial effects on the inhibition of cancer bone metastasis. The invention is achieved by combining an inhibition substance of the activation of osteoclast caused by the degradation of a signaling molecule, TRAF6, in the activation of osteoclast, a suppressive substance of the differentiation from osteoclast precursor cells to mature osteoclasts, and / or a bone resorption inhibitor and / or a Cox2 synthesis inhibitor. This combination was found to have an extremely high utility for the inhibition of cancer bone metastasis. Further, the invention is achieved by the inhibitor of cancer bone metastasis, wherein an IL-12 production inducer as an inhibition substance of the activation of osteoclast caused by the degradation of a signaling molecule, TRAF6, in the activation of osteoclast, a tyrosine kinase inhibitor as a suppressive substance of the differentiation from osteoclast precursor cells to mature osteoclasts, and / or a bisphosphonate as a bone resorption inhibitor and / or a Cox2 synthesis inhibitor for inhibiting the stimulation of RANKL / RANK receptor are combined.
Owner:ORIENT CANCER THERAPY
Cathepsin K inhibitor and application thereof
The invention relates to a cathepsin K inhibitor and an application thereof, and specifically, relates to a compound and a drug composition thereof used for treating or preventing cathepsin dependence diseases, and the compound and the composition containing the compound can be used as a bone resorption inhibitor to treat relevant diseases. The cathepsin includes but is not limited to cathepsin K.
Owner:SUNSHINE LAKE PHARM CO LTD
Injectable Calcium Phosphate Solid Rods and Pastes for Delivery of Osteogenic Proteins
ActiveUS20100273706A1Surgical adhesivesPeptide/protein ingredientsCalcium biphosphateOsteogenic proteins
Osteogenic proteins are delivered via an injectable solid rod or hardenable paste. The formulation comprises a calcium phosphate material, an osteogenic protein, and optional additives and active ingredients such as a bone resorption inhibitor. Methods of making injectable pharmaceutical compositions and methods of using the osteogenic compositions to treat bone defects are also disclosed.
Owner:ETEX +1
2,3-diaminopropionic acid derivative
The present invention relates to a 2,3-diaminopropionic acid derivative of the formula (1): or a pharmaceutically acceptable salt thereof. The compounds of the present invention are useful as a platelet aggregation inhibitor, a cancer metastasis inhibitor, a wound healing agent or a bone resorption inhibitor.
Owner:SUMITOMO DAINIPPON PHARMA CO LTD
Stable effervescent bisphosphonate formulations with rapid solubilization characteristics
A stable effervescent tablet, granule or powder composition free from excipients that may react with an effervescing organic acid component, comprising, an effective amount of a bisphosphonate bone resorption inhibitor, an effervescing organic acid component, an effervescing base component; wherein said composition is free of polyol binders and tableting lubricants; has a loss on drying of 0.25% (m / m) or less; has a complete disintegration time of no more than 180 seconds when placed in 3 to 8 fluid ounces of water at between 5-20° C.; and said bisphosphonate is incorporated as a micronized particle or by spray drying and is completely solubdised in water within 2 minutes without stirring.
Owner:EFFRX PHARMA SA
Α-oxoacyl amino-caprolactam derivative
ActiveUS9481707B2Lower levelHigh activityPeptide/protein ingredientsMetabolism disorderDiseaseSide effect
Owner:SEIKAGAKU KOGYO CO LTD
Cathepsin k inhibitors and uses thereof
The invention relates to a cathepsin K inhibitor and an application thereof, and specifically, relates to a compound and a drug composition thereof used for treating or preventing cathepsin dependence diseases, and the compound and the composition containing the compound can be used as a bone resorption inhibitor to treat relevant diseases. The cathepsin includes but is not limited to cathepsin K.
Owner:SUNSHINE LAKE PHARM CO LTD
Cathepsin k inhibitors and uses thereof
The invention relates to compounds and pharmaceutical compositions thereof for treatment or prevention of cathepsin dependent diseases; the compounds and the compositions comprising the compounds can be used as bone absorption inhibitors for treatment of related diseases, wherein the cathepsin includes, but is not limited to, cathepsin K.
Owner:SUNSHINE LAKE PHARM CO LTD
Amorphous calcium carbonate for the treatment of calcium malabsorption and metabolic bone disorders
ActiveUS20190022133A1Prevent decrease delay onsetIncrease bone formationOrganic active ingredientsSkeletal disorderDiseaseIncreased bone mineral density
Owner:AMORPHICAL LTD
Alpha-oxoacyl amino-caprolactam derivative
ActiveUS20160137691A1High cathepsin K inhibitory activityLower levelDipeptide ingredientsAntipyreticDiseaseSide effect
The purpose of the present invention is to provide a pharmaceutical composition that is useful for the treatment of diseases that are caused by an increase in bone resorption and that does not cause serious side effects even when used in combination with another drug. The present invention relates to: an α-oxoacyl aminocaprolactam derivative that is represented by formula (I)(in the formula, X is —O— or —N(R1)— and R1 represents an alkoxycarbonyl group having 1-10 carbon atoms); and a bone resorption inhibitor containing the α-oxoacyl aminocaprolactam derivative.
Owner:SEIKAGAKU KOGYO CO LTD
Cathepsin K inhibitors and application thereof
ActiveUS10494364B2Cause effect and serious side effectStrong inhibitory activityOrganic compound preparationPreparation by N-O/N-N bondsDiseaseCathepsin K
The invention relates to capthepsin K inhibitors and uses thereof, specifically relates to a class of compounds having the formula (I) which are used for treating or preventing cathepsin dependent diseases or conditions, specifically, wherein the cathepsin is capthepsin K. The compounds and compositions thereof can be used as bone resorption inhibitors for the treatment of associated diseases.
Owner:SUNSHINE LAKE PHARM CO LTD
Cathepsin k inhibitors and uses thereof
The present invention relates to cathepsin K inhibitors and uses thereof, specifically a class of compounds (represented by formula (I)) for treating or preventing cathepsin-dependent diseases, including but not limited to cathepsin K inhibitors. The compound and its pharmaceutical composition can be used as a bone resorption inhibitor to treat related diseases.
Owner:SUNSHINE LAKE PHARM CO LTD
Alpha-oxoacyl amino-caprolactam body
The purpose of the present invention is to provide a pharmaceutical composition that is useful for the treatment of diseases that are caused by an increase in bone resorption and that does not cause serious side effects even when used in combination with another drug. The present invention relates to: an α-oxoacyl amino-caprolactam that is represented by formula (I)(in formula (I), X represents N or CH, Y represents O or CH2, and Z represents S or CH2); and a bone resorption inhibitor containing the α-oxoacyl amino-caprolactam.
Owner:SEIKAGAKU KOGYO CO LTD
Amorphous calcium carbonate for the treatment of calcium malabsorption and metabolic bone disorders
ActiveUS10688124B2Prevent decrease delay onsetIncrease bone formationOrganic active ingredientsSkeletal disorderDiseaseMedicine
Provided are methods for treating calcium malabsorption and conditions associated with calcium malabsorption, employing the administration of a composition containing stable amorphous calcium carbonate. Further provided are methods for increasing bone mineral density in a bone metabolism associated disorders, diseases or conditions, employing the administration of said composition in combination with a bone resorption inhibitor.
Owner:AMORPHICAL LTD
Heteroaryl estrogen receptor regulating agent and application thereof
InactiveCN110256410AExtended shelf lifeHigh activityOrganic active ingredientsOrganic chemistryDiseaseHormone Receptor Modulators
The invention provides a compound as shown in a formula (I) and a heteroaryl estrogen receptor regulating agent including the same, belonging to the technical field of medicines. The heteroaryl estrogen receptor regulating agent further comprises a calcium phosphosilicate microsphere carrier, wherein the carrier includes silicon oxide, calcium oxide and phosphorus pentoxide in a weight ratio of 20: 4: 1, and the diameters of the microspheres are in a range of 300 to 1100 nm. The compound and the regulating agent provided by the invention can be used as estrogen receptor degrading agents, AKR1C3 inhibitors and bone resorption inhibitors for treating estrogen receptor related diseases or illnesses of patients and can be applied to the treatment of breast cancer, endometriosis and osteoporosis. The preparation method for the regulating agent can prolong the shelf life of the compound, increase the loading amount of a microsphere product, and enhance the stability and sustained release performance of the regulating agent; and the regulating agent provides a supersaturation amount of Ca<2+> in a biological fluid environment for binding to bone tissue nucleation sites so as to promote regeneration or repairing of bone tissue.
Owner:嘉兴市爵拓科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com