Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
45 results about "Implantable rod" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Birth control implants are devices that go under a woman's skin. They release a hormone that prevents pregnancy. The implant available in the U.S. is Nexplanon. It’s a newer version of the implant Implanon. The implant is a plastic rod about the size of a matchstick.
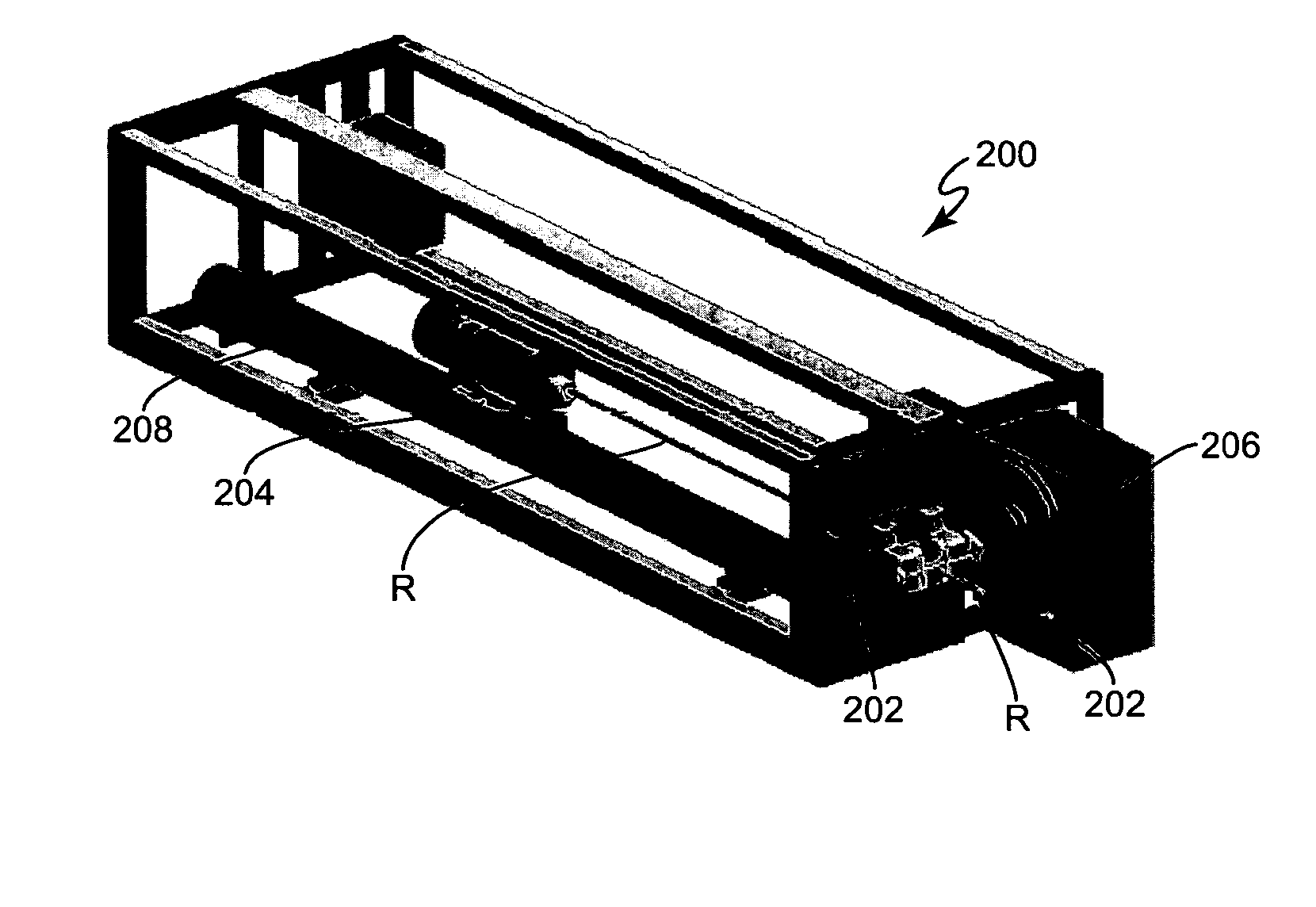
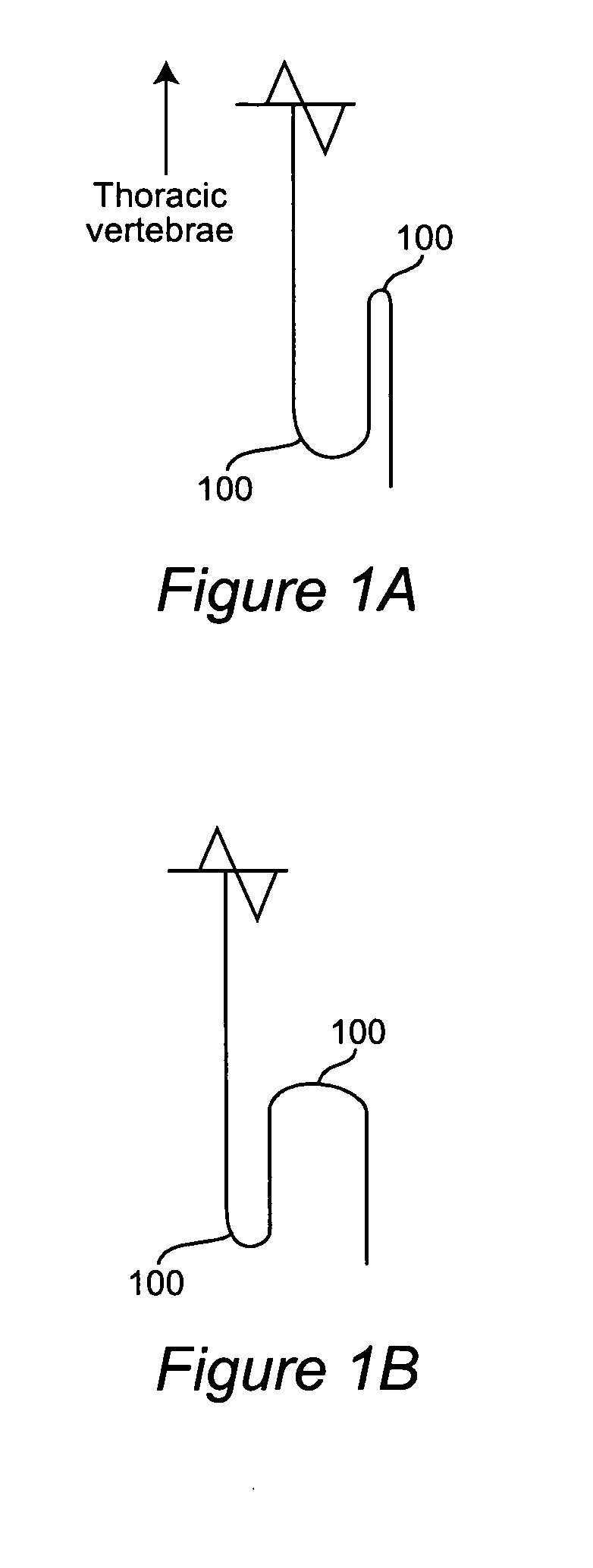
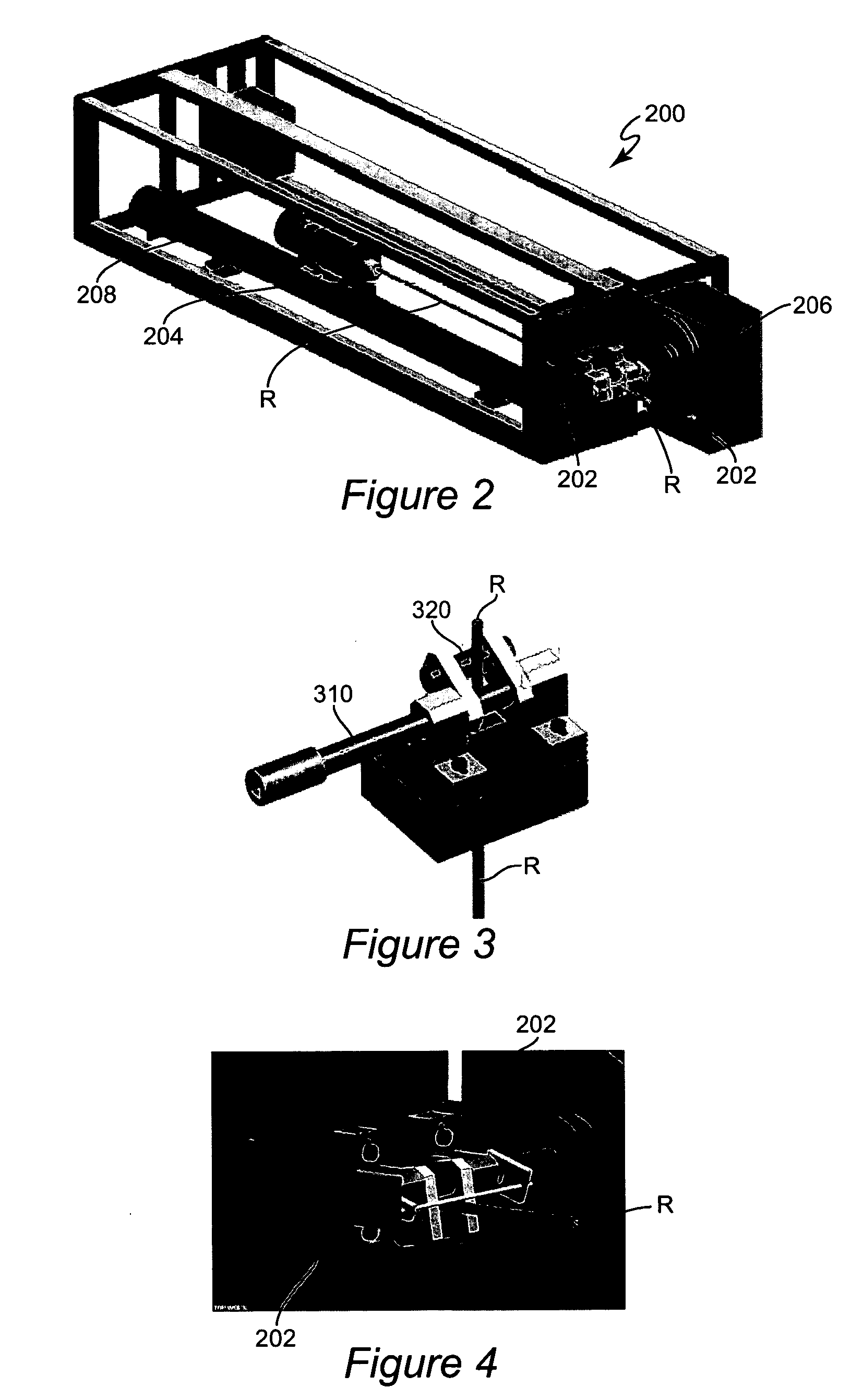
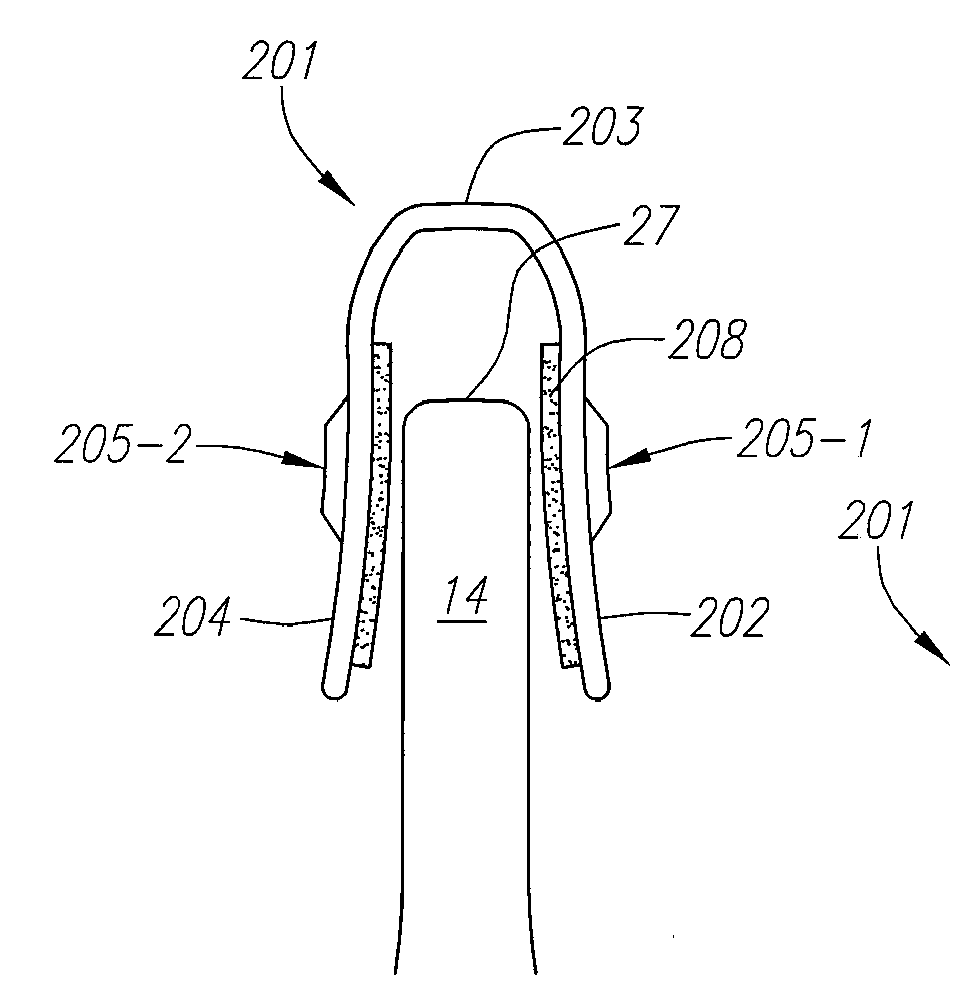
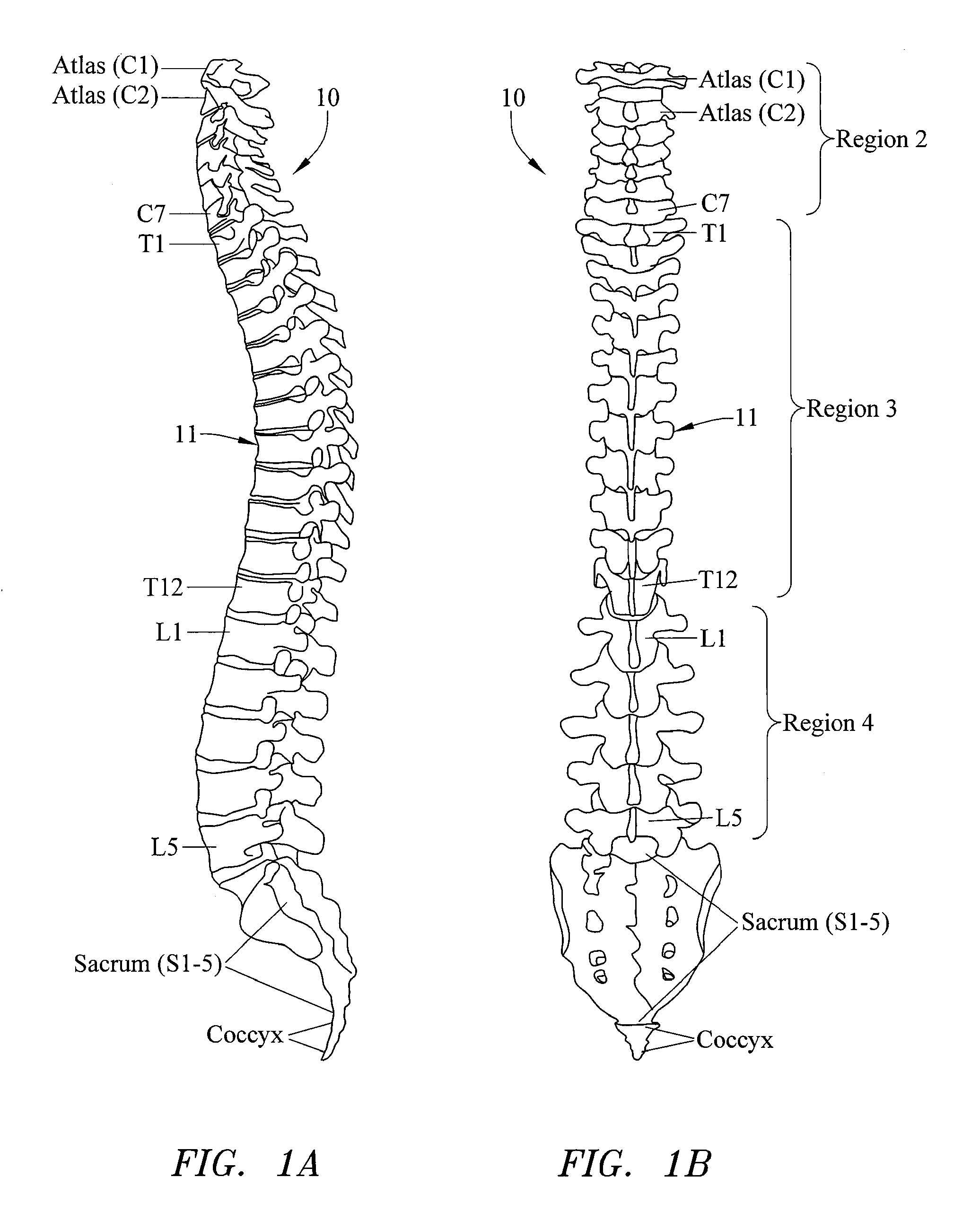
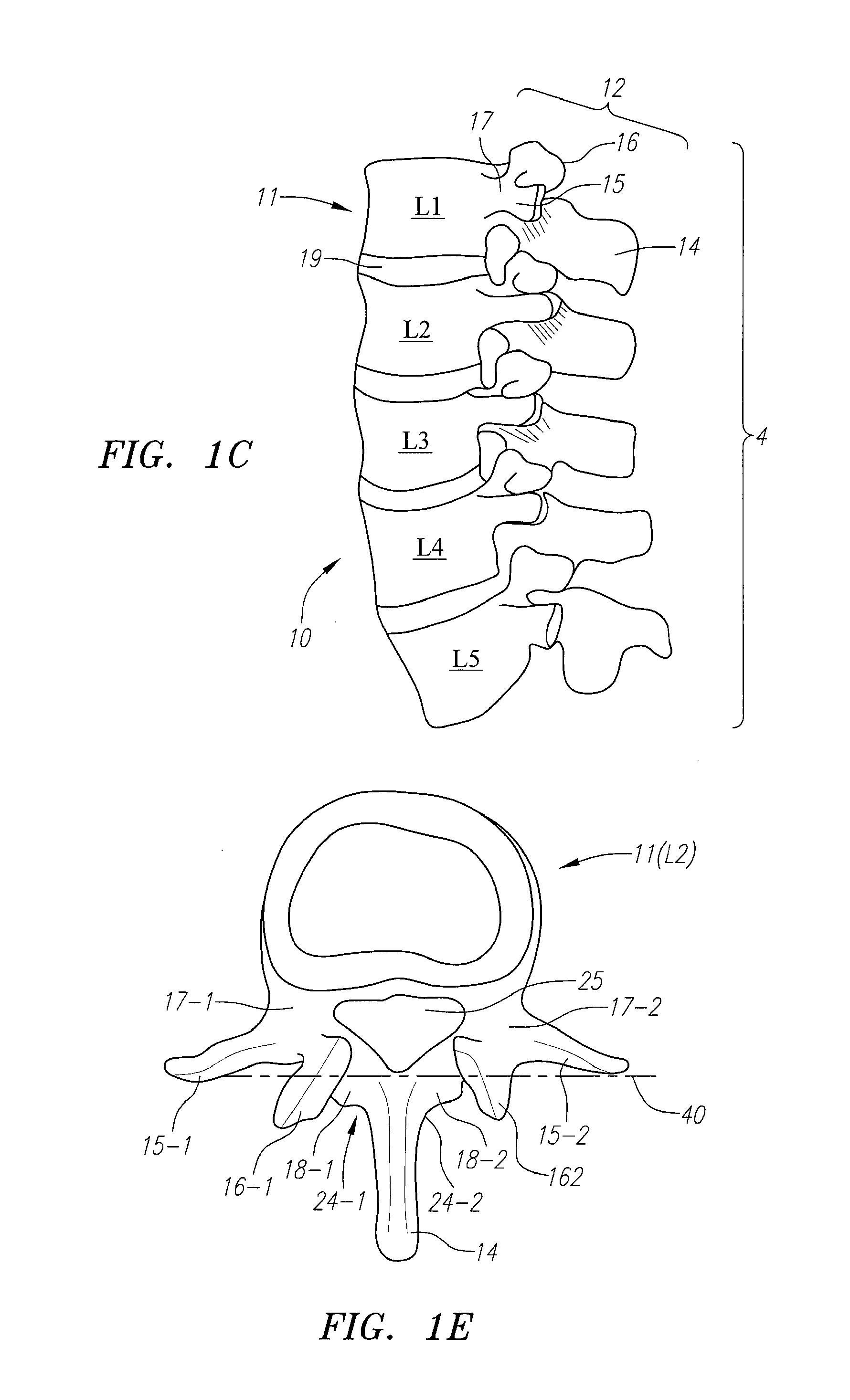
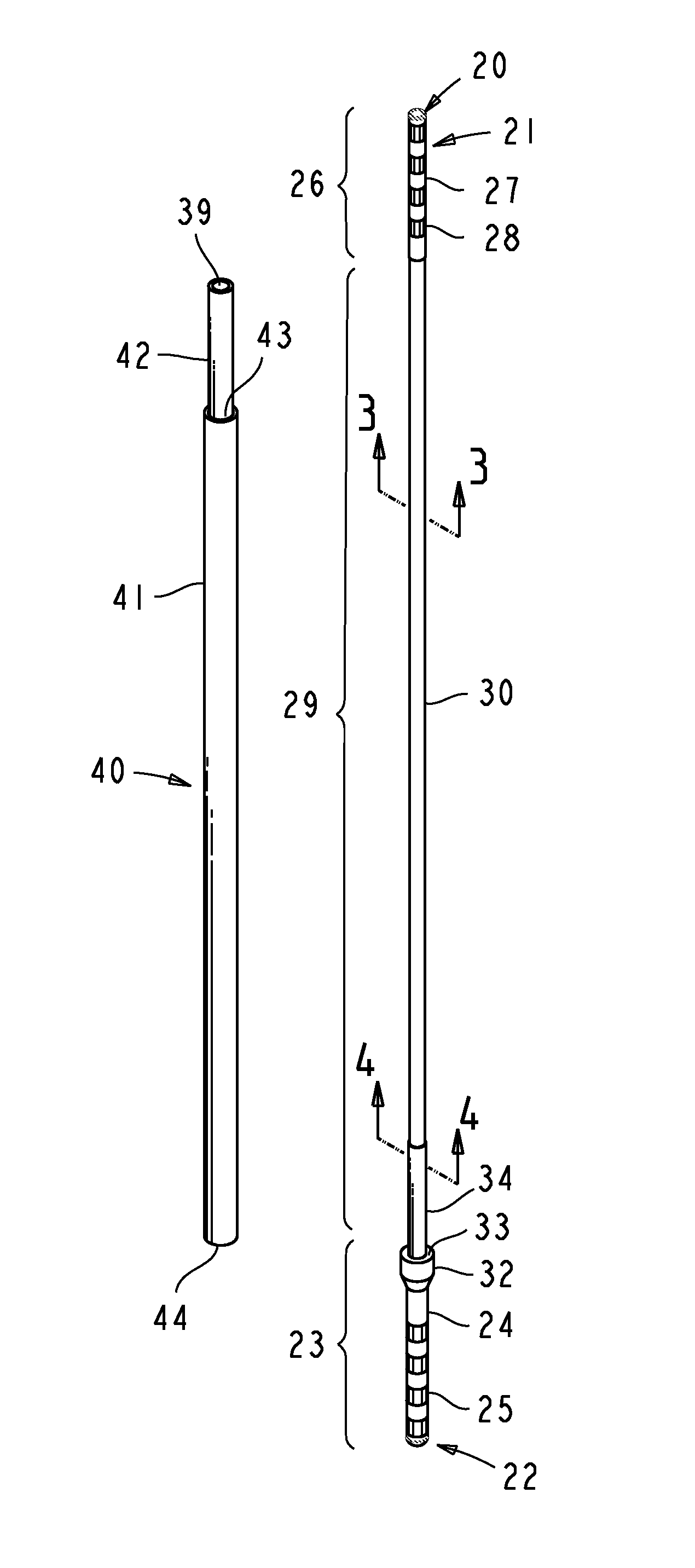
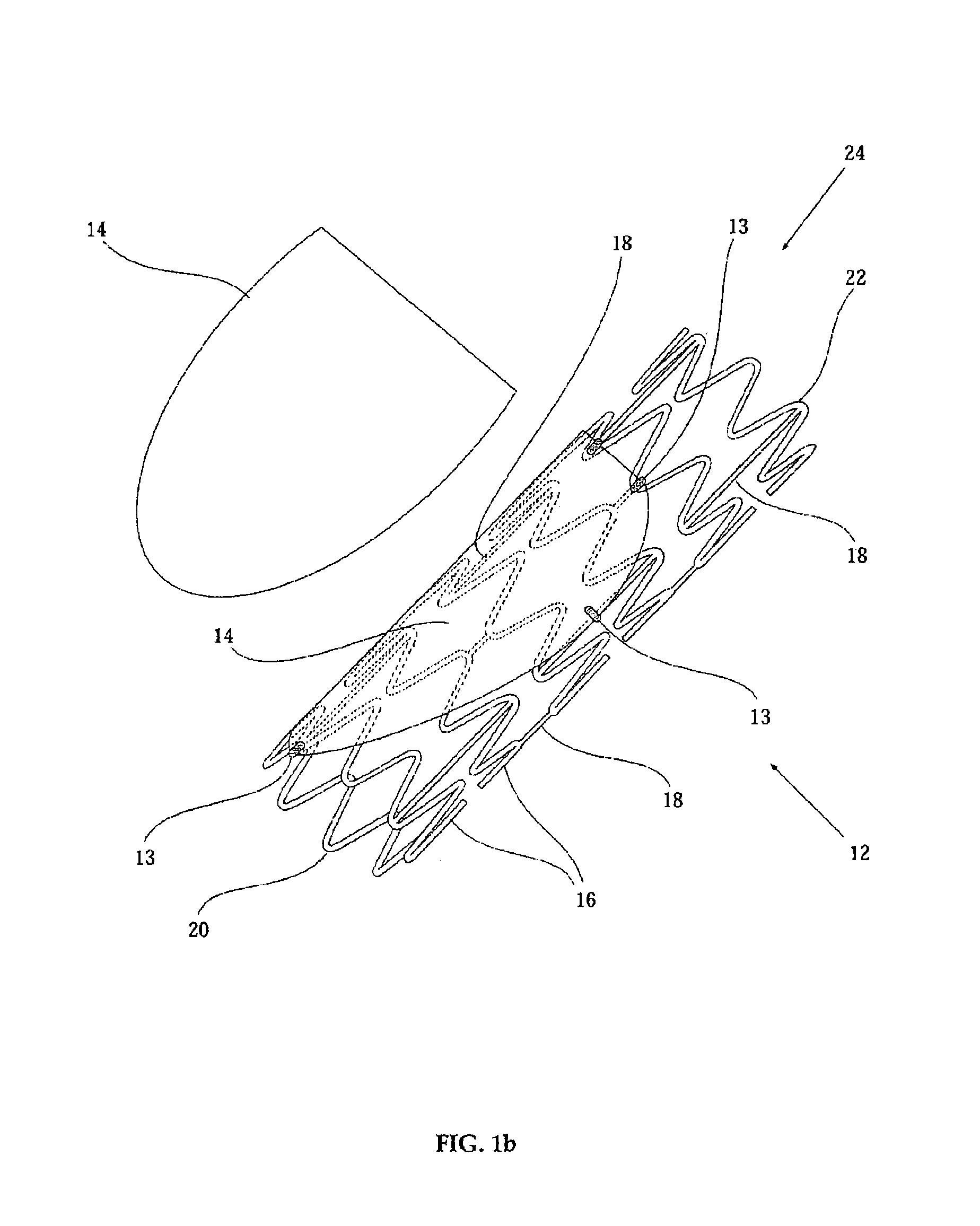
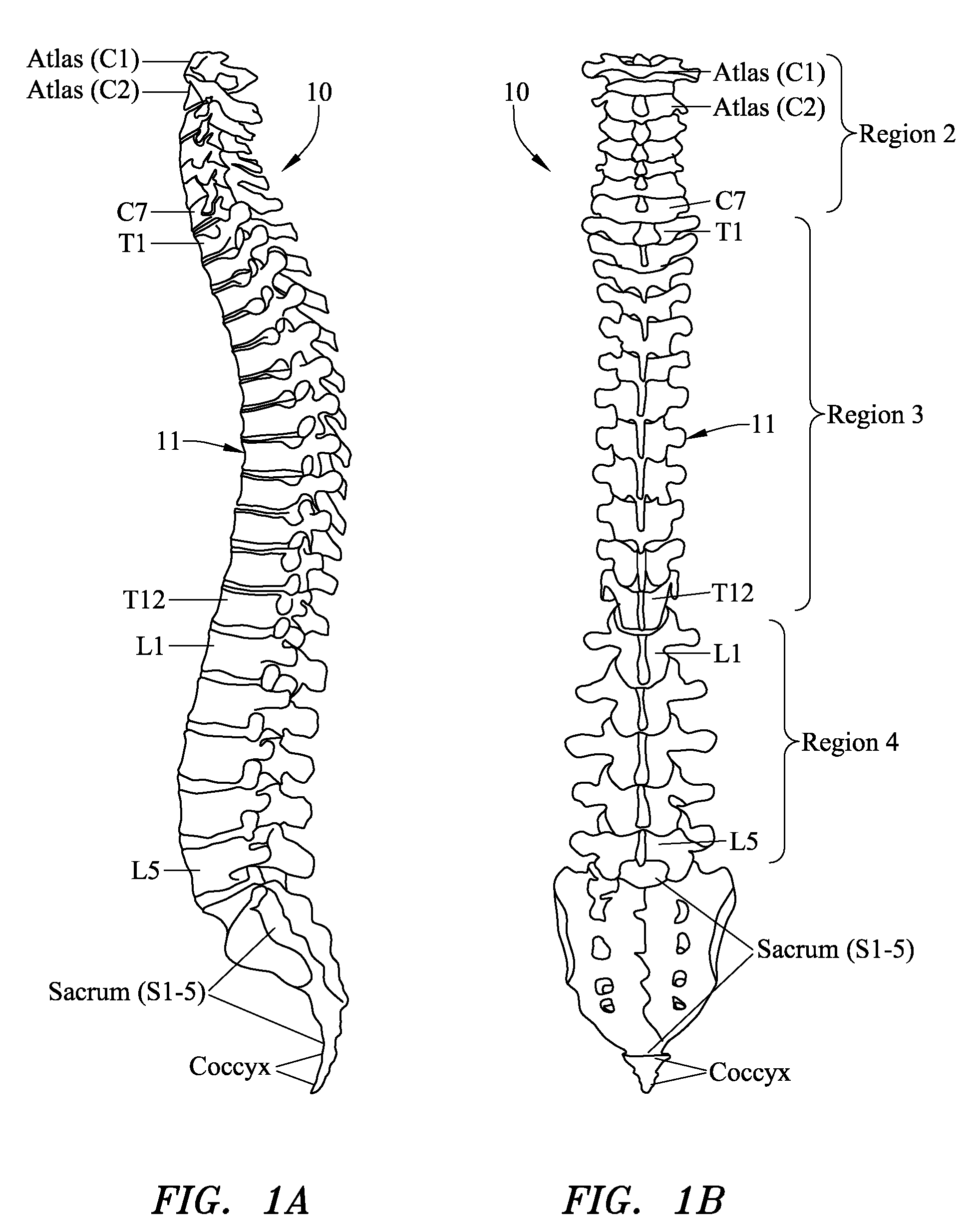
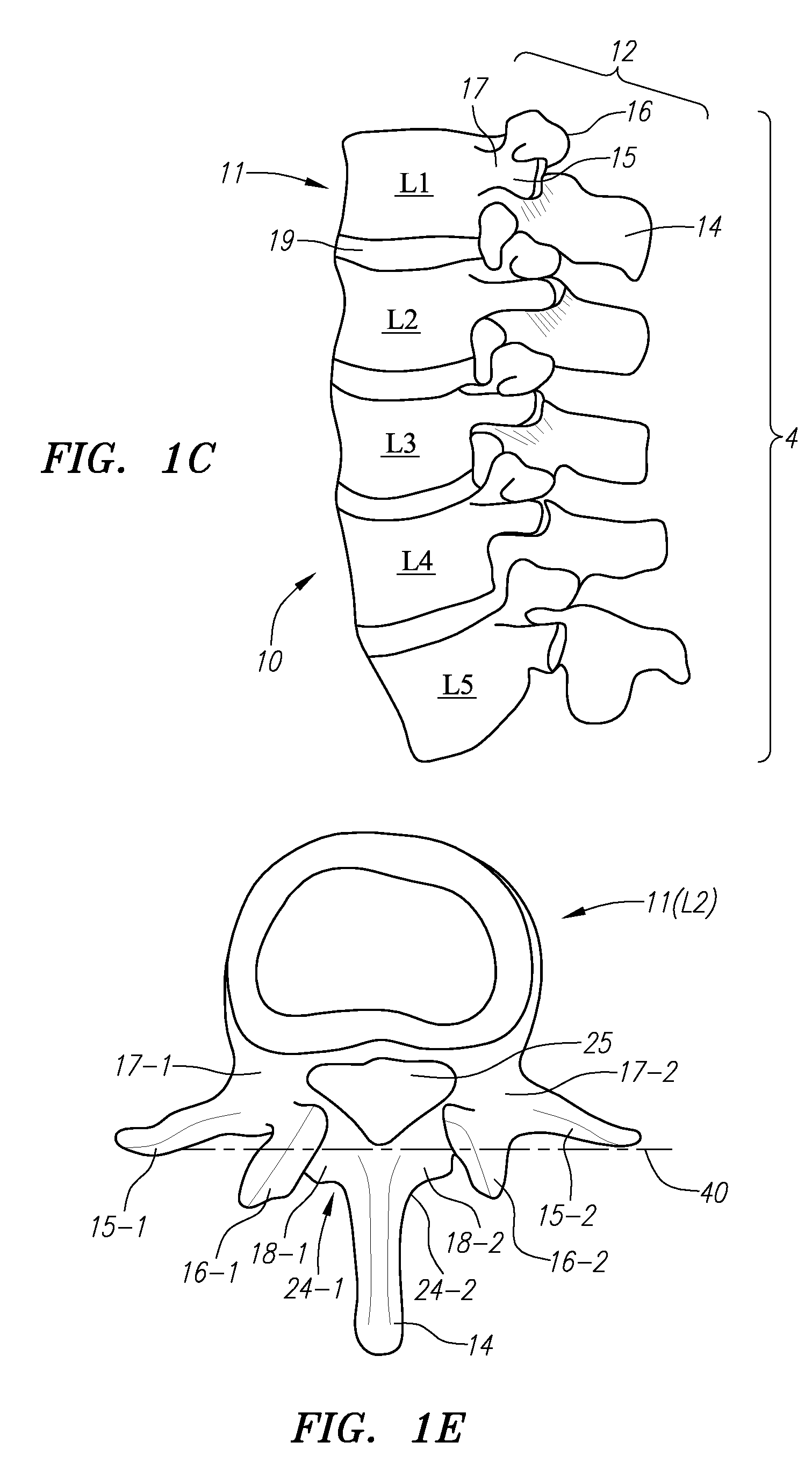
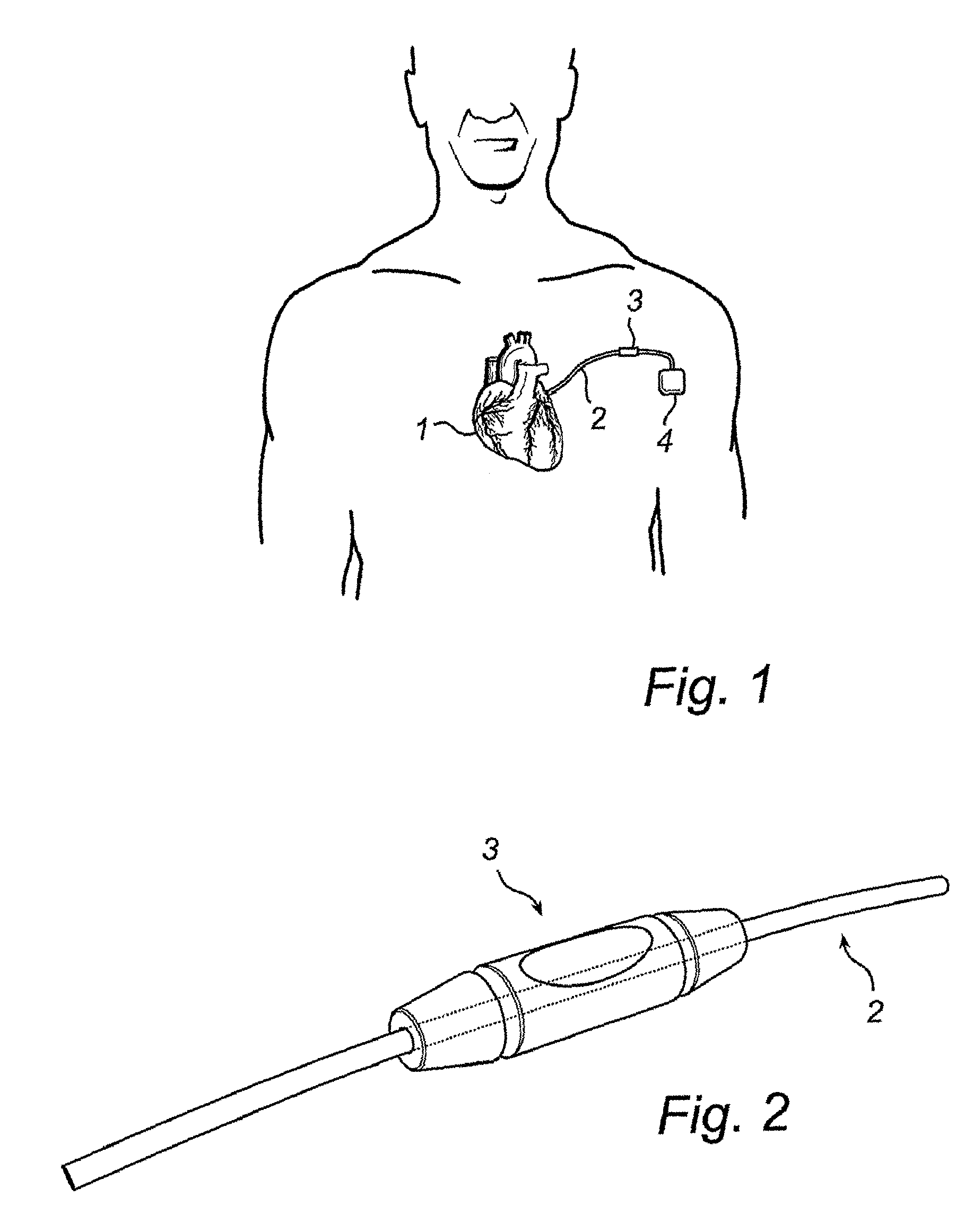
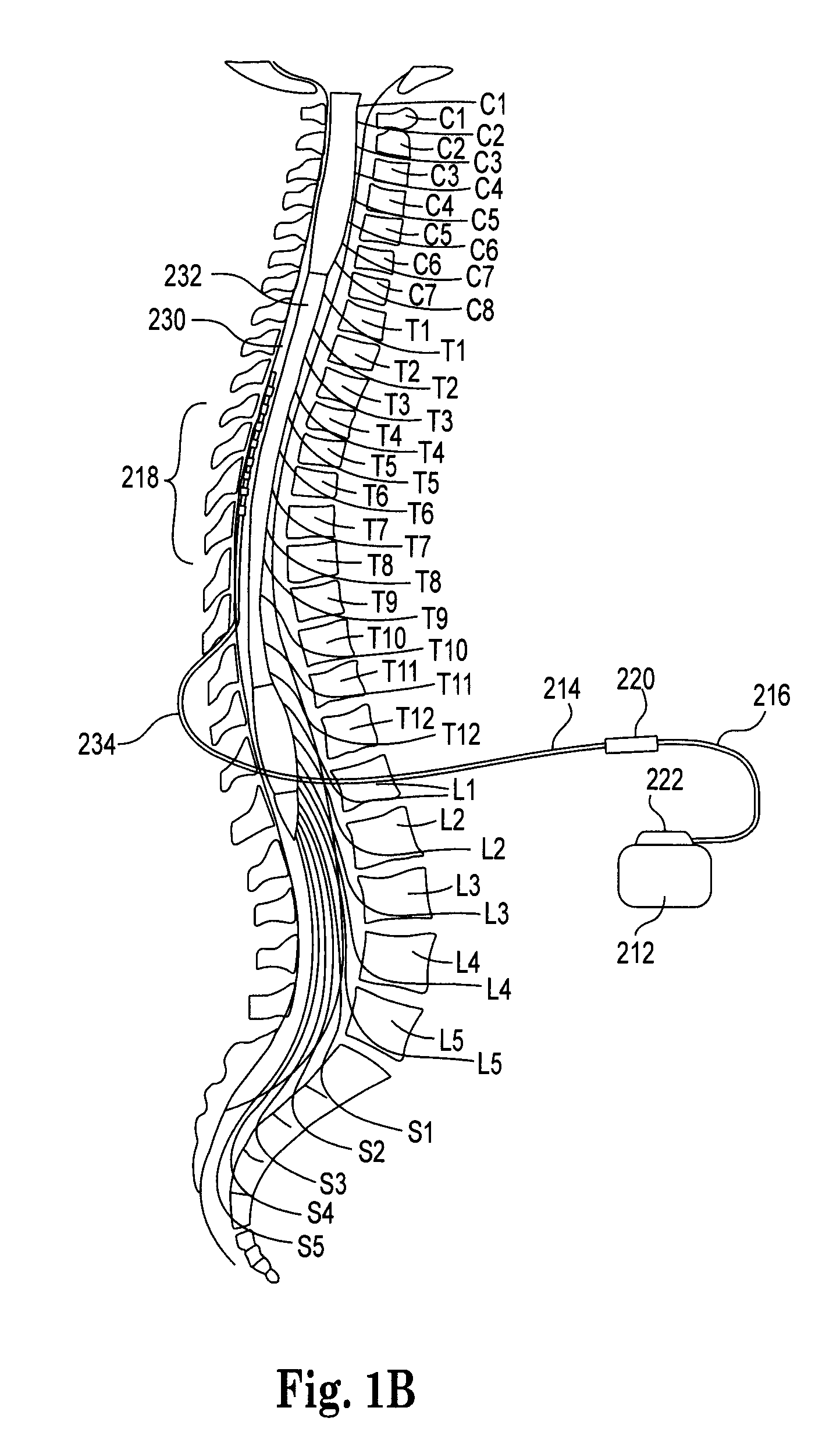
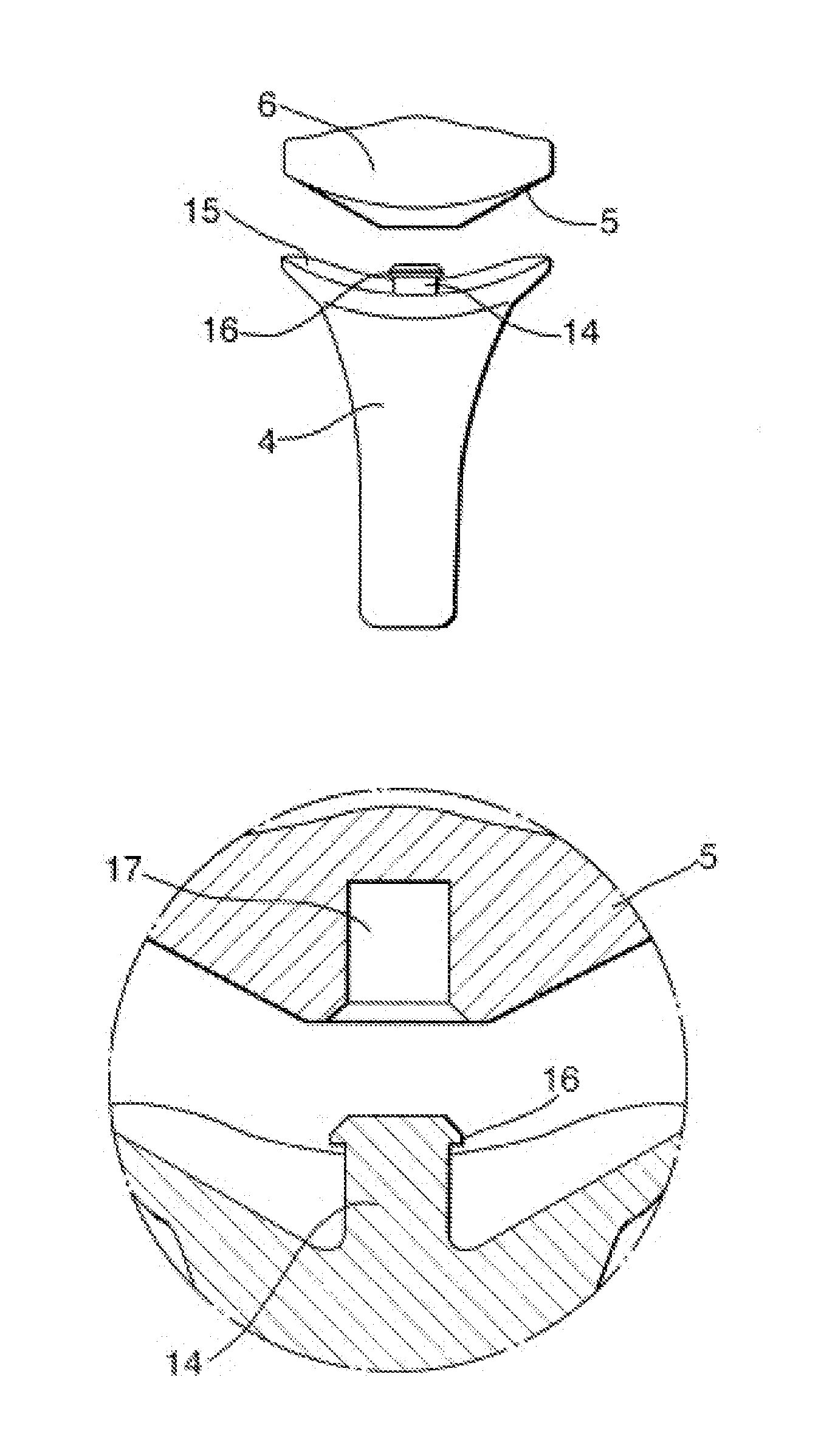
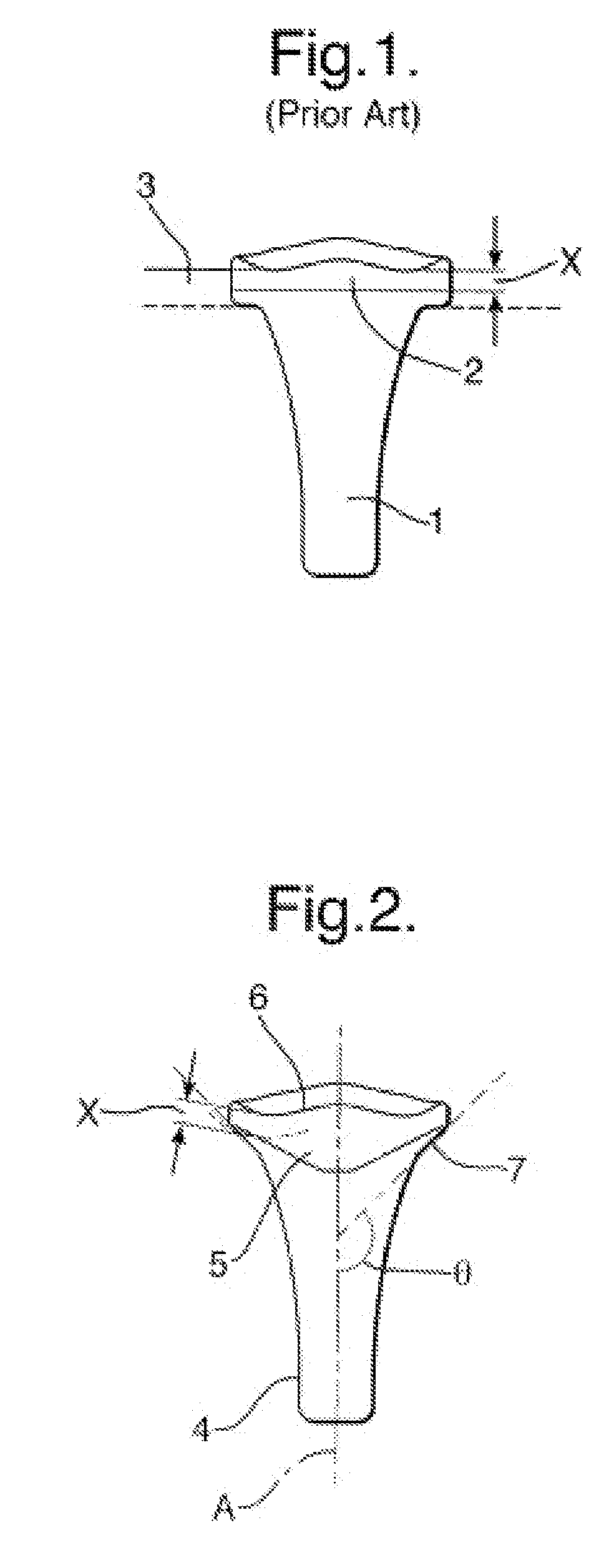
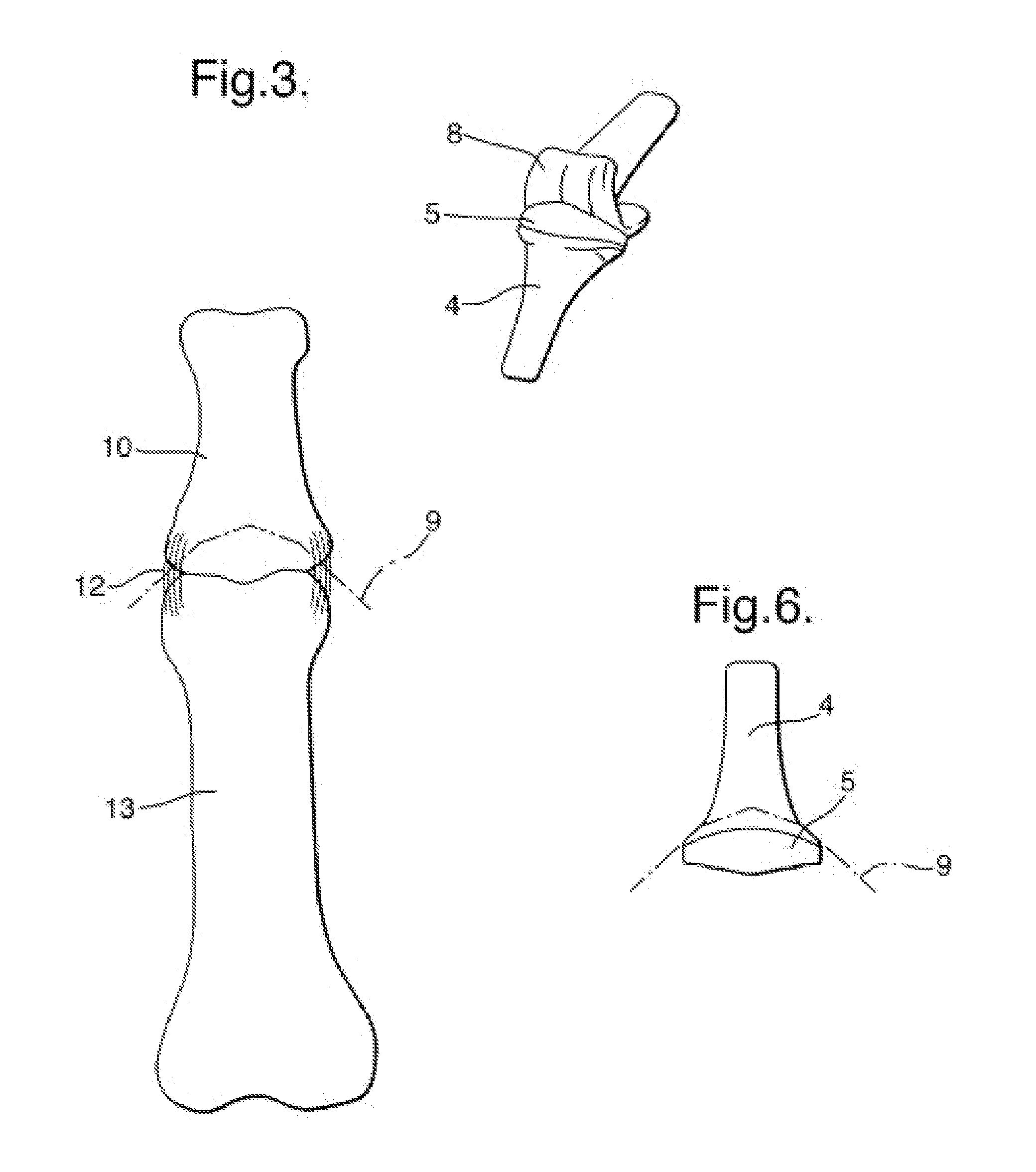
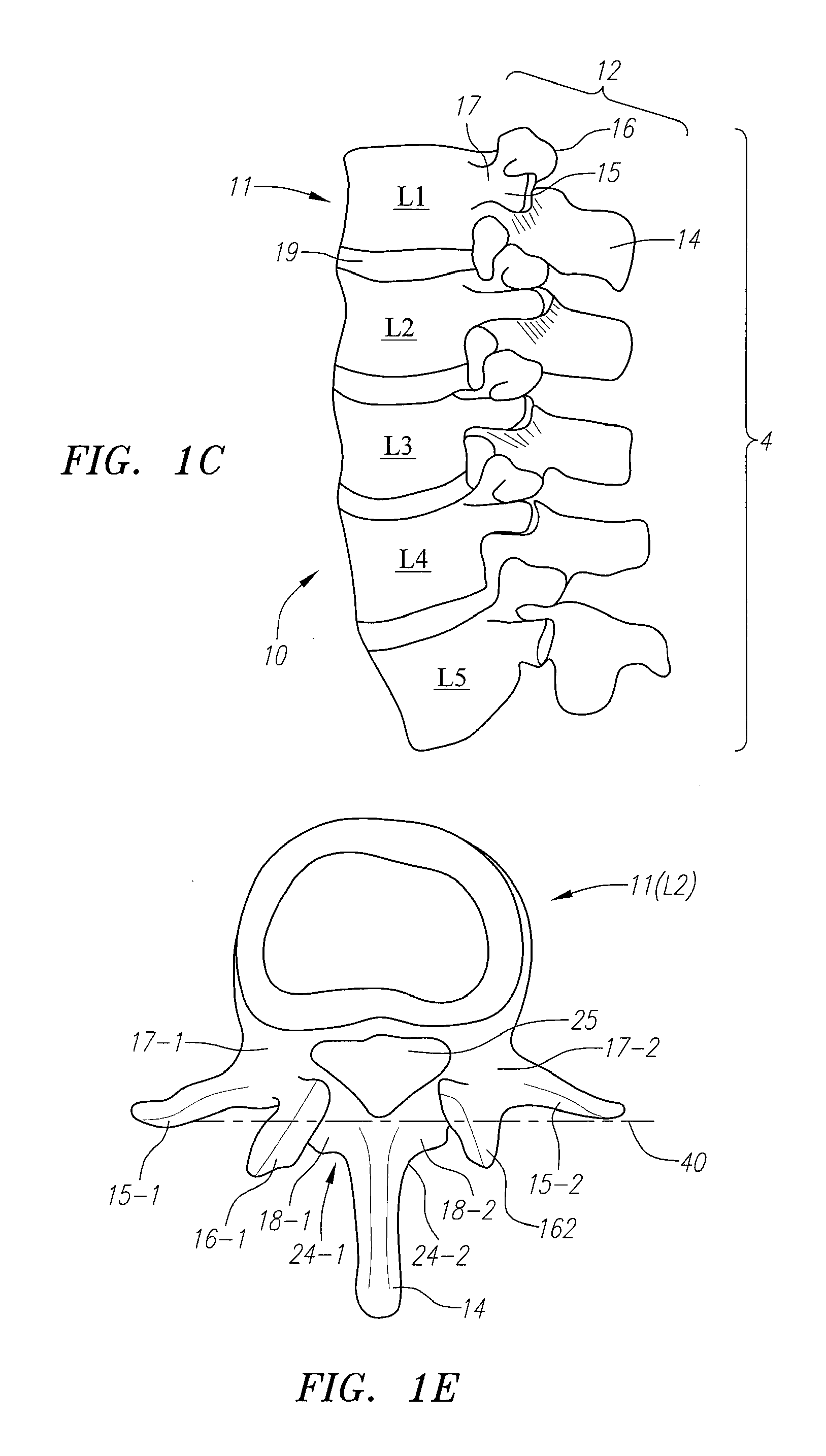
Computer-aided three-dimensional bending of spinal rod implants, other surgical implants and other articles, systems for three-dimensional shaping, and apparatuses therefor
InactiveUS20050262911A1Satisfactory reproductionGlobal accuracyOsteosynthesis devicesImplantable rodComputer aid
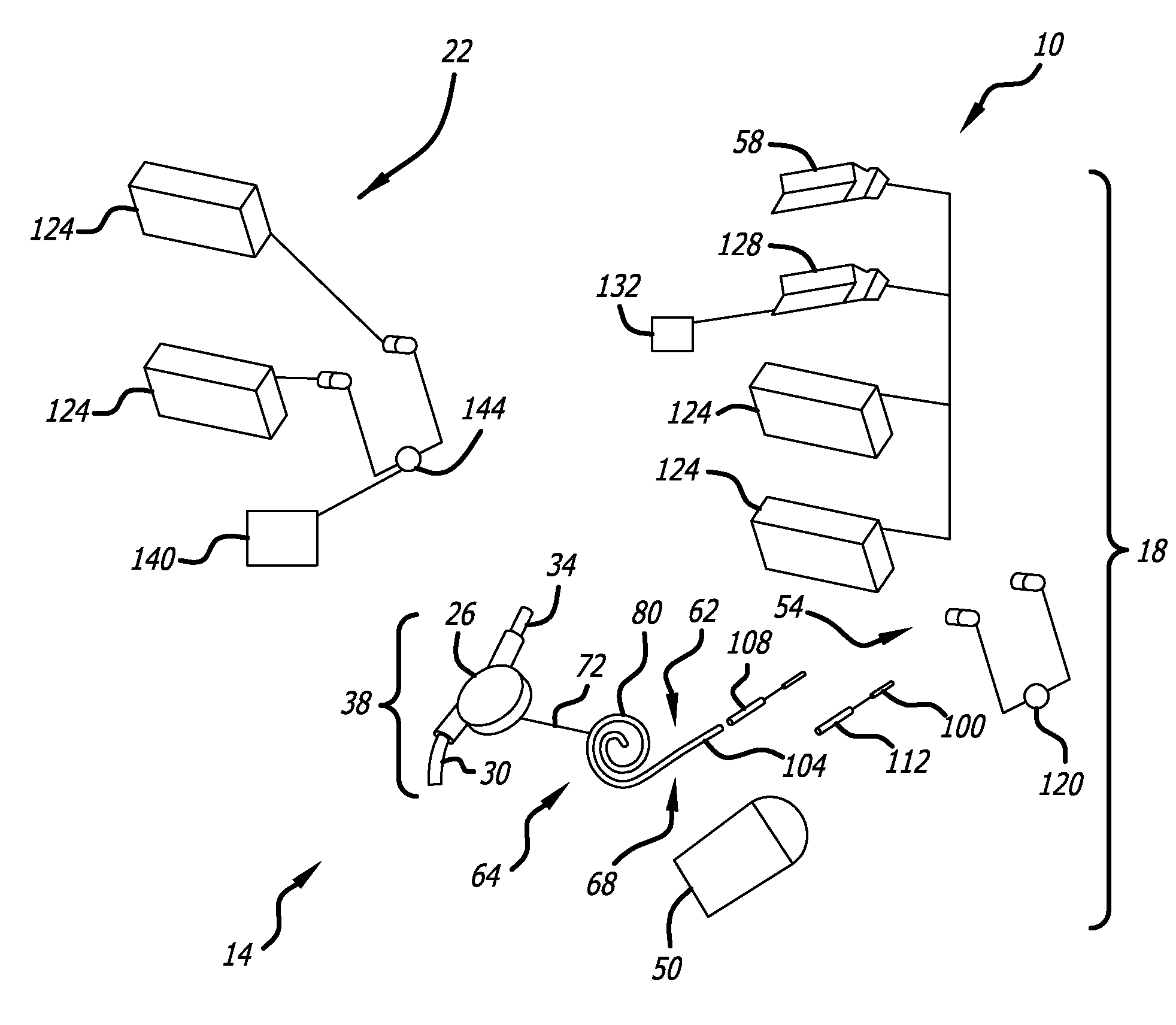
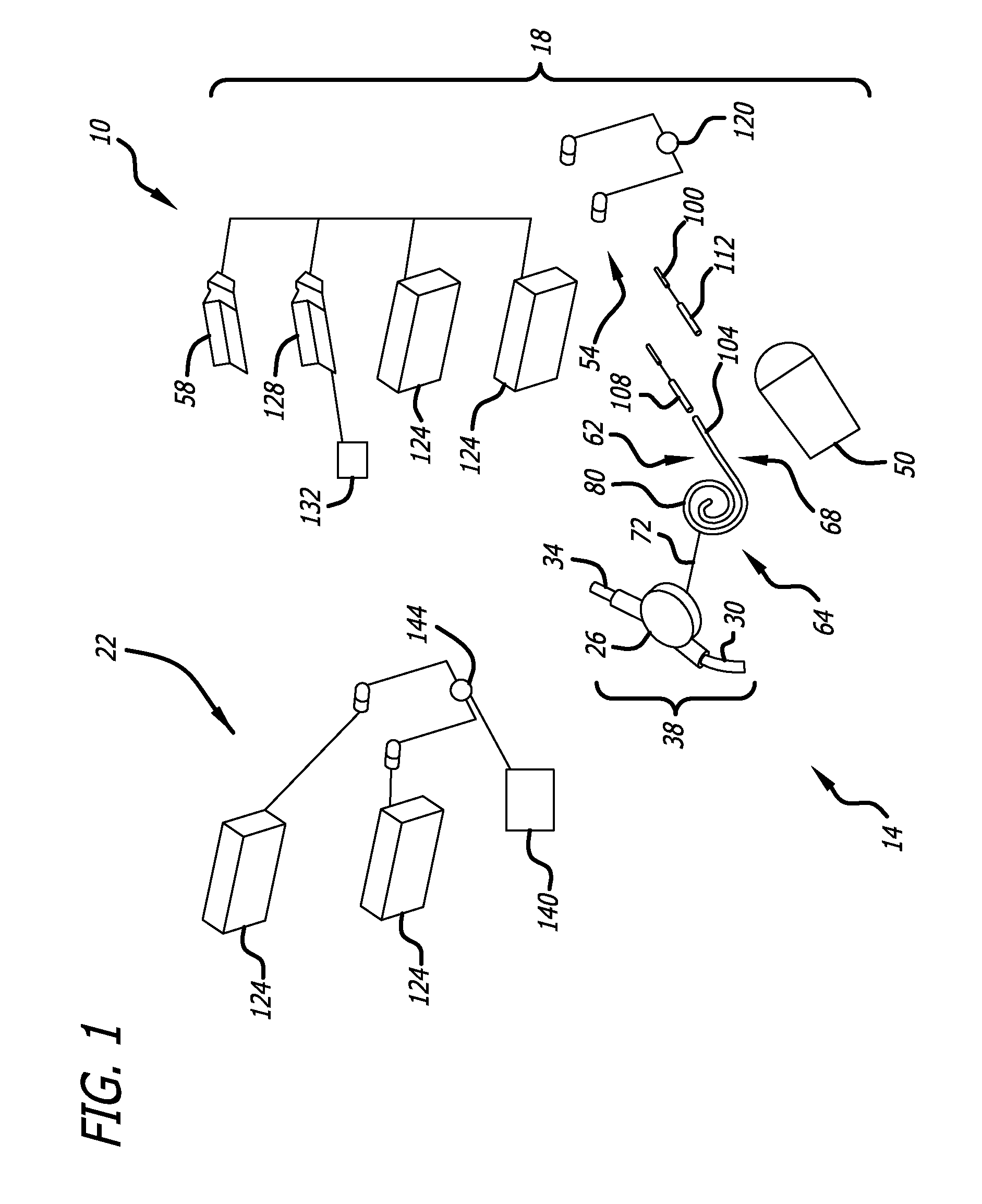
An implantable rod can be bent three-dimensionally in an automated system, which is especially useful for pre-surgical formation of implantable spinal rods. When local and / or global feedback processing accompanies a series of shaping steps automatically imposed on a rod or other article being shaped into three-dimensional form, formation time may be expedited compared to manual creation, and shapes difficult or impractical to create manually may be constructed simply.
Owner:VIRGINIA POLYTECHNIC INSTITUTE AND STATE UNIVERSITY +1
Systems, Devices and Methods for the Correction of Spinal Deformities
Provided herein are systems, devices and methods for the correction of spinal deformities with the use one or more implantable rods configured to apply a corrective force to the spine. Methods of minimally invasive implantation of a corrective system are provided, such as where the corrective system is attached only to the spinous process of one or more vertebral bodies. Various corrective systems as well as components thereof are also provided.
Owner:REDUCTION TECH
Implantable lead and surgical accessories
InactiveUS8968331B1Improve stress resistanceEliminate useHead electrodesDiagnosticsImplantable rodElectrical conductor
Leads for chronic implantation in the brain or other anatomical targets utilize tubular stylet means which are external to the lead. The lead comprises a distal electrode terminal, a proximal connector terminal, and a conductor cable having a reinforced distal portion and a stepped outside diameter providing a shoulder which cooperates with the distal end of the external stylet means. The substantial stiffness of the external stylet allows implantation of the lead without a brain-entering cannula. A tubular stylet spacer is employed to minimize lead dislodgement due to disassembly and removal of the lead introduction tools. Externalized stylet allows the conductor cable to have a small outside diameter and a desirably short length. A method of terminating conductors to electrodes using inserts is suitable for very fine wires and stranded conductors. A reinforced electrode terminal construction enables robust small-dimensioned terminal and high localization accuracy when introduced with external stylet means.
Owner:SOCHOR JERZY ROMAN
Implantable VAD with replaceable percutaneous cable
There are disclosed apparatus and methods for replacing a percutaneous cable in connection with a vascular device. In an embodiment, the apparatus includes a distal disconnect coupler, a distal connector portion of the cable configured for removable connection with the distal disconnect coupler, and a connector cap configured for removable connection with the distal disconnect coupler and for tunneling through skin and tissue. In one embodiment, a method of repositioning a percutaneous cable in connection with a vascular device includes providing the cable with a distal disconnect coupler, disconnecting the cable at the distal disconnect coupler, attaching a connector cap to the distal disconnect coupler, removing the percutaneous cable from a first exit site, tunneling the connector cap together with the distal disconnect coupler through skin and tissue to form a new exit site, disconnecting the connector cap, and connecting the cable to the distal disconnect coupler.
Owner:WORLD HEART
Prosthesis
A distal component for an interphalangeal prosthesis suitable for insertion into a proximal end of a distal phalangeal bone includes a stem portion shaped to be received within a surgically prepared bore in the proximal end of the distal bone. A head portion has an under-surface and an upper-surface, the under-surface of which in use will bear on the bone into which the stem is implanted.
Owner:MATORTHO
Systems, methods and devices for correcting spinal deformities
Provided herein are systems, devices and methods for the correction of spinal deformities with the use one or more implantable rods configured to apply a corrective force to the spine. Methods of minimally invasive implantation of a corrective system are provided, such as where the corrective system is attached only to the spinous process of one or more vertebral bodies. Various corrective systems as well as components thereof are also provided, such as those that allow limited movement with respect to the spinal column.
Owner:REDUCTION TECH
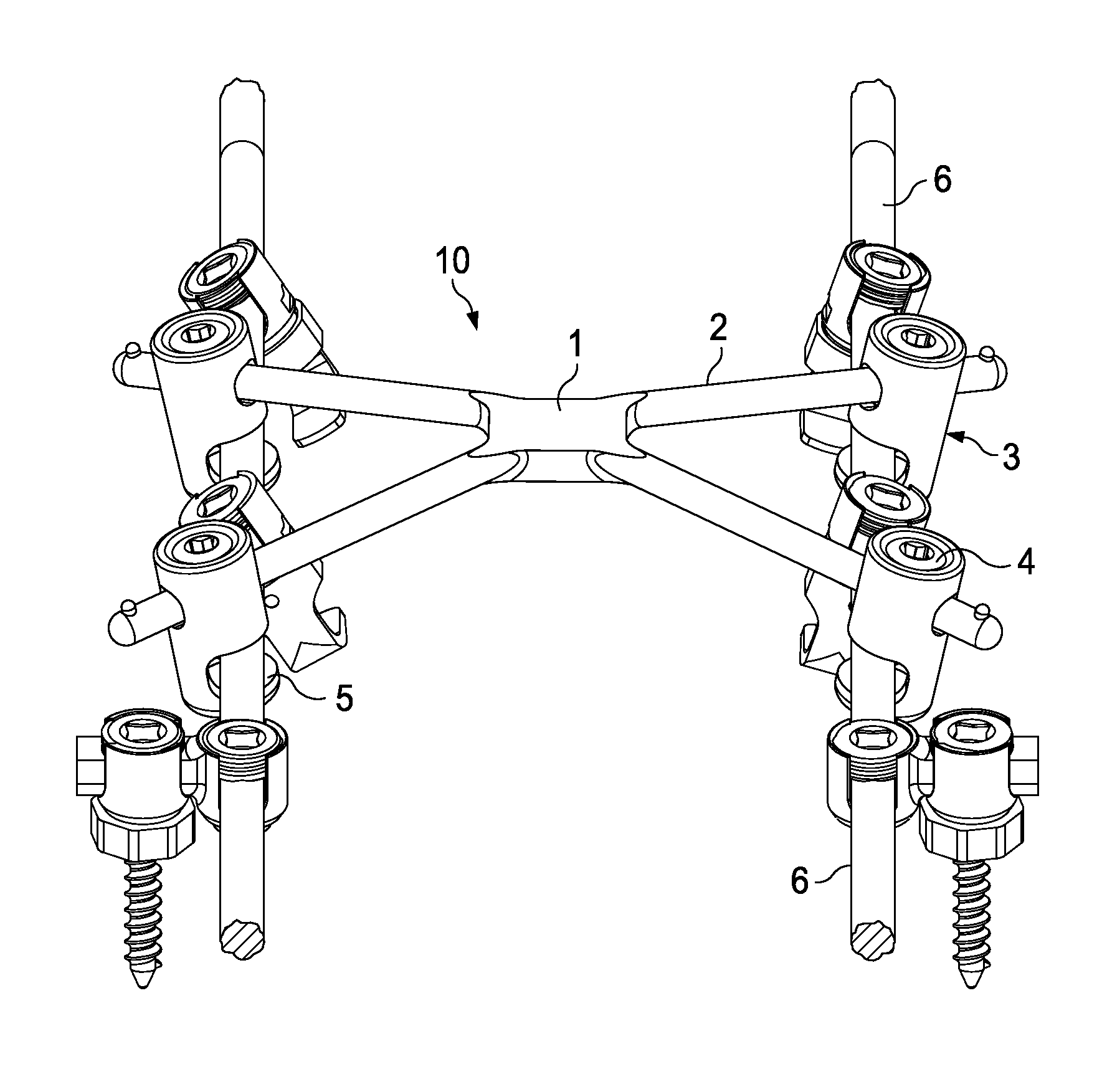
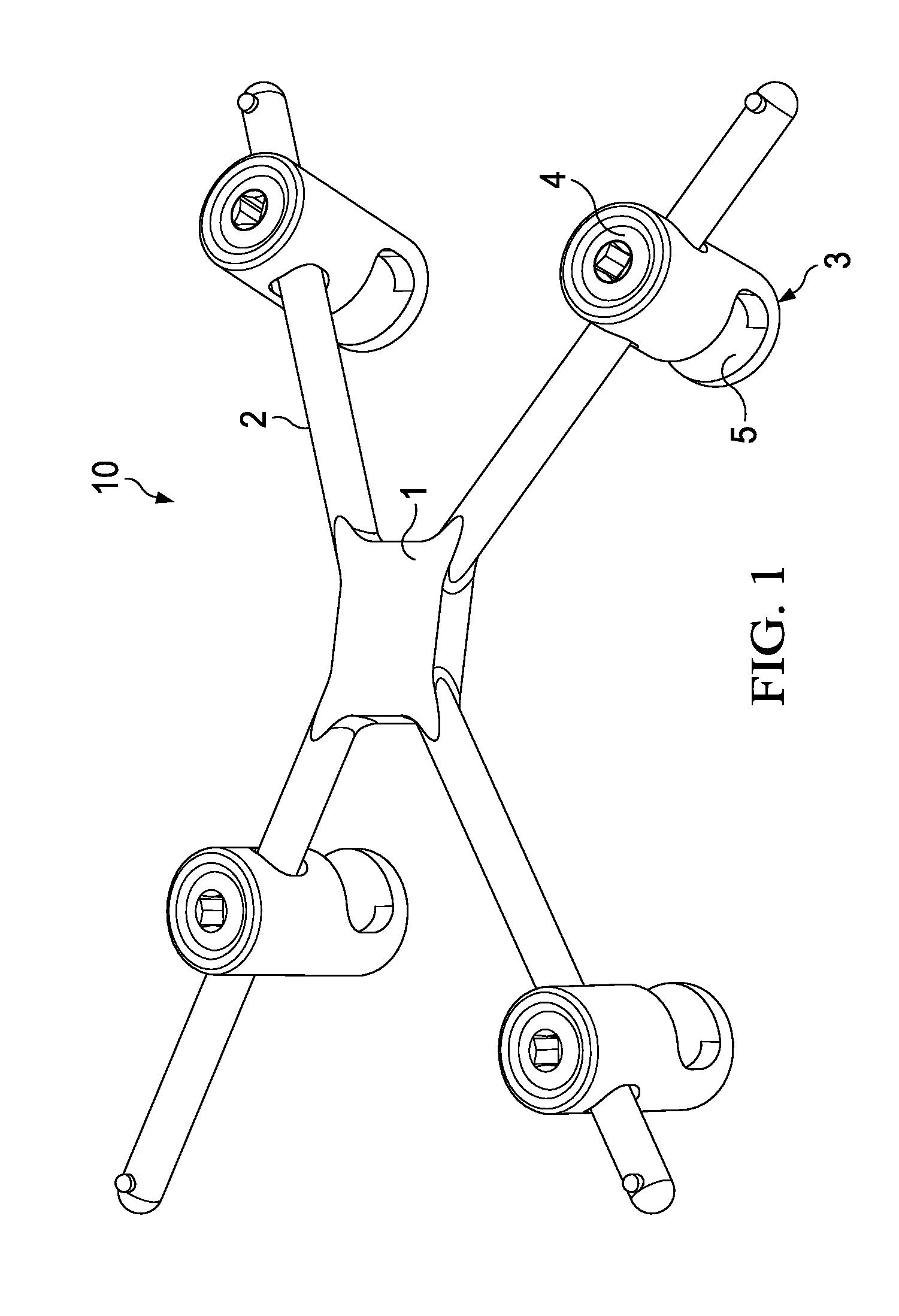
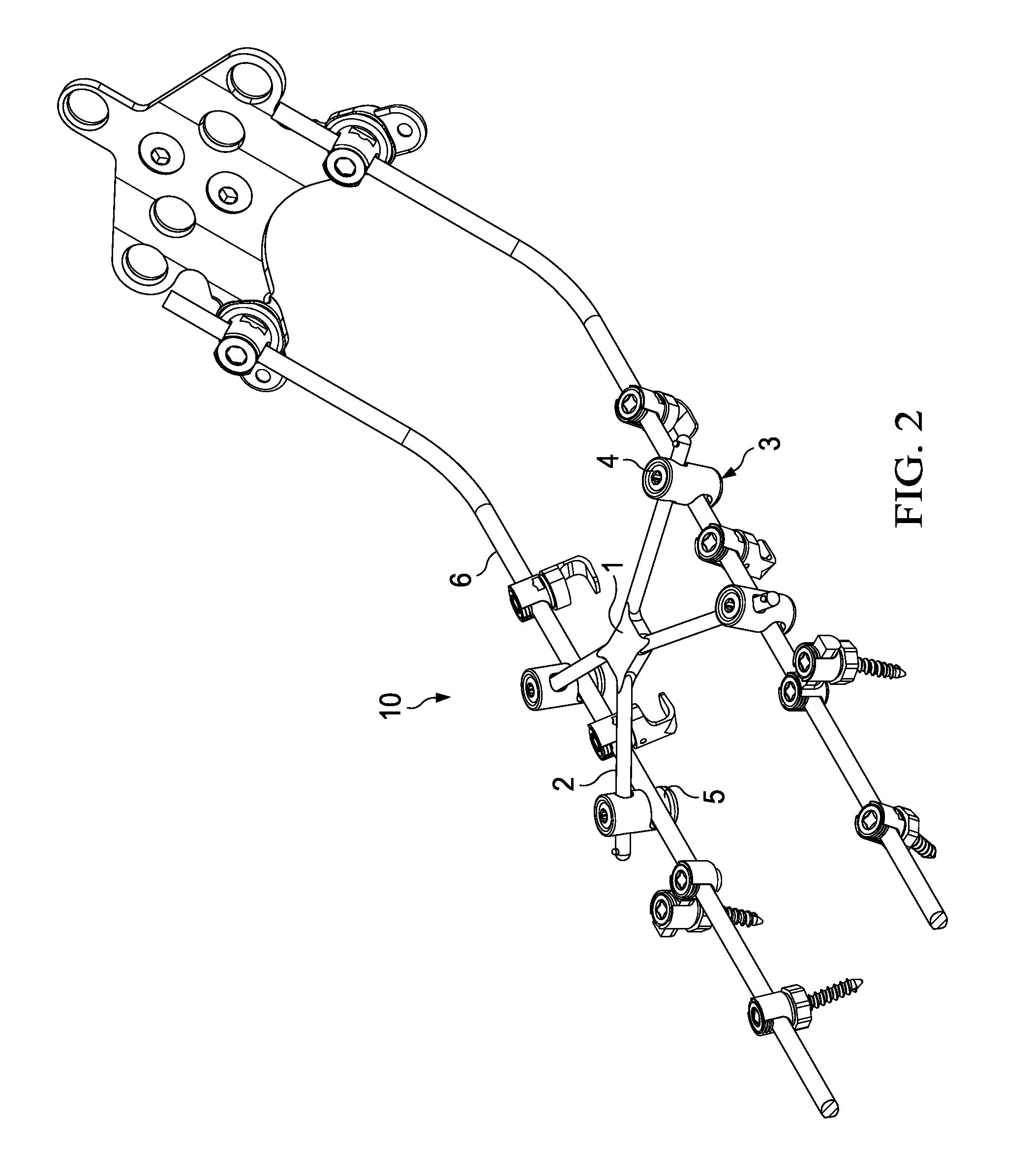
Cross-braced bilateral spinal rod connector
InactiveUS20140277146A1Alleviates stress shieldingStiffer vertebral bodyInternal osteosythesisJoint implantsImplantable rodCross bracing
A spinal rod connector apparatus for use with spinal implantation rods and methods of securing implantation rods using a cross-braced bilateral spinal rod connector apparatus. The spinal rod connector apparatus comprises a central member having two or more extension legs. The extension legs can be of varying lengths and disposed at varying angles with respect to the monolithic central member, depending upon physiological sizing requirements. Attached to each extension leg is a connecting member capable of connecting to a bilateral spinal rod construct.
Owner:BLACKSTONE MEDICAL
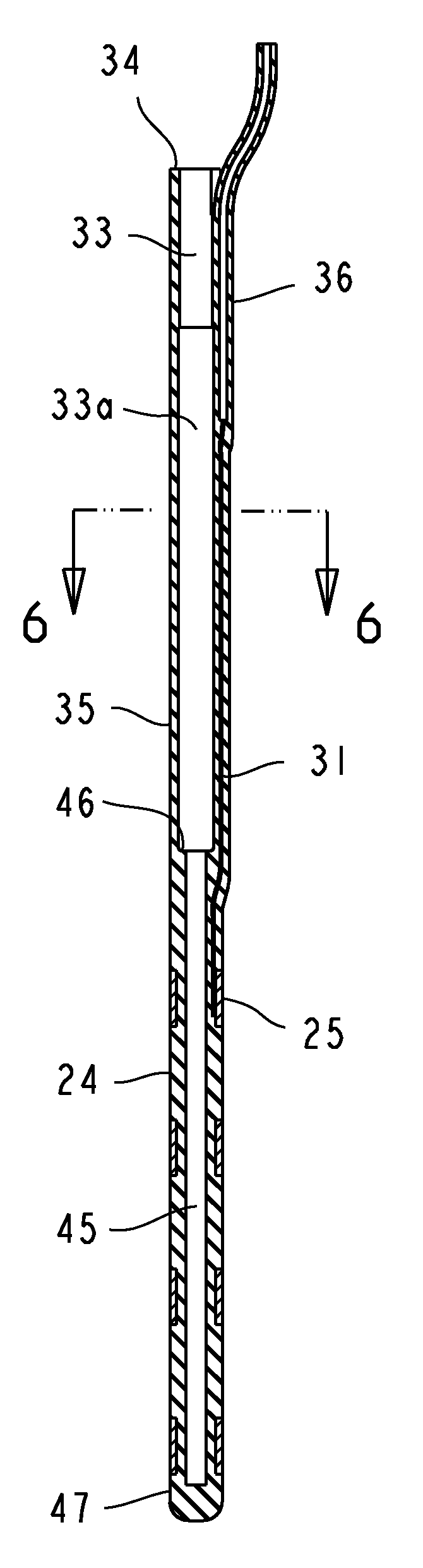
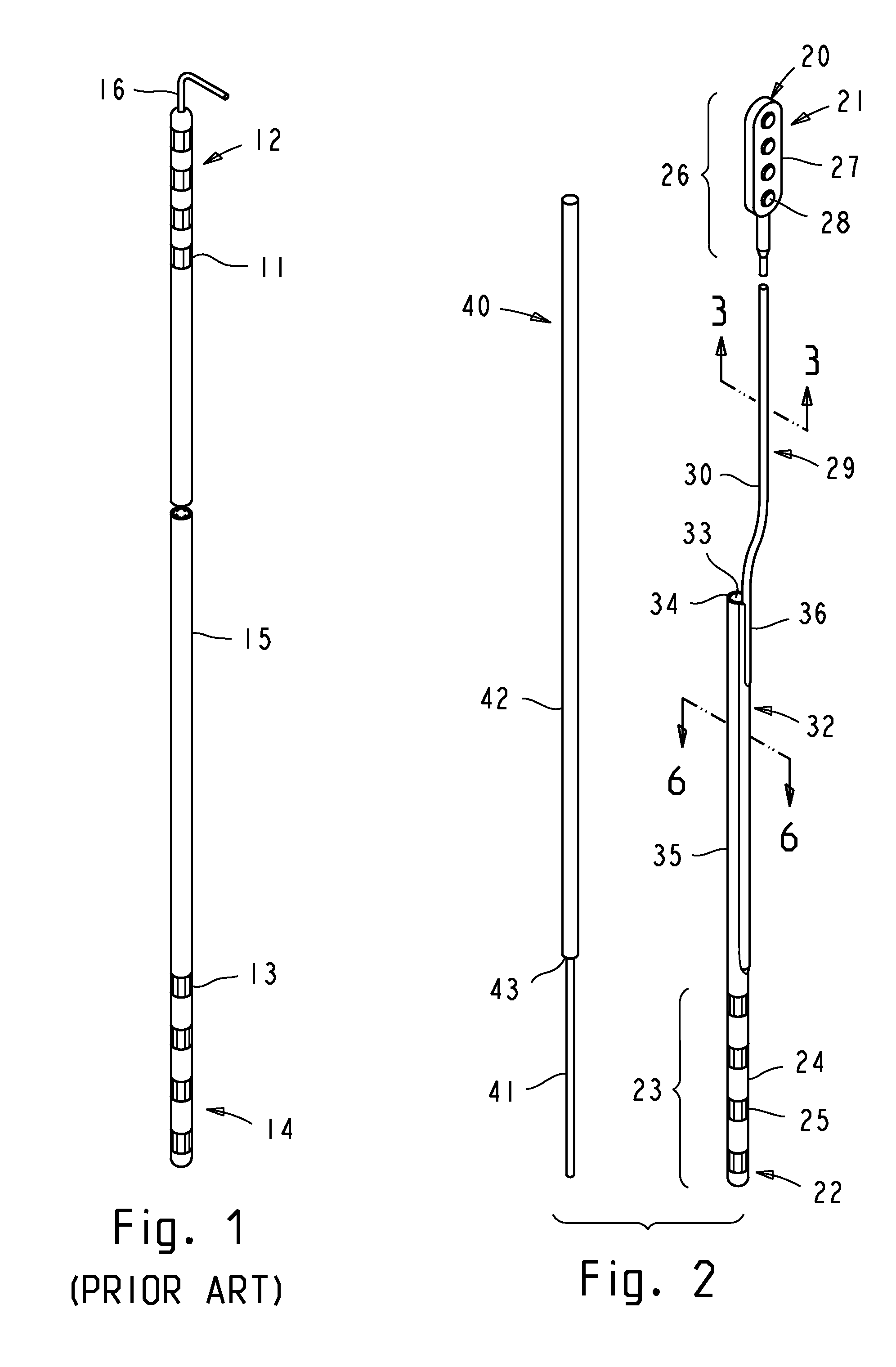
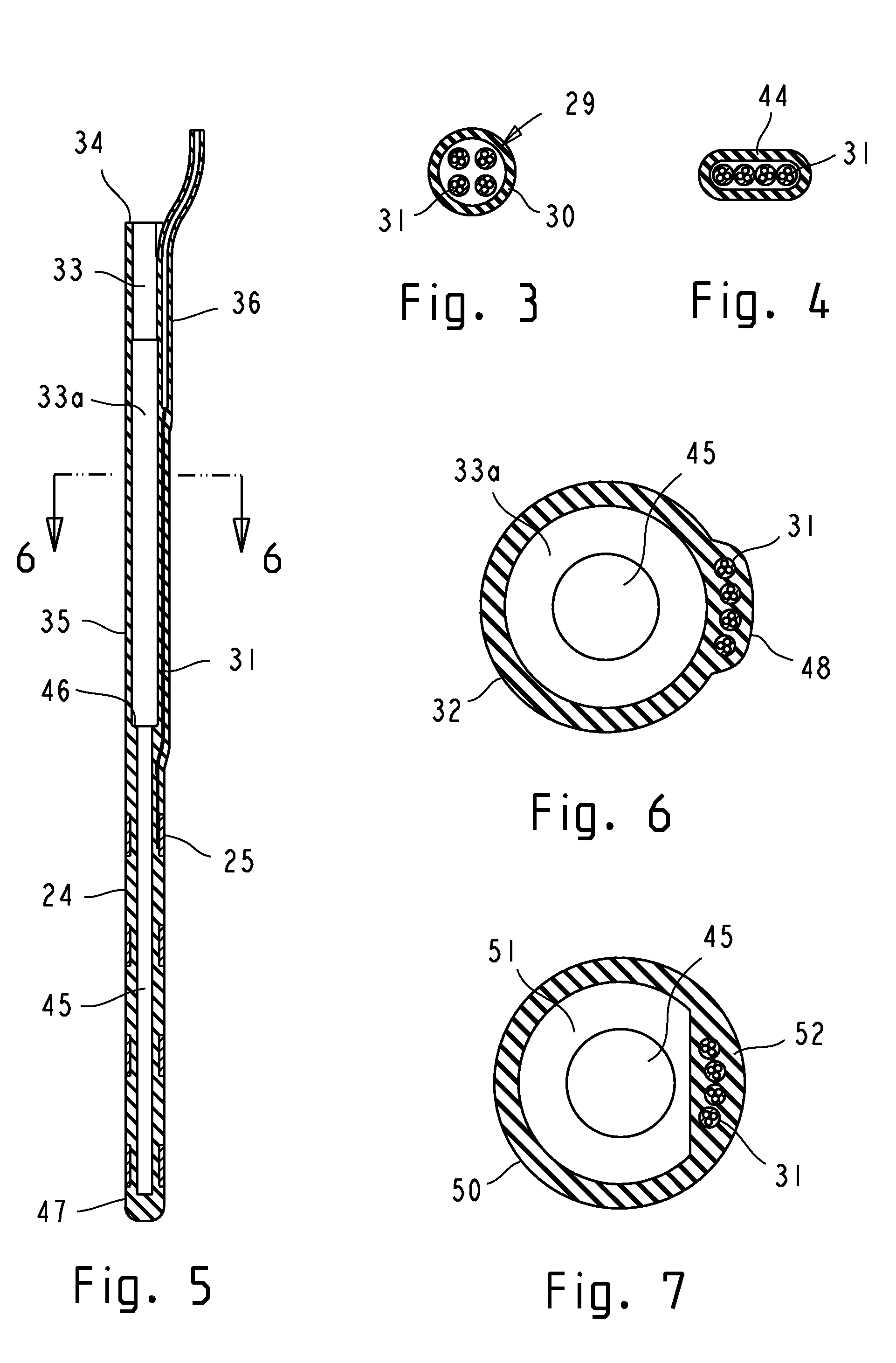
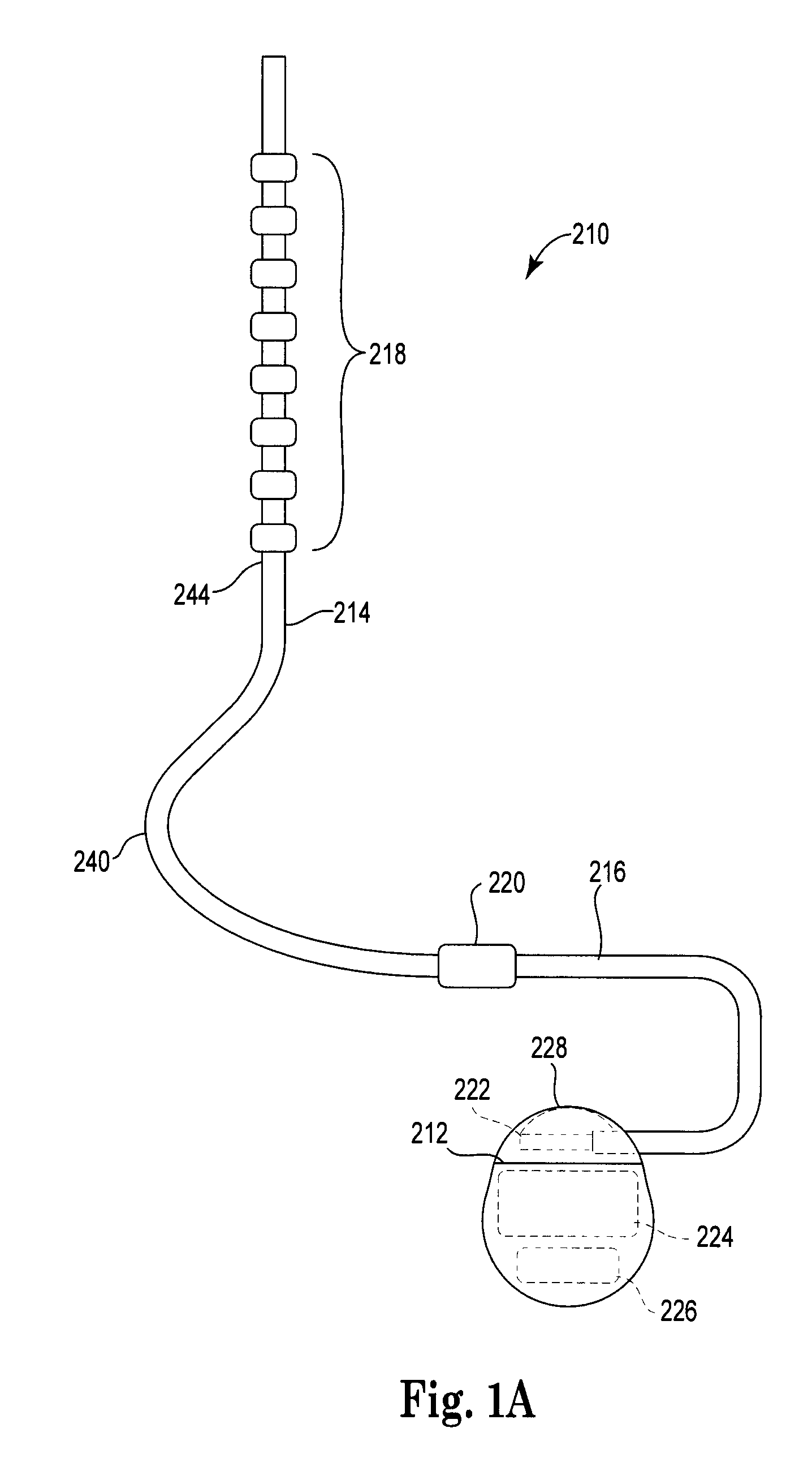
Implantable leads with a low profile distal portion
A stimulation lead includes an elongate body having a proximal portion and a distal portion and has a multilumen tube extending along the elongate body and defining a central lumen and a plurality of peripheral lumens disposed circumferentially around the central lumen; a plurality of conductors, at least one of the conductors extending along the central lumen and a remainder of the conductors extending along the plurality of peripheral lumens with at least one of the conductors in each peripheral lumen; a plurality of terminals disposed along the proximal portion of the elongate body and electrically coupled to proximal ends of the conductors; and a plurality of electrodes disposed along the distal portion of the elongate body and electrically coupled to distal ends of the conductors. Each of the conductors is coupled to at least one terminal and at least one electrode.
Owner:BOSTON SCI NEUROMODULATION CORP
Implantable graft assembly
InactiveUS20110160833A1Small surface areaLow profileStentsBlood vesselsImplantable rodCatheter device
An implantable graft-assembly has a) a radially expandable substantially tubular frame (e.g., a stent); and b) a graft having an at least partially curved periphery, such as an oval or circular graft. Also provided are methods of treating aneurysms using such graft assemblies, methods of making the graft assemblies, use of sheets of materials for making the graft assemblies, and methods of mounting graft-assemblies having partial covers such as grafts on delivery devices such as delivery catheters or inside delivery sheaths.
Owner:DESIGN & PERFORMANCE CYPRUS
Implantable lead and accessories
ActiveUS8543222B1Flexible cuttingPromote robustnessHead electrodesDiagnosticsStereotactic localizationElectrical conductor
Owner:SOCHOR JERZY ROMAN
Implantable heart assist system
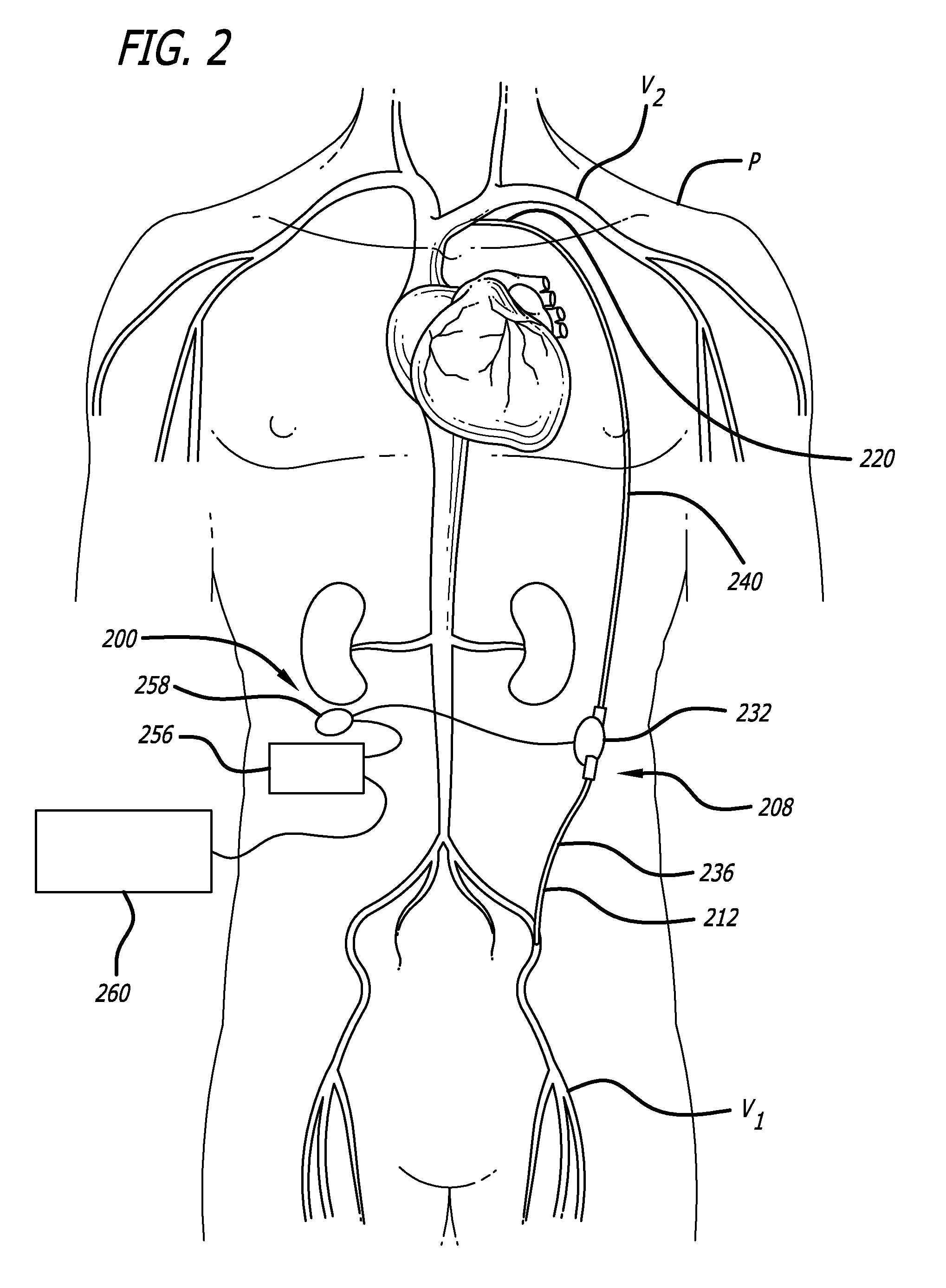
InactiveUS20110152600A1Reduce electrical noiseRelieve pressureControl devicesMedical devicesImplantable rodControl signal
A heart assist system having an implantable pump conveying blood between two vascular locations and an extracorporeal system providing power and control signals to the pump. The system also includes a communication link having an implantable portion coupled to the implantable pump, an extracorporeal portion coupled to the extracorporeal system and an isolation portion between the implantable portion and the extracorporeal portion that minimizes the transmission of movement and forces from the extracorporeal portion to the implantable portion.
Owner:TC1 LLC
Systems, Methods And Devices For Correcting Spinal Deformities
Provided herein are systems, devices and methods for the correction of spinal deformities with the use one or more implantable rods configured to apply a corrective force to the spine. Methods of minimally invasive implantation of a corrective system are provided, such as where the corrective system is attached only to the spinous process of one or more vertebral bodies. Various corrective systems as well as components thereof are also provided, such as those that allow limited movement with respect to the spinal column.
Owner:REDUCTION TECH
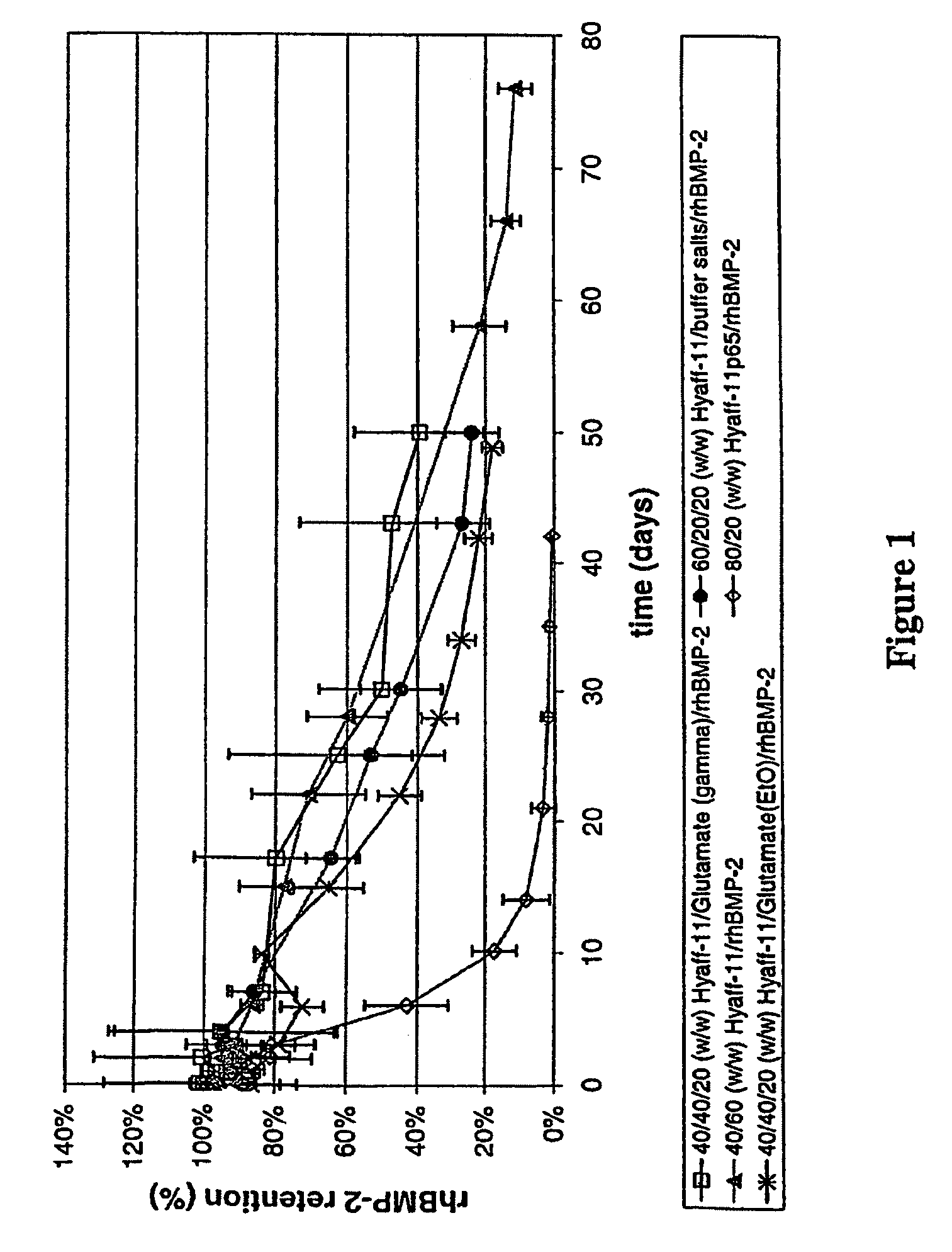
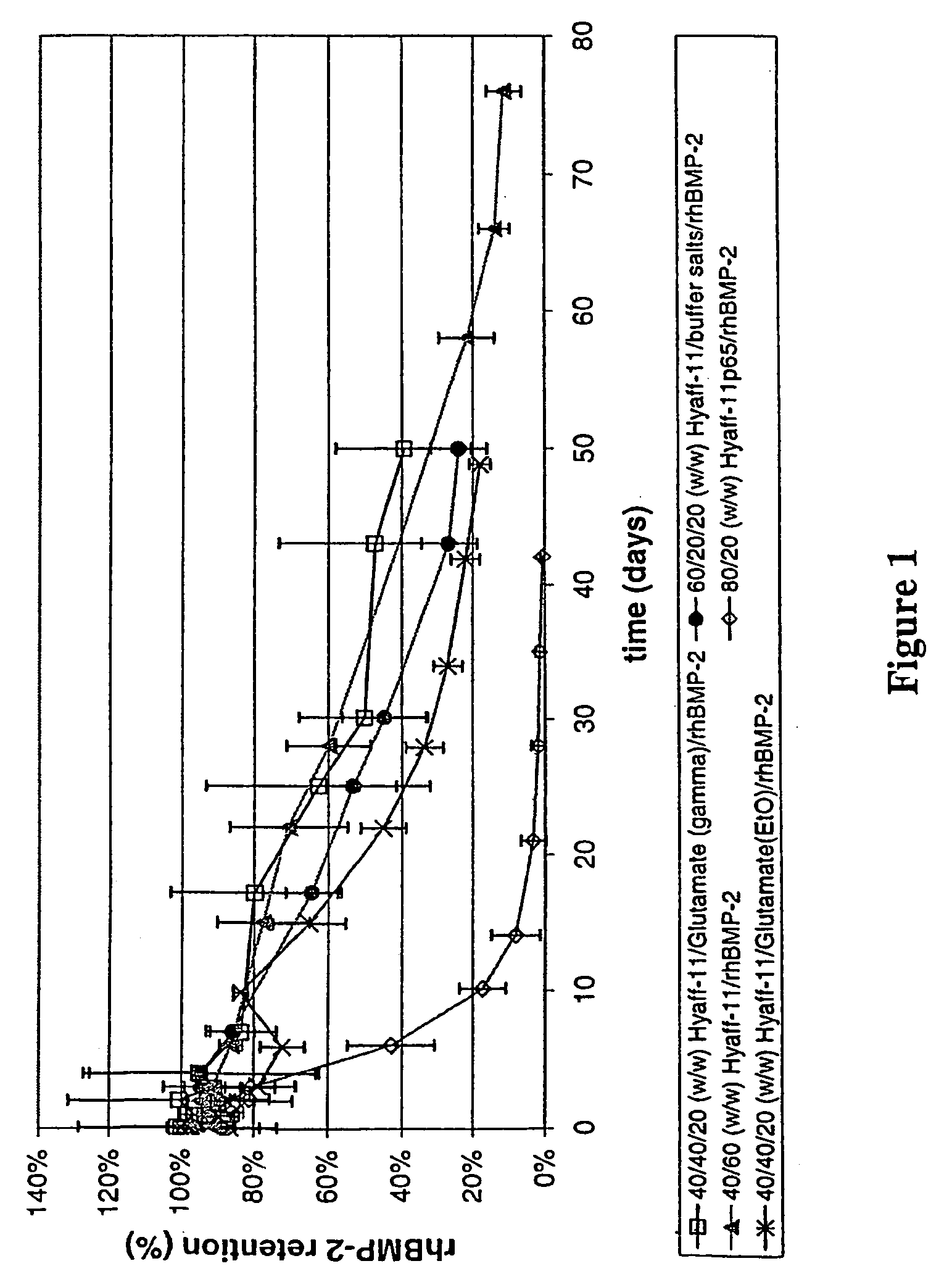
Injectable solid hyaluronic acid carriers for delivery of osteogenic proteins
InactiveUS20090181058A1High retention rateMinimizing and reducing incidence and severityOrganic active ingredientsPeptide/protein ingredientsImplantable rodOsteogenic proteins
Methods of using an injectable or implantable rod-shaped formulation for delivery of osteogenic proteins to treat osteoporotic and / or osteopenic bone are disclosed. The formulation comprises hyaluronic acid derivatives and osteogenic proteins, and optional excipients and active ingredients such as a bone resorption inhibitor.
Owner:WYETH LLC +1
Injectable solid hyaluronic acid carriers for delivery of osteogenic proteins
InactiveUS20050287135A1High retention rateMinimizing and reducing incidence and severityBiocidePeptide/protein ingredientsImplantable rodOsteogenic proteins
An injectable or implantable rod-shaped formulation is disclosed for delivery of osteogenic proteins. The formulation comprises hyaluronic acid derivatives and osteogenic proteins, and optional excipients and active ingredients such as a bone resorption inhibitor. Methods of making injectable rod-shaped pharmaceutical compositions and methods of using the osteogenic compositions to treat osteoporotic and / or osteopenic bone are also disclosed.
Owner:WYETH +1
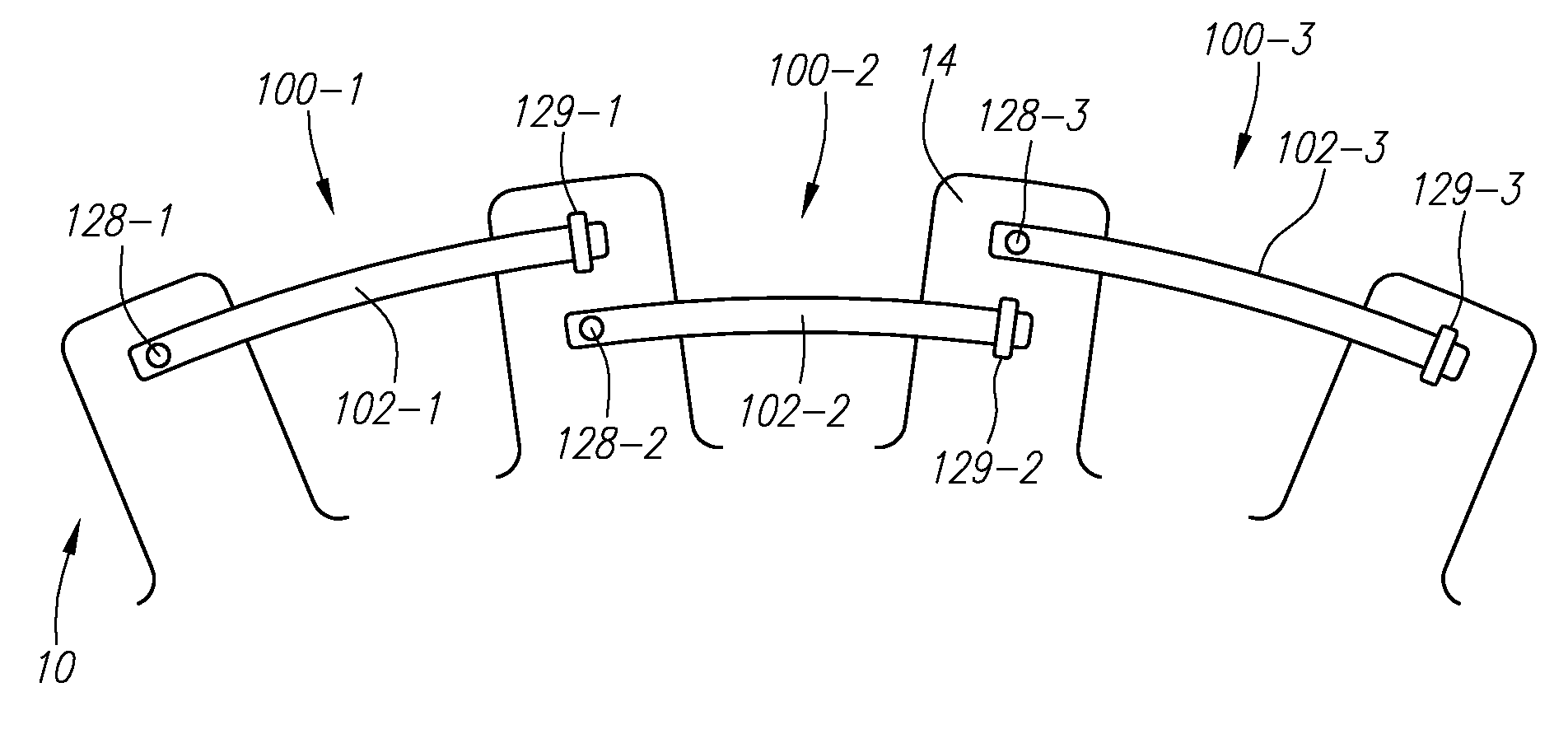
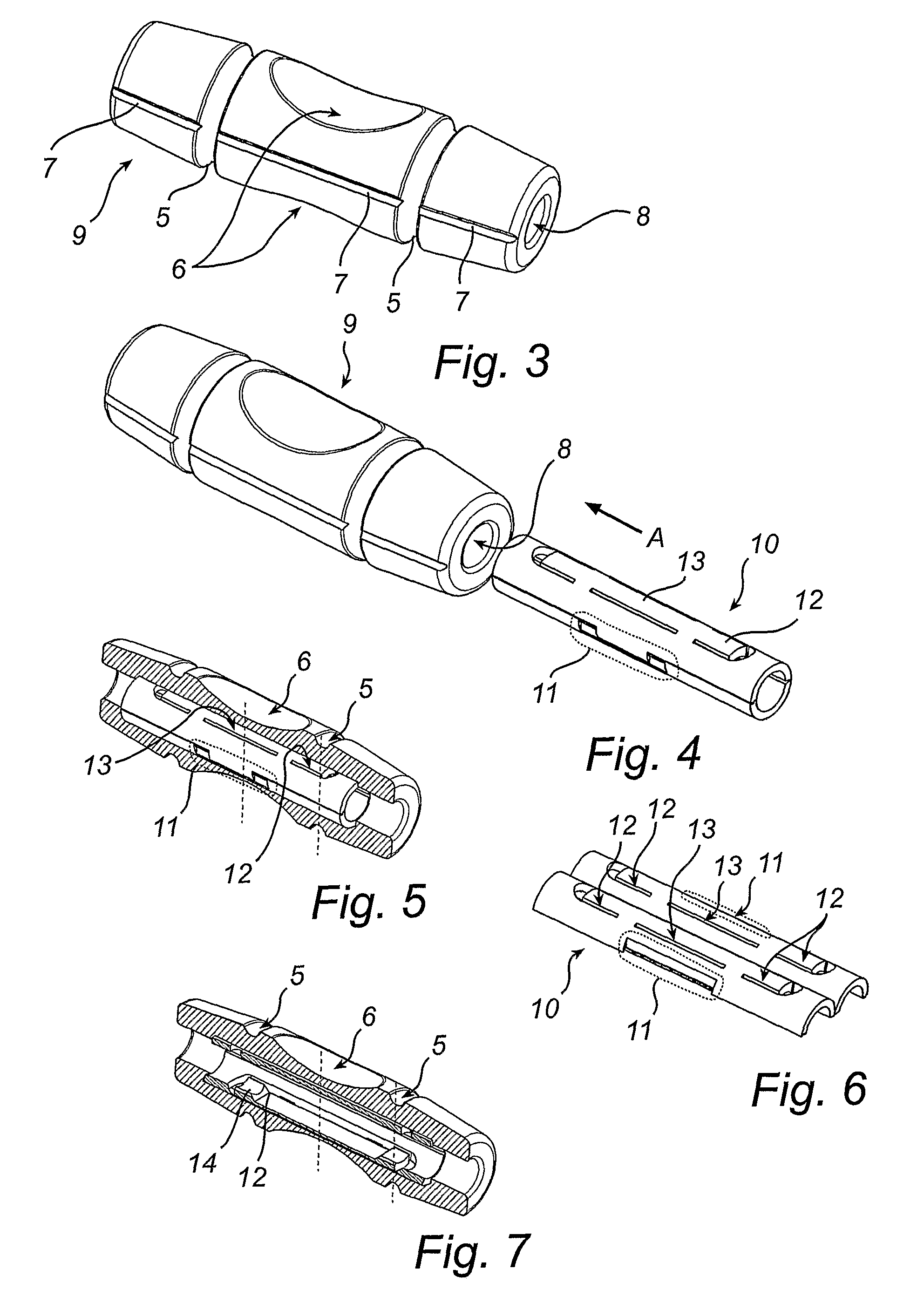
Percutaneous rod-to-rod cross connector
A percutaneous cross connector system for use with implantation rods comprising a rod attachment device comprising a first jaw portion and a second jaw portion. The first jaw portion comprises a first concave surface to engage with a surface of a rod, a first biasing member, and a first pivoting mechanism. The second jaw portion comprises a second concave surface to engage with an opposite surface of the rod from the first jaw portion, a second pivoting mechanism, a second biasing surface, and a second locking surface. The first and second jaw portions can be mated so that the first and second concave surfaces are positioned on opposite sides of the rod, the first and second pivoting mechanisms are aligned to pivot on a common axis, and the first biasing member is engaged against the second biasing surface to drive the second jaw portion against the rod.
Owner:NEUROVENT
Suture sleeve
InactiveUS8000811B2Easy to moveSmall sizeTransvascular endocardial electrodesMedical devicesImplantable rodSurgery
Owner:ST JUDE MEDICAL
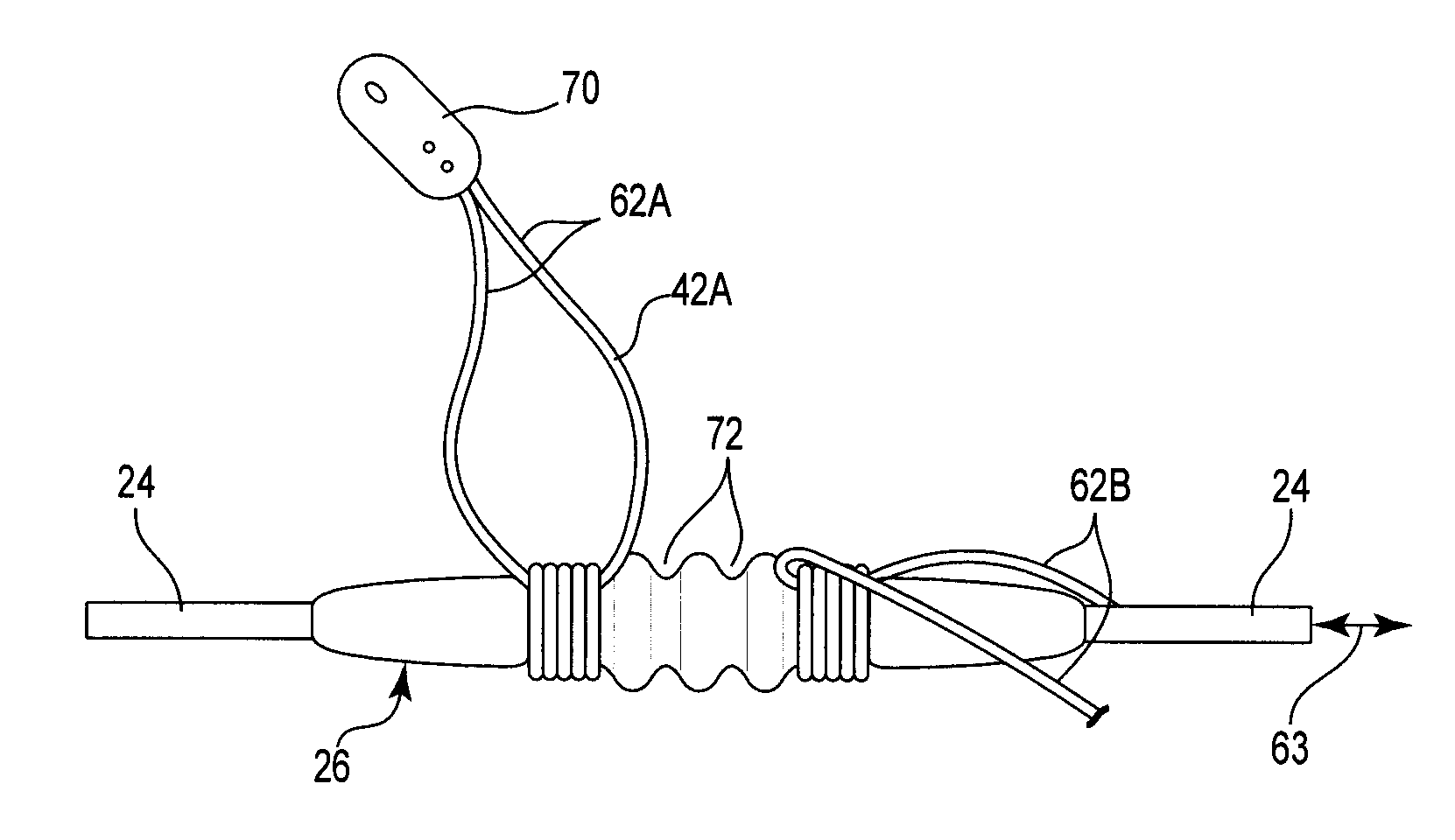
Pre-sutured anchor for implantable leads
ActiveUS8437846B2Prevent movementConvenient amountSuture equipmentsSpinal electrodesImplantable rodSuture anchors
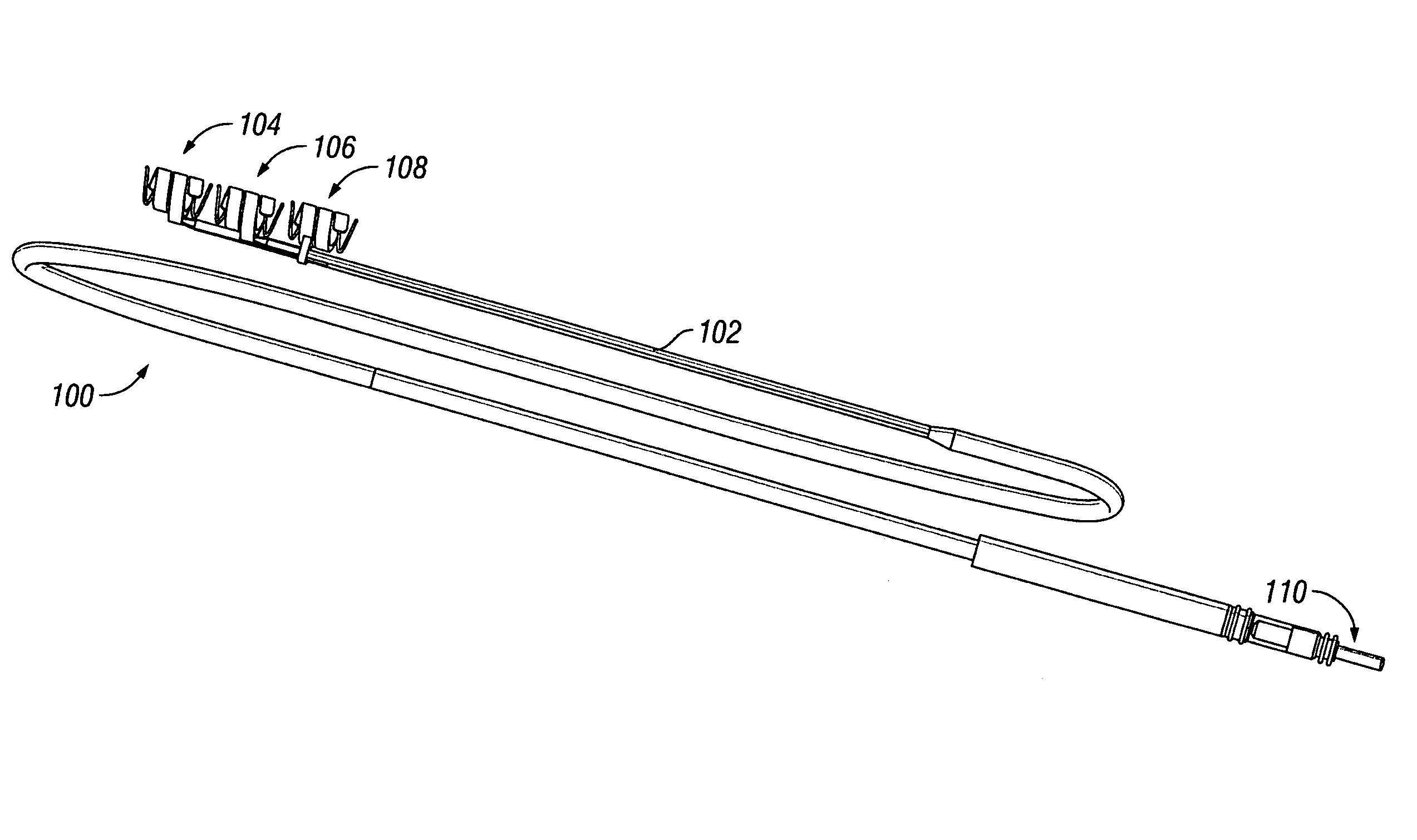
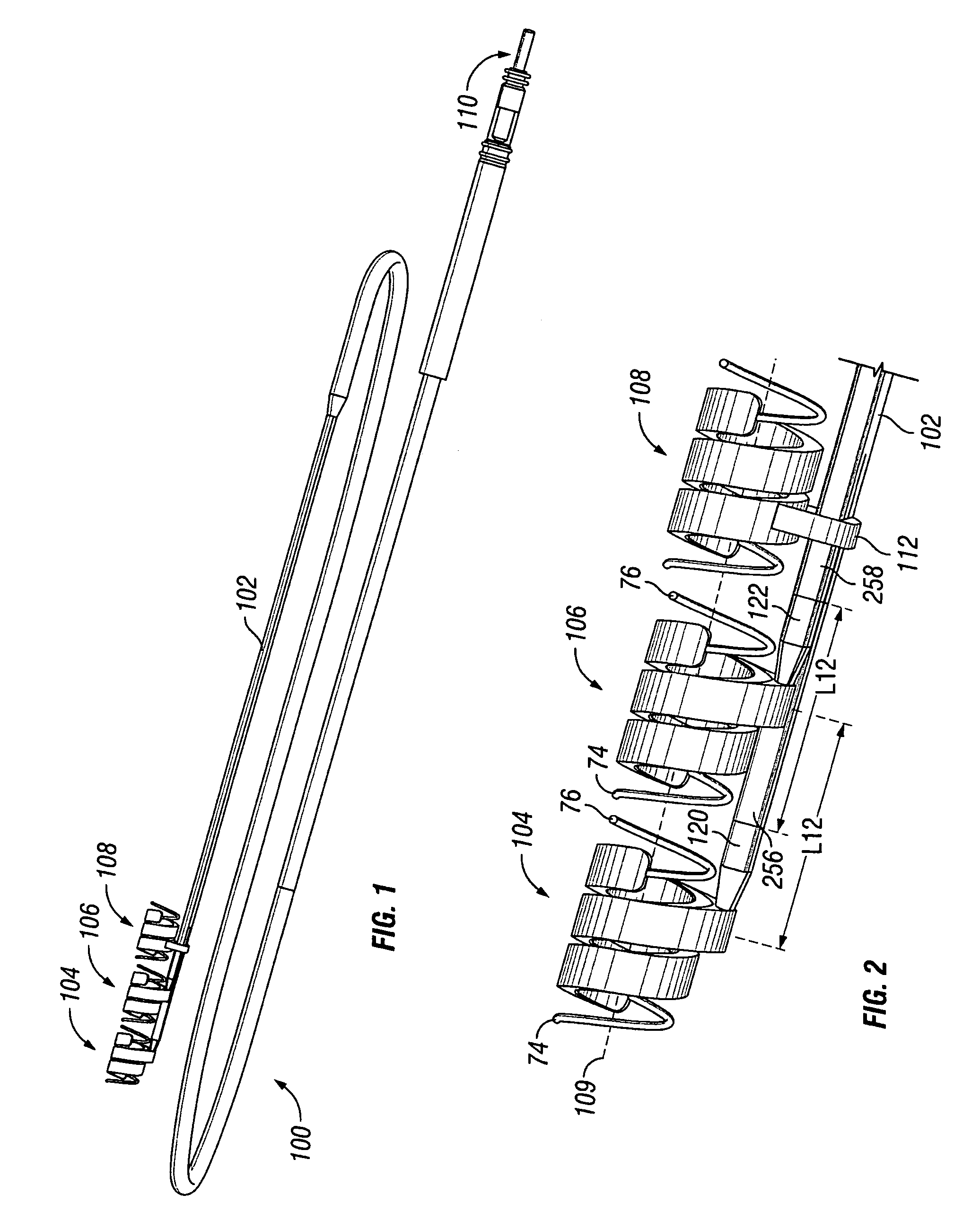
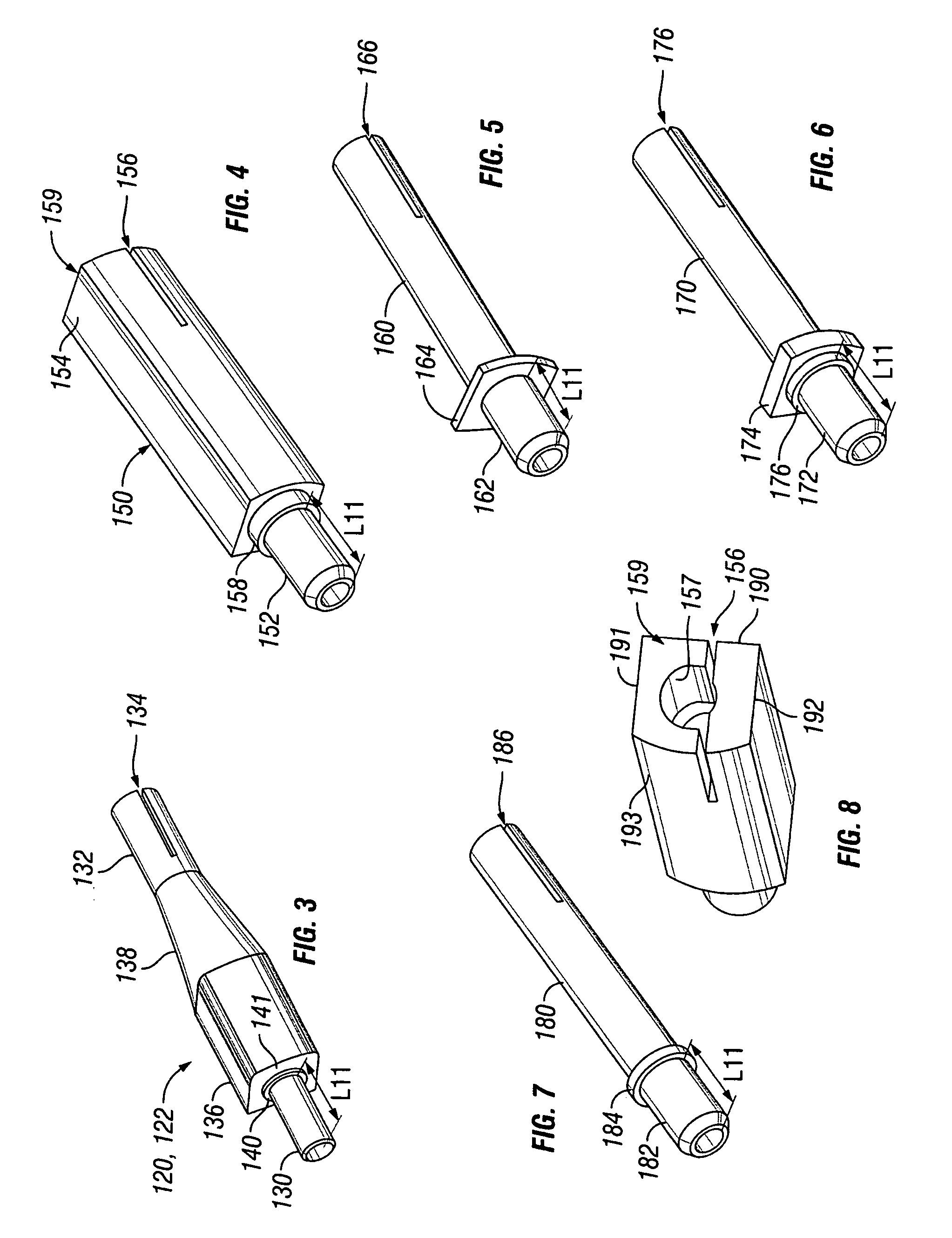
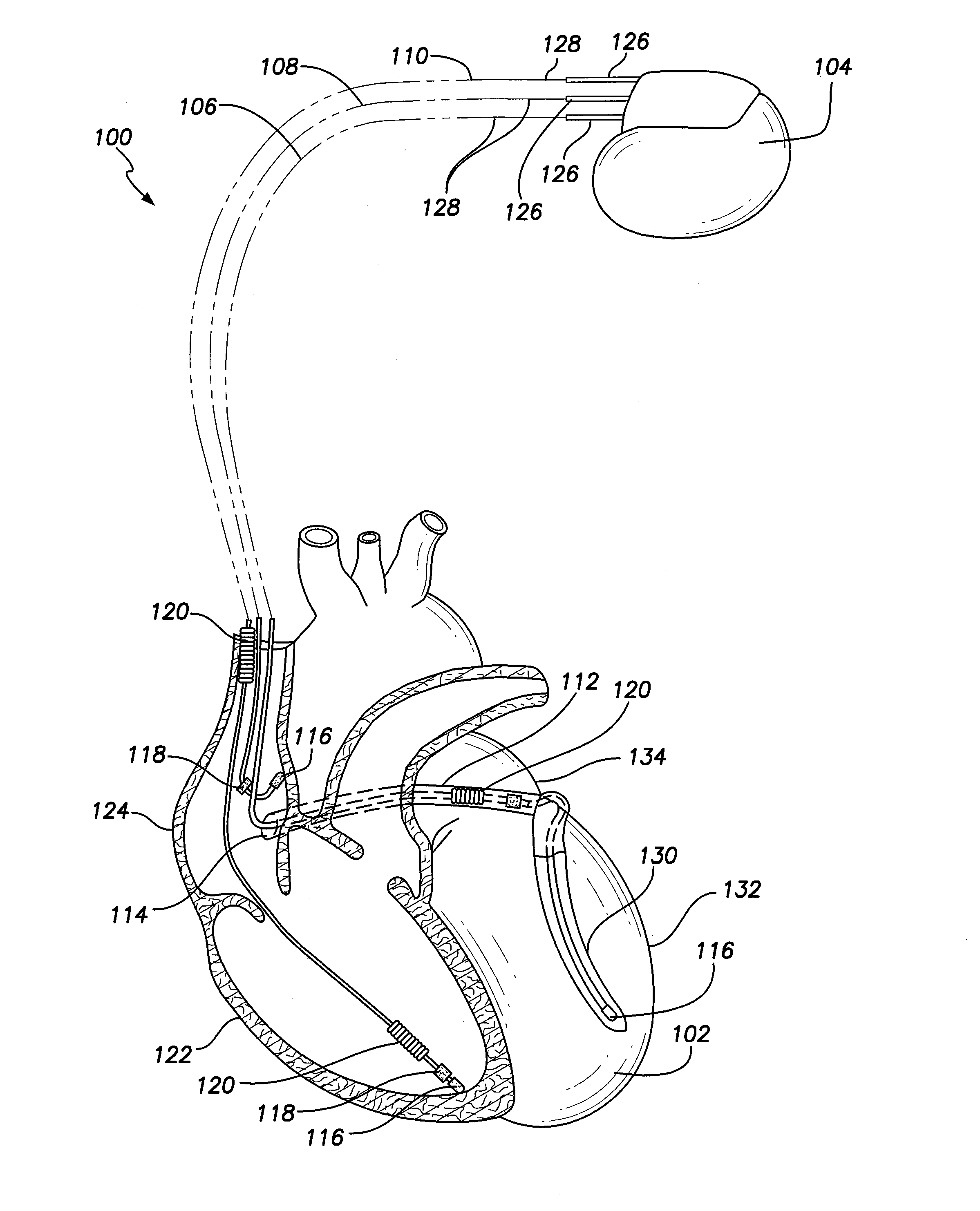
A pre-sutured anchor including a deformable anchor sleeve with a lumen sized to receive the therapy delivery element. An outer surface of the anchor sleeve including one or more annular compression grooves oriented generally co-axial to the lumen. At least one compression member is located in a compression groove in an open configuration. The compression member includes at least one stop. A suture material pre-tied in a self-locking compression knot extends around each compression member. The suture material includes distal ends adapted to receive a tension force that is transmitted as a radial compression force to deform the compression members and substantially engage the stop in a compressed configuration. The anchor sleeve compressively engages the therapy delivery element in the compressed configuration.
Owner:CIRTEC MEDICAL CORP
Prosthesis
A distal component for an interphalangeal prosthesis suitable for insertion into a proximal end of a distal phalangeal bone includes a stem portion shaped to be received within a surgically prepared bore in the proximal end of the distal bone. A head portion has an under-surface and an upper-surface, the under-surface of which in use will bear on the bone into which the stem is implanted.
Owner:MATORTHO
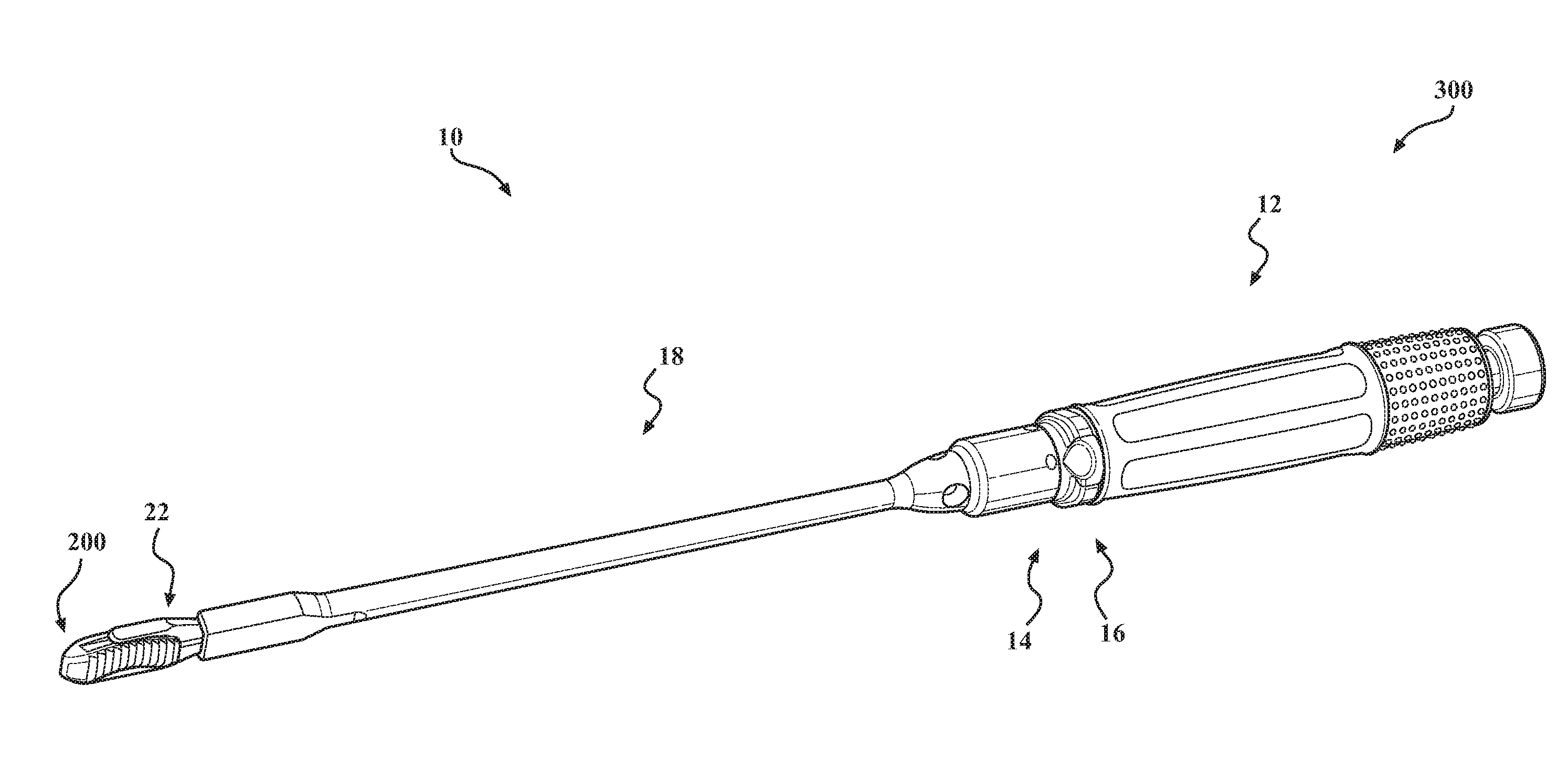
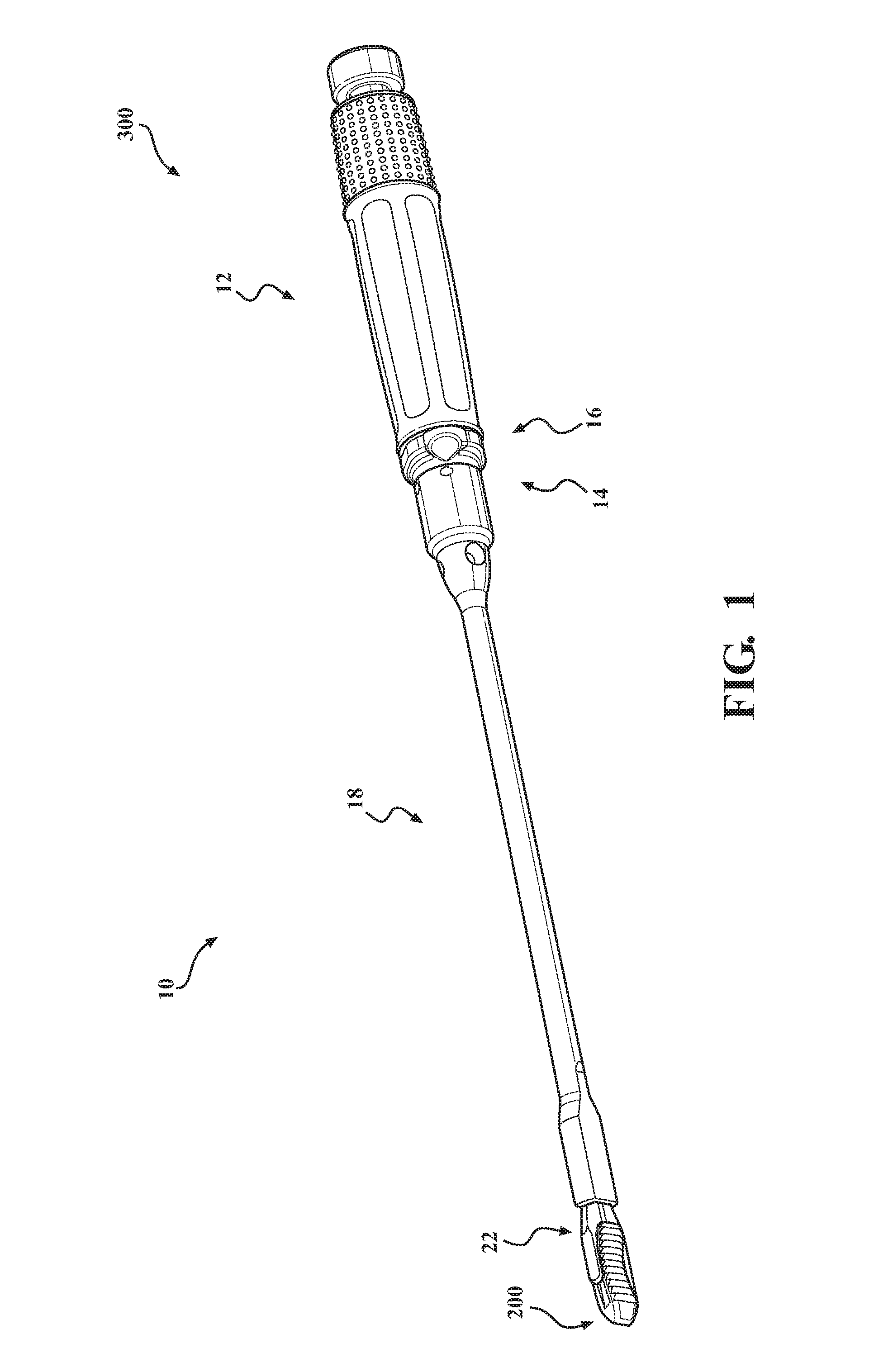
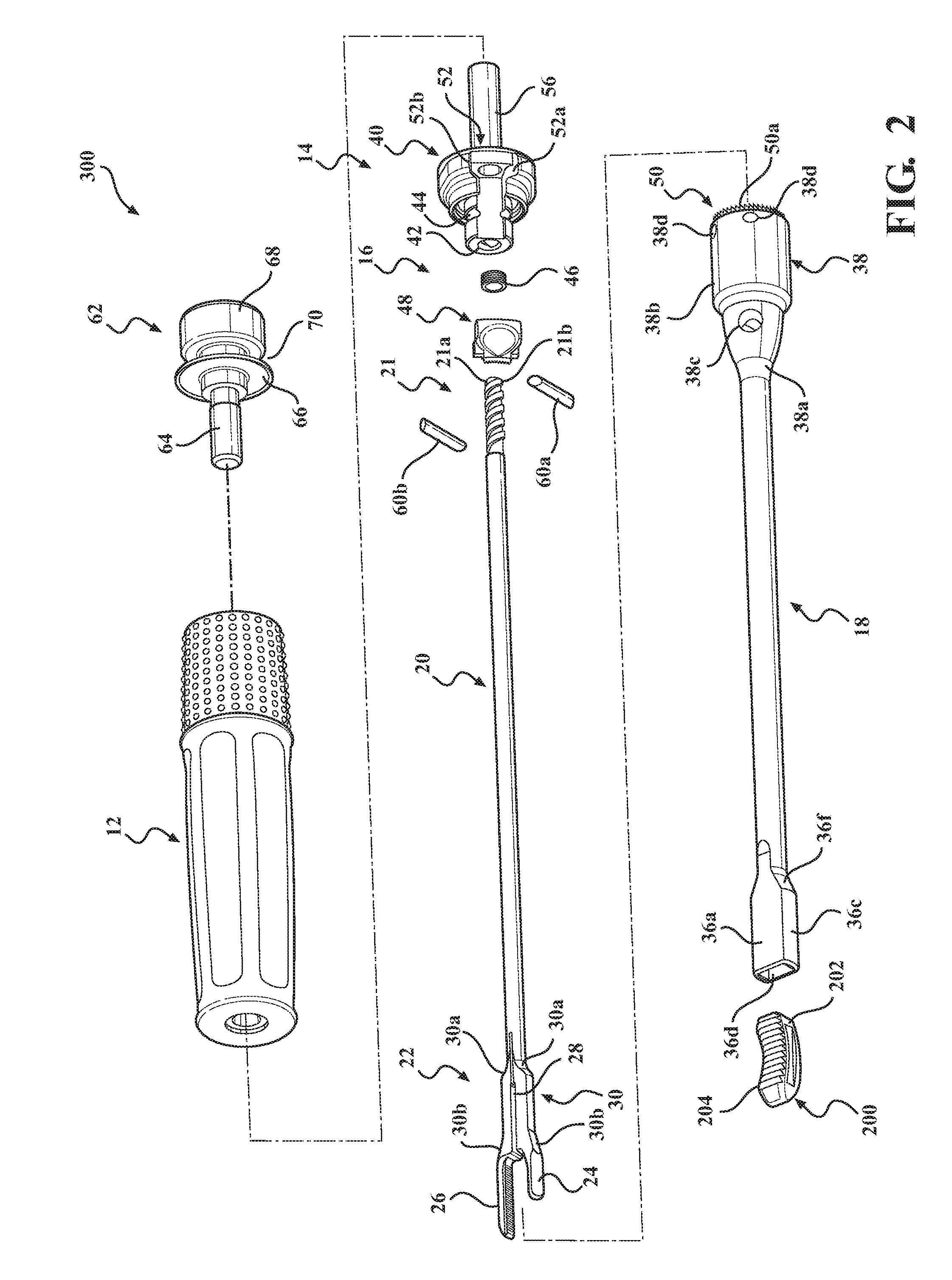
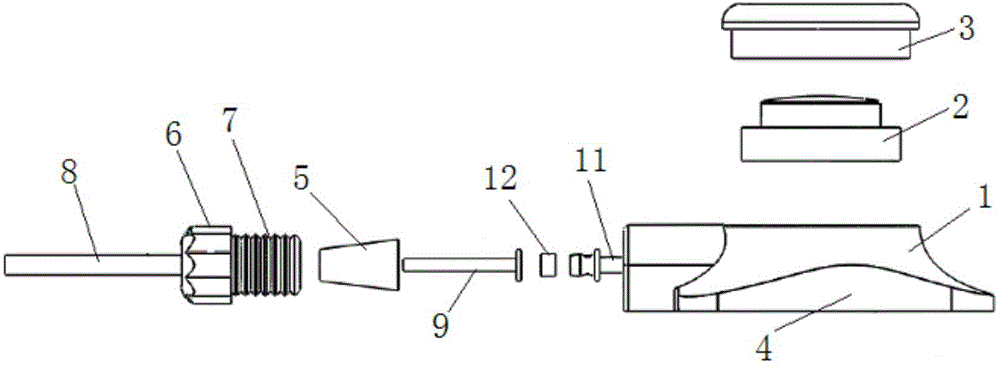
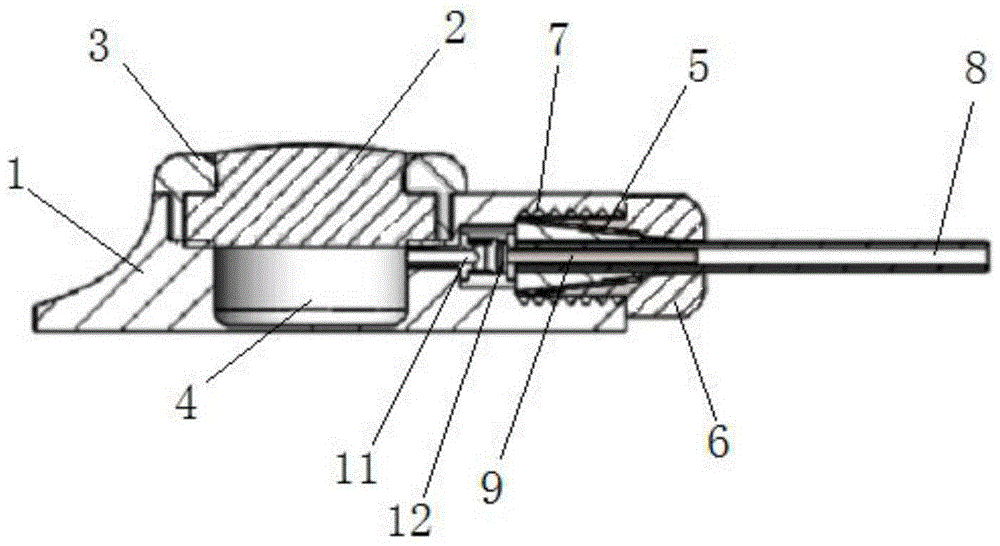
Implant insertion instrument
An implant insertion instrument, and an implant are provided. The instrument includes a handle, a sleeve mounted to a distal end of the handle, and an implant rod disposed within the sleeve. The implant rod includes a gripper disposed on a distal end of the implant rod. The gripper has a first prong spaced apart from a second prong, the second prong being longer than the first prong. The implant includes an implant body, the implant body having a convex surface opposite a concave surface wherein an outer surface of the implant is continuous so as to prevent bone growth within the implant. Accordingly, the instrument facilitates the engagement of the implant by a simple push of the sleeve. Further, the implant has a continuous surface so as to inhibit bone growth and facilitate removal of the implant from the surgical site.
Owner:ALPHATEC SPINE INC
Knot-free anchor and implantation device thereof
InactiveCN109223076AAvoid axial misalignmentImproving Implantation EfficiencySuture equipmentsImplantable rodMedical device
The invention relates to the field of medical devices, and discloses a knot-free anchor and an implantation device thereof. The knot-free anchor comprises an anchor body and a threading portion located at a top end position of the anchor body, a first external thread is formed on an outer peripheral wall of the anchor body, and a threading hole is formed on the threading portion. The structure ofthe implantation device comprises: a rotary handle including an implantation rod for connecting with a knot-free anchor and an implantation handle coaxially connected with the implantation rod; And asupport handle including a support sleeve and a grip handle coaxially connected to the support sleeve. The knot-free anchor of the invention can make the fixation effect of the suture better, so thatthe knot-free anchor can more stably and effectively fix the soft tissue on the bone, thereby promoting the rapid repair and reconstruction of the soft tissue. The knot-free anchor of the invention can make the fixation effect of the suture better, so that the knot-free anchor can more stably and effectively fix the soft tissue on the bone, thereby promoting the rapid repair and reconstruction ofthe soft tissue.
Owner:BEIJING CHUNLIZHENGDA MEDICAL INSTR
Systems, Devices and Methods for the Correction of Spinal Deformities
Provided herein are systems, devices and methods for the correction of spinal deformities with the use one or more implantable rods configured to apply a corrective force to the spine. Methods of minimally invasive implantation of a corrective system are provided, such as where the corrective system is attached only to the spinous process of one or more vertebral bodies. Various corrective systems as well as components thereof are also provided.
Owner:THOMPSON MATTHEW +3
Implantable drug-supplying device of check valve seal-free pipe
InactiveCN104800966AIncrease lethalityAvoid cloggingMedical devicesIntravenous devicesImplantable rodCurative effect
Owner:BEIJING ZHAOSHI MEDICAL EQUIP CO LTD
Implantable Electrode
InactiveUS20170246448A1Phenomenon of bio-fouling described above is reducedReduce biofoulingHead electrodesPharmaceutical delivery mechanismBiological cellImplantable rod
According to the present invention, an implantable device comprising an electrode for carrying an electric signal to or from a biological cell or tissue provided. The electrode material is chosen to exhibit desirable properties in terms of electrical conductivity, biocompatibility and bio-fouling. The invention further provides implantable devices comprising such implantable electrodes.
Owner:LUXEMBOURG INST OF SCI & TECH LIST
Implantable lead with flexible paddle electrode array
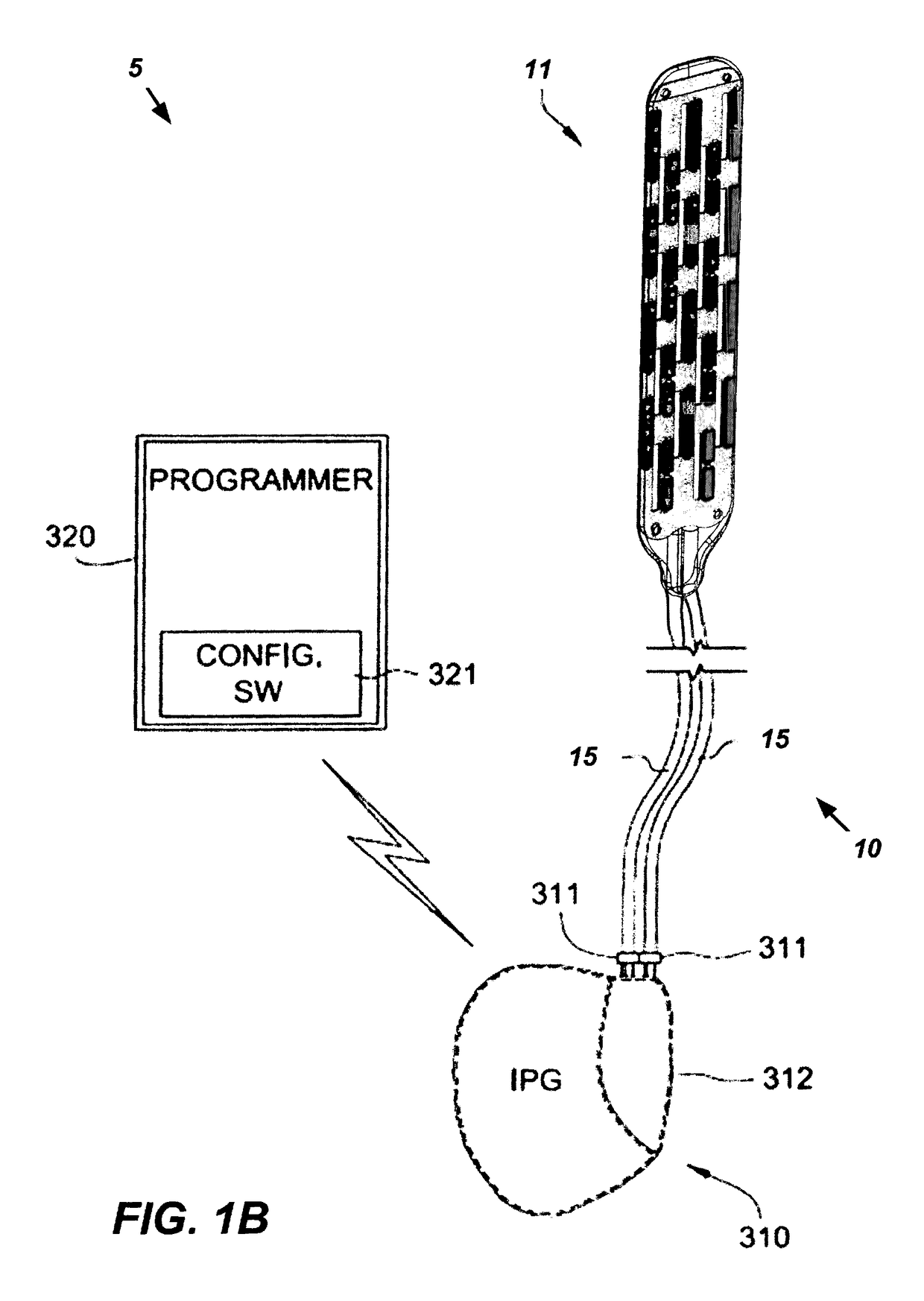
A neurostimulation system is disclosed herein. The neurostimulation system includes an implantable pulse generator and an implantable therapy lead configured to be electrically coupled to the implantable pulse generator. The implantable therapy lead includes a flexible paddle electrode array with flexible electrodes. Each flexible electrode has a segmented configuration having first and second electrode segments and a flexible bridge or living hinge joining together the first and second electrode segments.
Owner:ADVANCED NEUROMODULATION SYST INC
Implantation and recovery device for adsorption wire by surface geochemical exploration, and use method
InactiveCN106769247AAvoid damageEasy to carryWithdrawing sample devicesRecovery methodImplantable rod
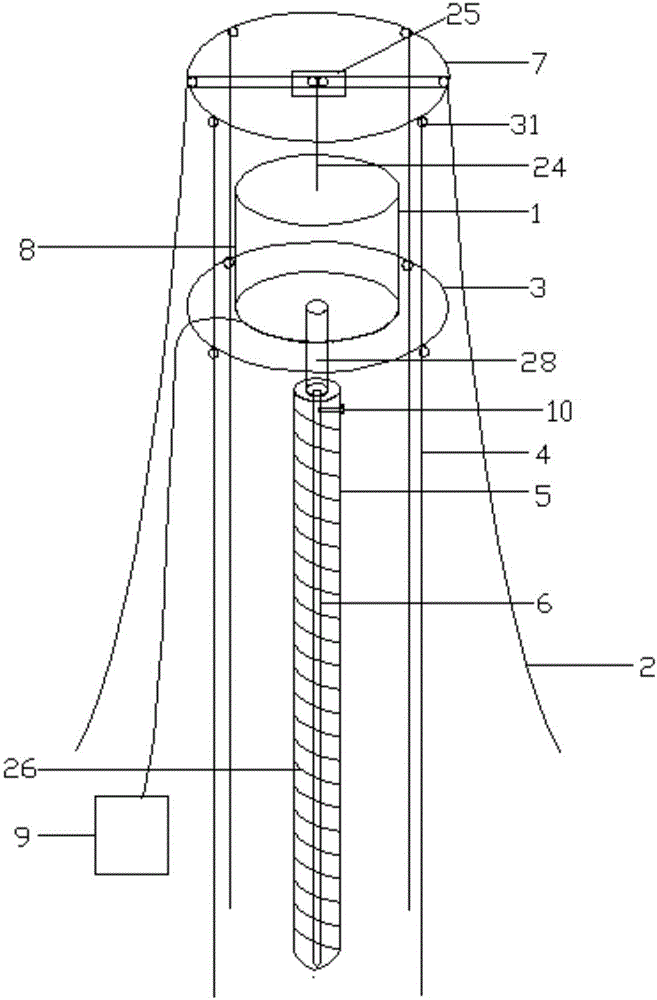
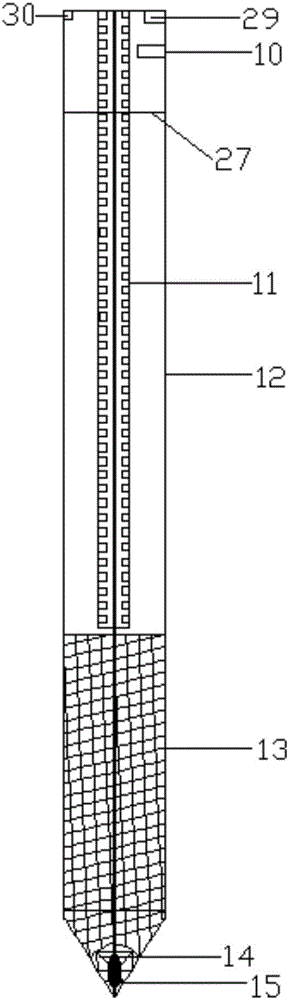
The invention provides an implantation and recovery device for an adsorption wire by surface geochemical exploration. The implantation and recovery device comprises a drilling machine fixing device, a drilling machine lifting device, a drilling machine, a drilling rod, an implantation rod and a signal device, wherein the drilling machine consists of a drilling machine main body, clamping rods, a remote controller and lower inserting feet; a pin hole is formed at the side part of the top end of the drilling rod; clamping holes correspond to the clamping rods; a circular swinging iron sheet is arranged in the center of the bottom of the drilling rod; a swinging magnet is arranged in the drilling rod, in a circular ring shape, and fixed at the bottom of the drilling rod; an opening of the swinging iron sheet faces outwards; the implantation rod is located in the center of the interior of the drilling rod; a pin hole is formed at the side part of the top end of the implantation rod; a gear groove is formed in the implantation rod; a net structure is arranged outside the implantation rod, and provided with a large opening at the middle lower part of the implantation rod; the adsorption wire is arranged at the bottom of the implantation rod; the exterior of the implantation rod is surrounded by a dismountable large-opening protective net; and the signal device comprises a signal transmitting device, a signal positioning device and a signal lamp. In addition, the invention further provides an implantation and recovery method for the adsorption wire by the surface geochemical exploration.
Owner:SHANDONG UNIV OF SCI & TECH
Implantable lead
An implantable wireless lead includes an enclosure, the enclosure housing: one or more electrodes configured to apply one or more electrical pulses to a neural tissue; a first antenna configured to: receive, from a second antenna and through electrical radiative coupling, an input signal containing electrical energy, the second antenna being physically separate from the implantable neural stimulator lead; one or more circuits electrically connected to the first antenna, the circuits configured to: create the one or more electrical pulses suitable for stimulation of the neural tissue using the electrical energy contained in the input signal; and supply the one or more electrical pulses to the one or more electrodes, wherein the enclosure is shaped and arranged for delivery into a subject's body through an introducer or a needle.
Owner:MICRON DEVICES LLC
Insert for implantable electrode
A lead assembly comprises an outer body tubing, first and second conductors, and first and second tubing insert members. The outer body tubing comprises at least two longitudinal outer body tubing portions, each such portion comprising a through-hole and each portion comprising a keyed surface having a predetermined shape. Each of the tubing insert members receives a conductor and comprises a surface that substantially matches the predetermined shape of the keyed surface. When both of the conductors are inserted into corresponding longitudinal through-holes such that each of said first and second surfaces of the first and second hollow body members is substantially aligned with the keyed surface of a corresponding outer body tubing portion, electrodes formed on ends of the first and second conductors are encouraged to be substantially co-linear.
Owner:LIVANOVA USA INC
Implantable lead with body profile optimized for implant environment
InactiveUS20140155966A1Problem be addressEpicardial electrodesTransvascular endocardial electrodesInsulation layerImplantable rod
Implementations described and claimed herein provide an implantable lead optimized for an implant environment and methods of manufacturing such implantable leads. The implantable lead includes an insulation layer having one or more transitions along a length of the insulation layer from a proximal end to a distal end. Each of the transitions is a seamless change from a section of the insulation layer having a set of performance characteristics to another section of the insulation layer having a different set of performance characteristics.
Owner:PACESETTER INC
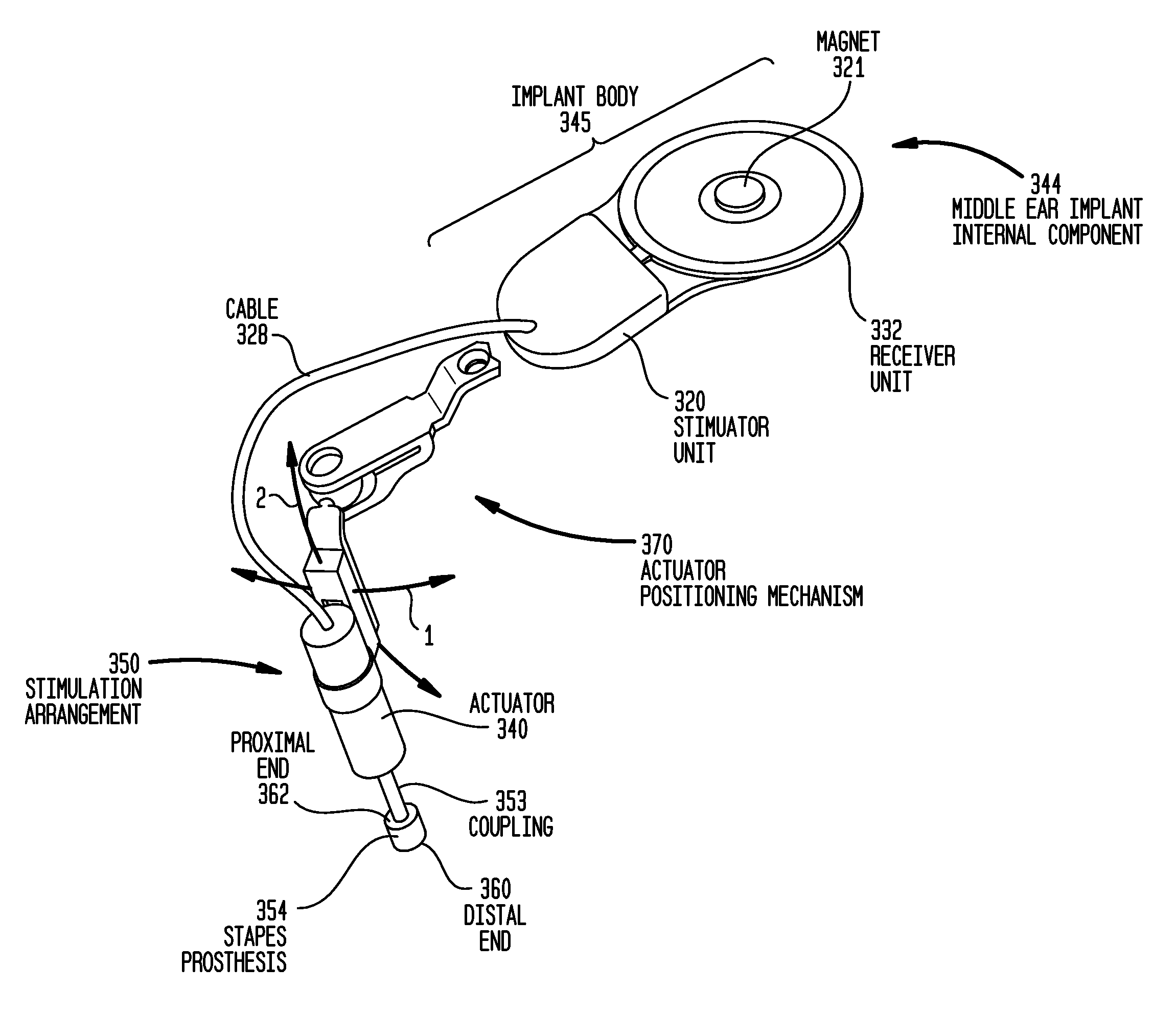
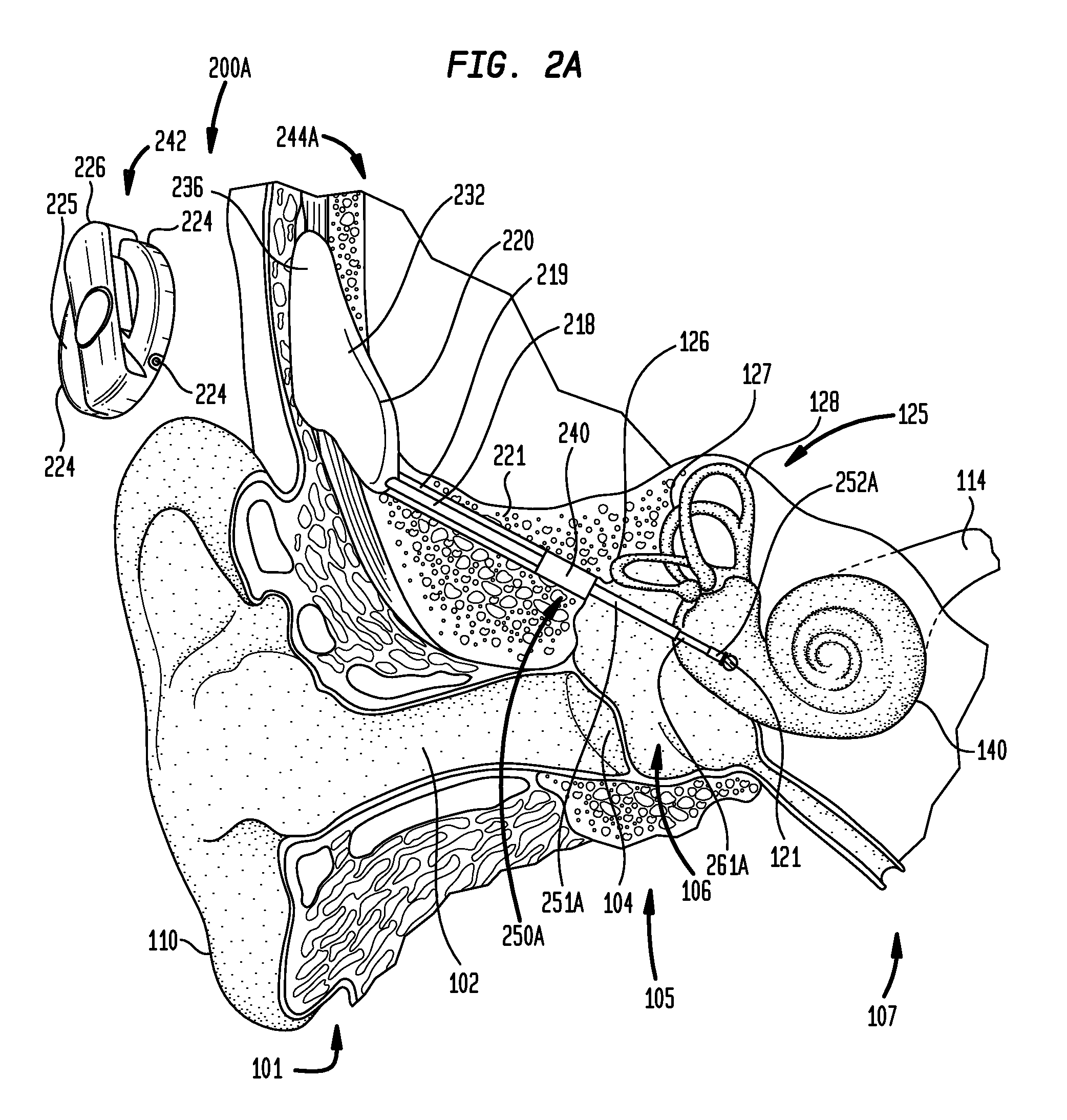
Combined functional component and implantable actuator positioning mechanism
An implantable component including an implantable body including a functional component of the implantable medical device, and implantable actuator positioning mechanism connected to and extending from the implantable body, the actuator positioning mechanism configured to removably receive an implantable actuator.
Owner:COCHLEAR LIMITED
Implantable electrode
InactiveCN106794281AMitigating Toxicity-Related IssuesLow level of toxicityHead electrodesPharmaceutical delivery mechanismBiological cellImplantable rod
According to the present invention, an implantable device comprising an electrode for carrying an electric signal to or from a biological cell or tissue provided. The electrode material is chosen to exhibit desirable properties in terms of electrical conductivity, biocompatibility and bio-fouling. The invention further provides implantable devices comprising such implantable electrodes.
Owner:LUXEMBOURG INST OF SCI & TECH LIST
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com