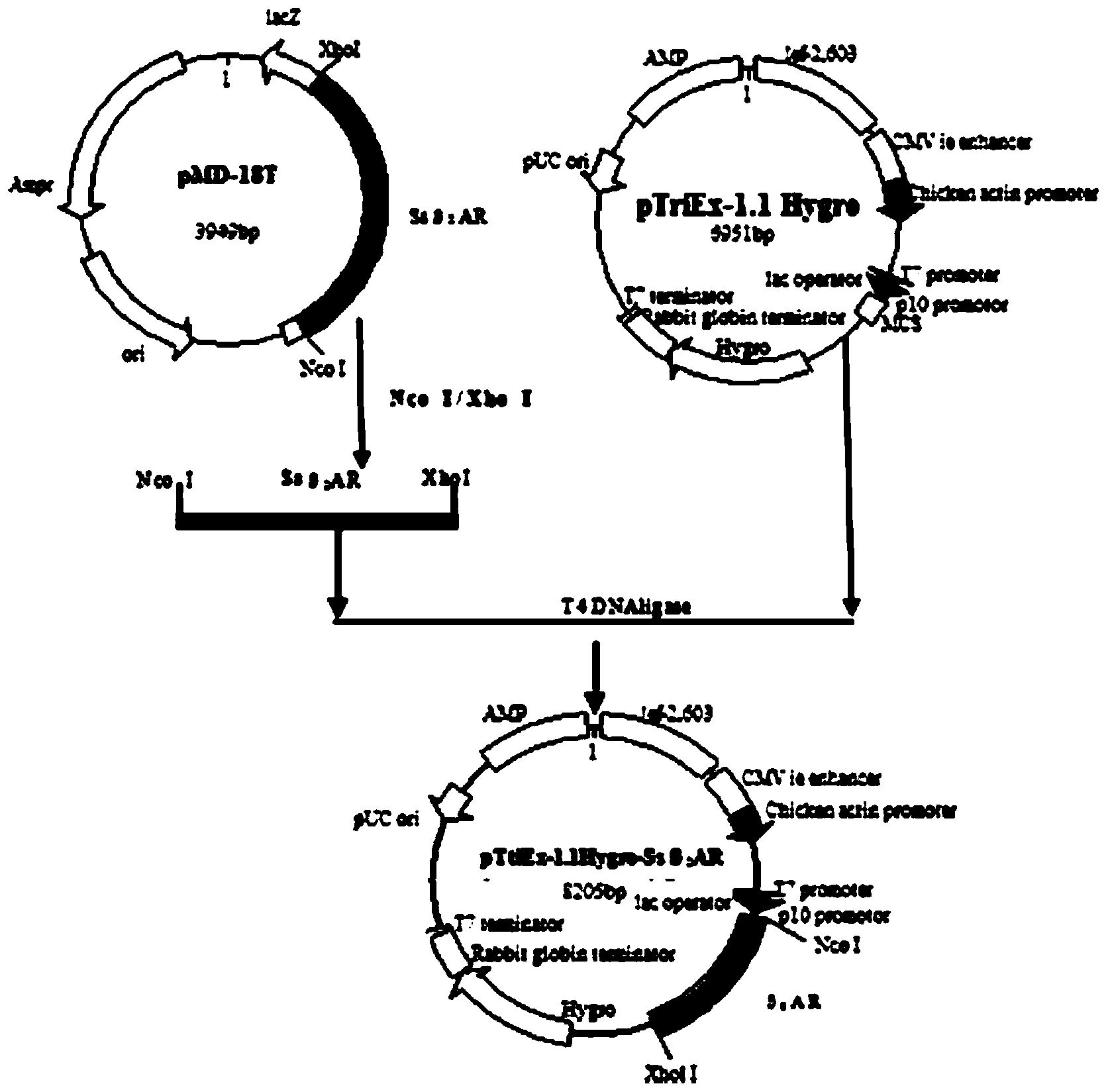
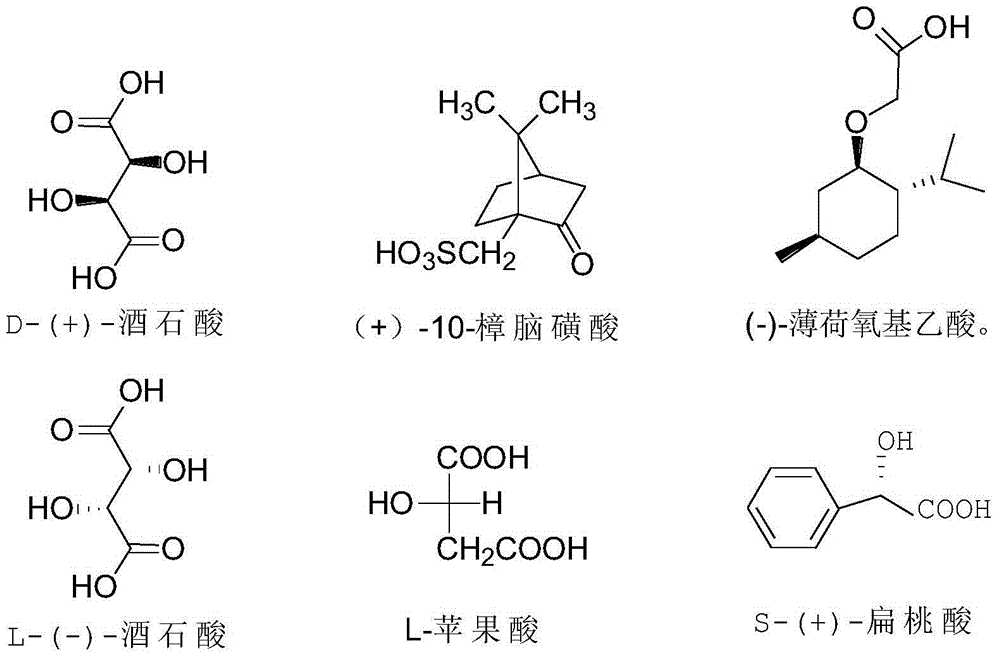
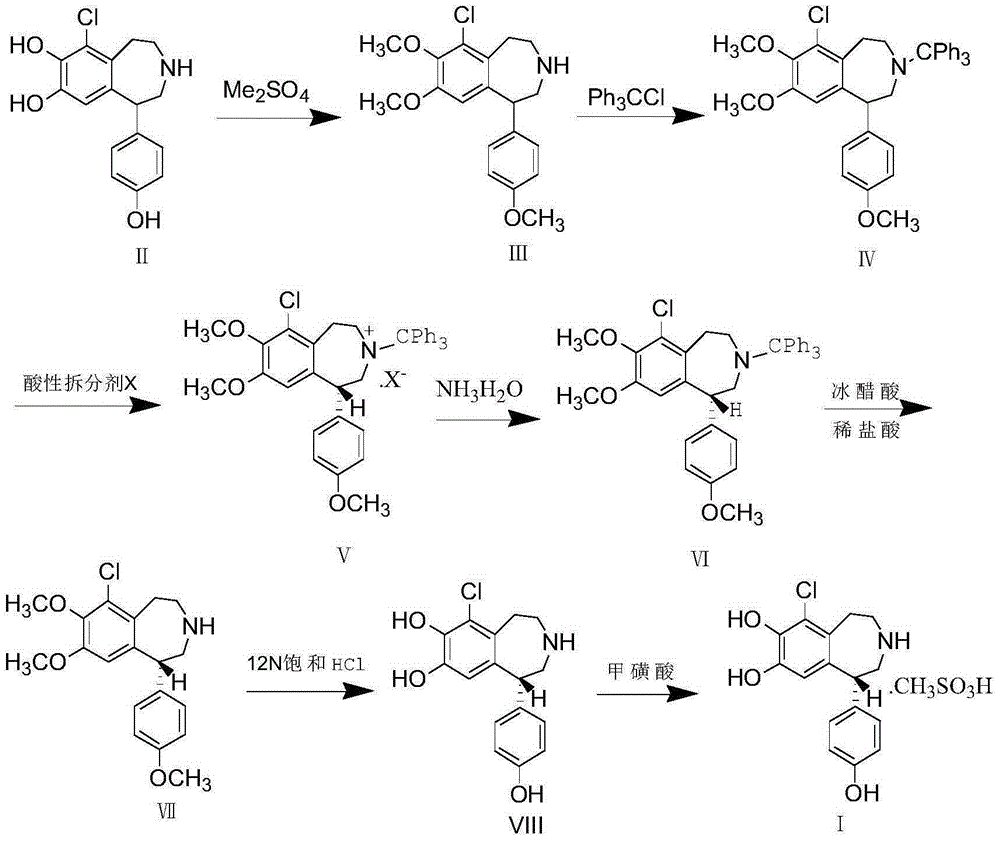
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
49 results about "Agonist drugs" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
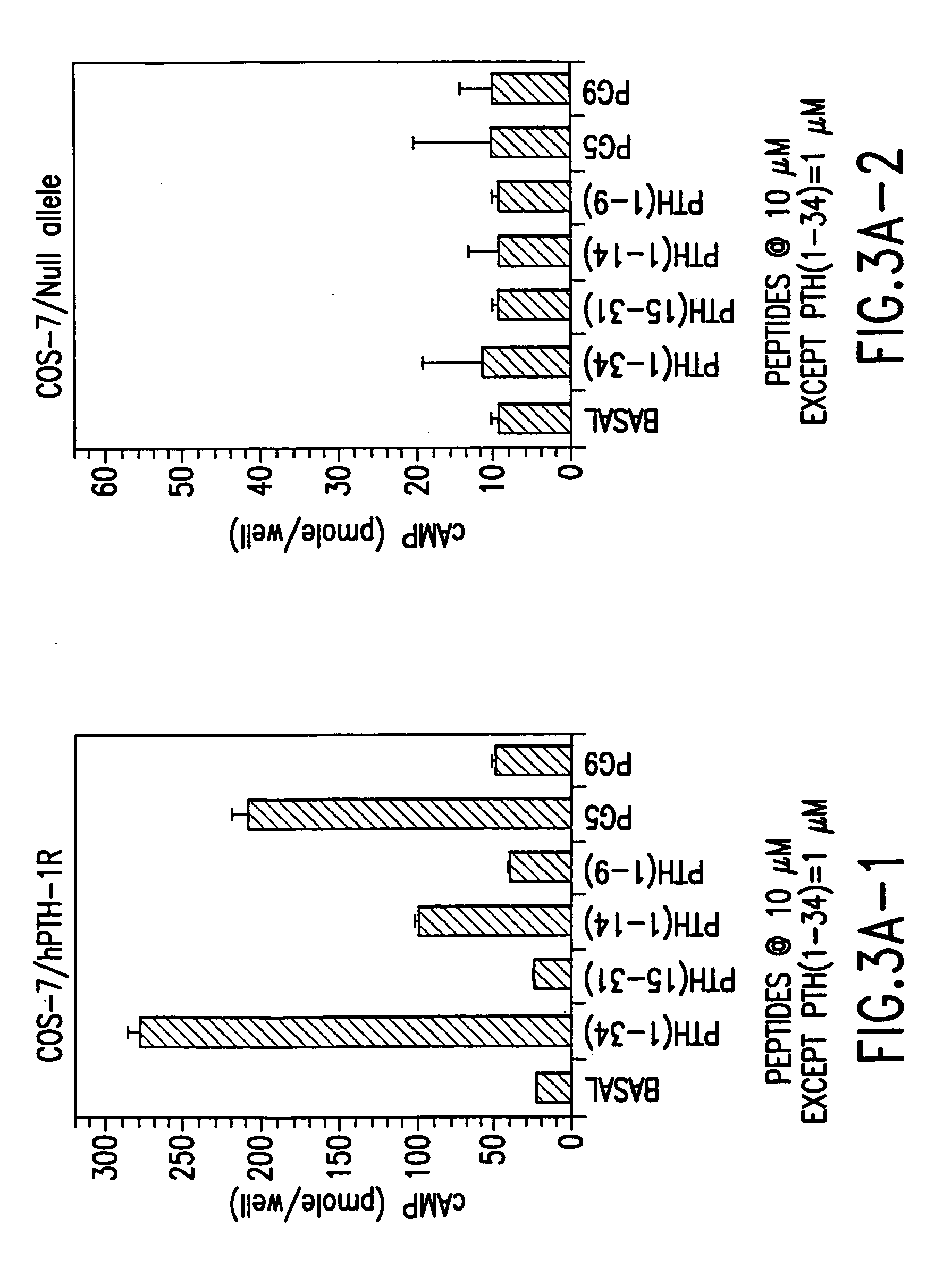
PTH functional domain conjugate peptides, derivatives thereof and novel tethered ligand-receptor molecules
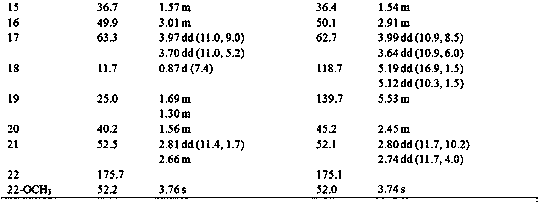
InactiveUS7057012B1Improve efficacyHigh potencyPeptide/protein ingredientsImmunoglobulinsAgonist drugsPharmaceutical drug
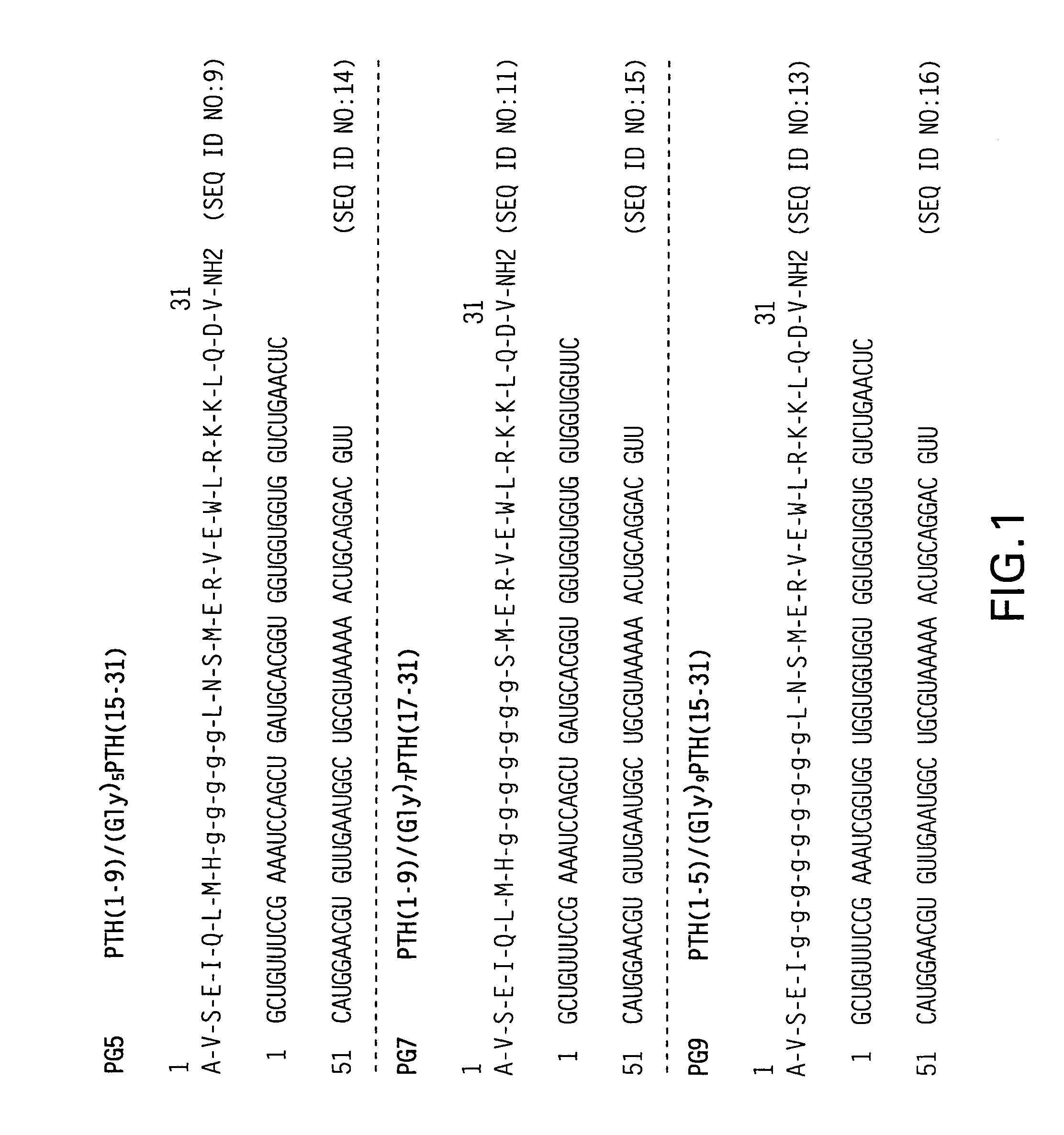
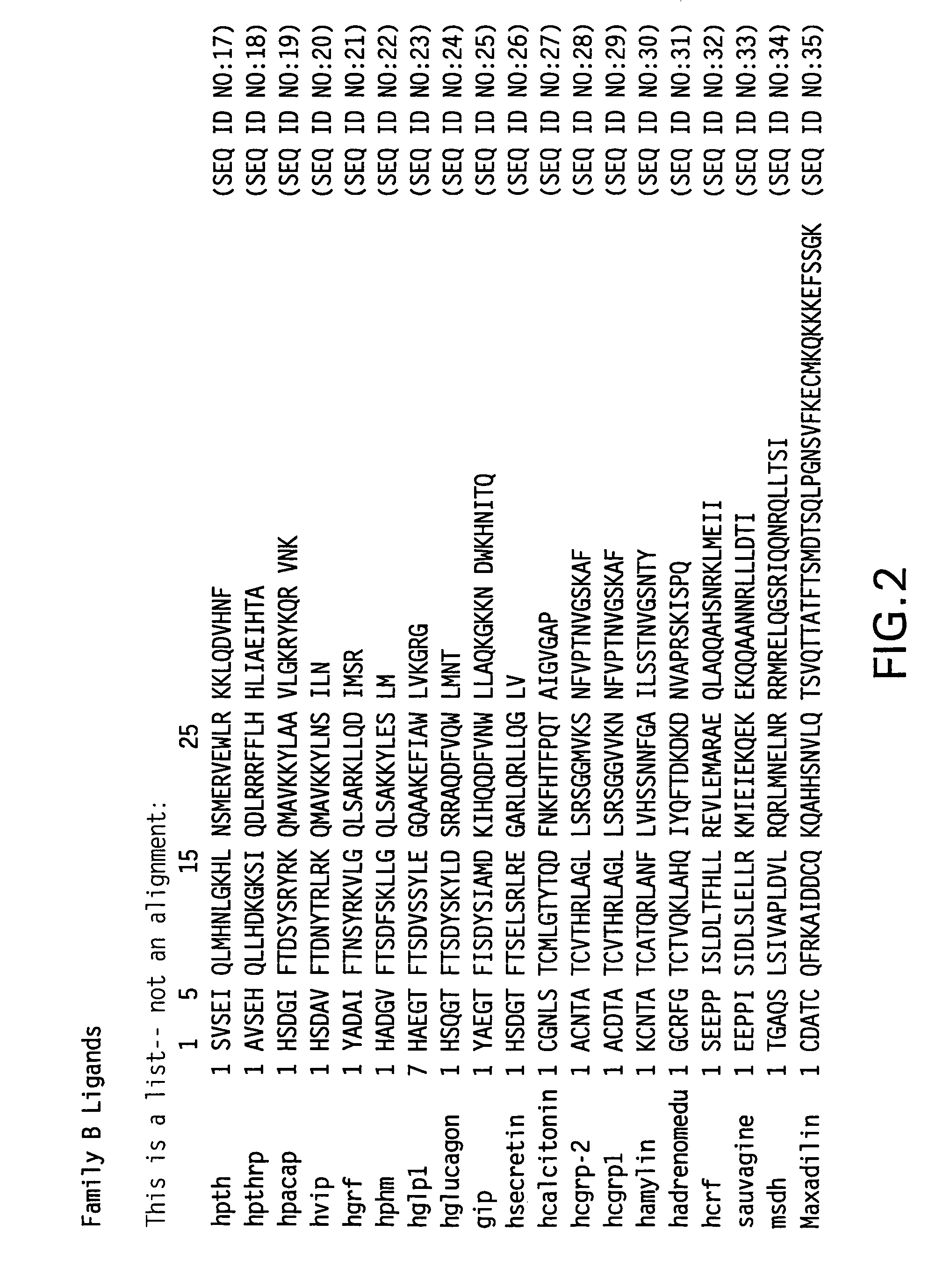
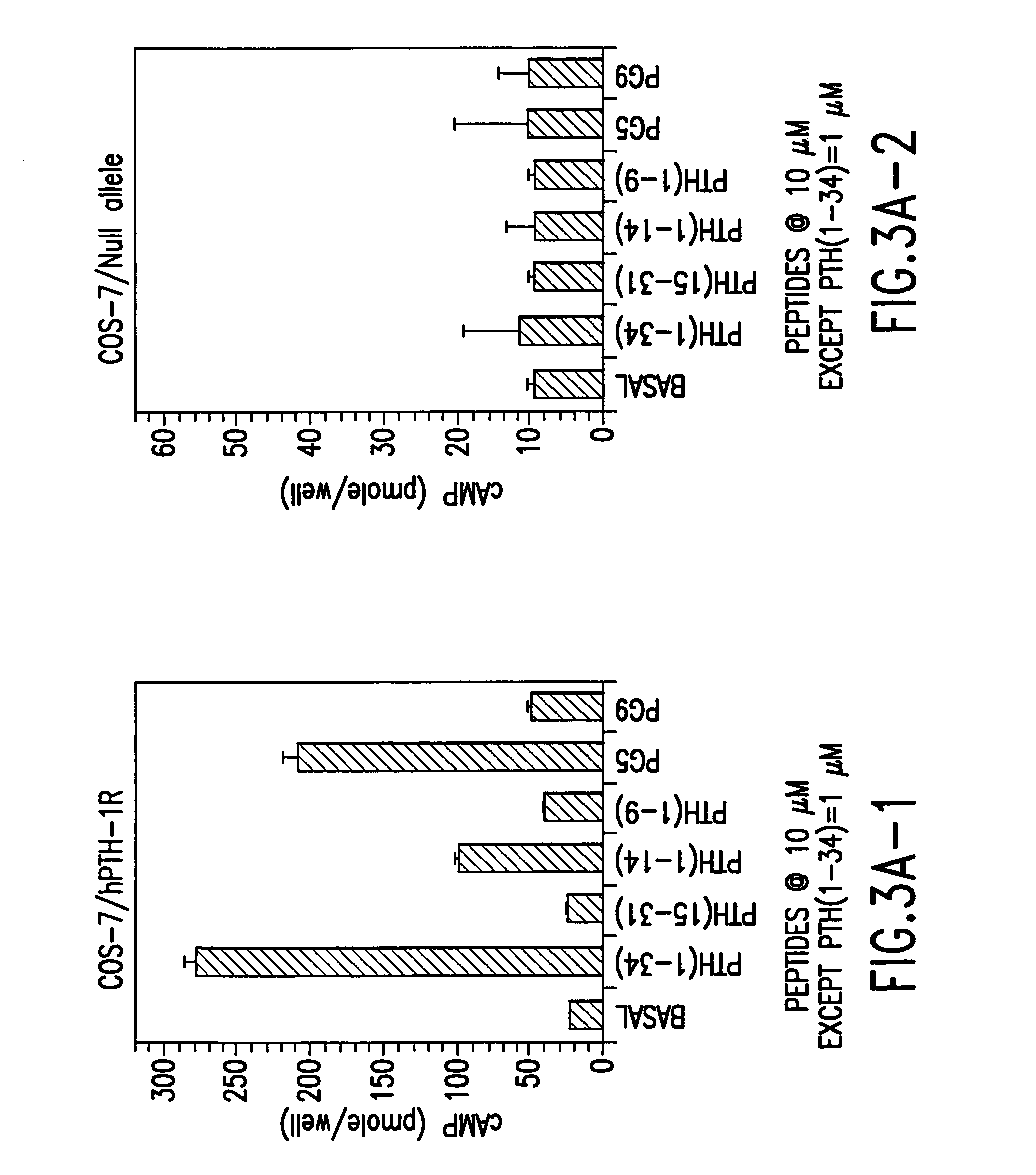
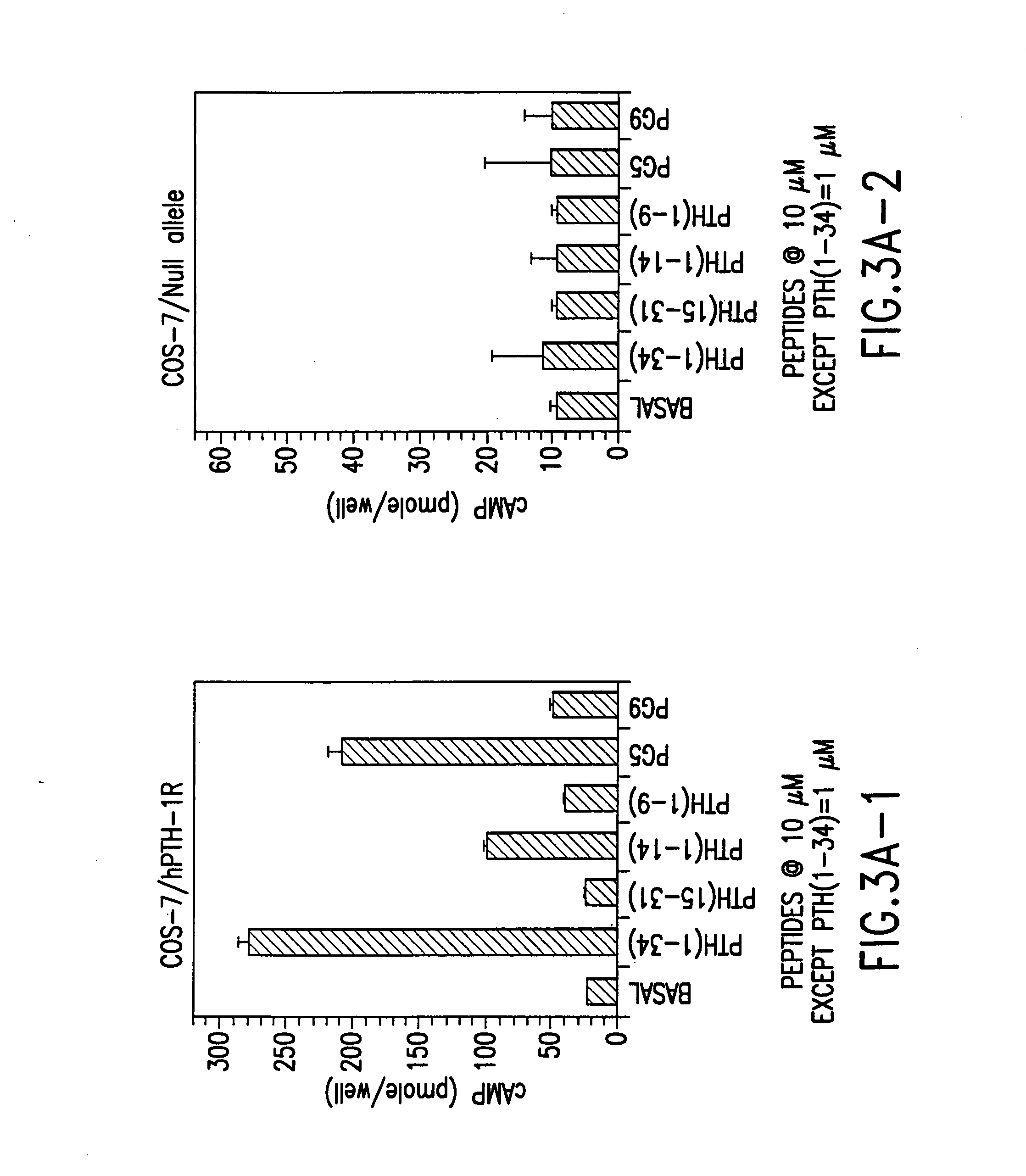
Novel parathyroid hormone (PTH) peptides and analogs thereof of the PTH(1–34) fragment are disclosed that combine the N-terminal signaling domain (residues 1–9) and the C-terminal binding domain (residues 15–31) via a linker. Nucleic acid molecules and peptides for PTH(1–9)-(Gly)5-PTH(15–31) (PG5) and (1–9)-(Gly)7-PTH(15–31) and a novel PTH receptor are disclosed. Additionally, methods of screening for PTH agonists, pharmaceutical compositions and methods of treatment are disclosed.
Owner:THE GENERAL HOSPITAL CORP
Extended release alpha-2 agonist pharmaceutical dosage forms
InactiveUS20050118256A1Sufficient effectFacilitated releaseBiocideDigestive systemAdrenergic receptor agonistsAgonist drugs
Disclosed is an extended release pharmaceutical formulation containing at least an alpha-2 adrenergic agonist, such as tizanidine, for the treatment and prevention of spasticity in a subject, e.g., painful inflammatory conditions associated with skeletal muscle spasms.
Owner:GLENMARK PHARMACEUTICALS LIMITED
Compositions to enhance the efficacy and safety of bio-pharmaceutical drugs
InactiveUS6593094B2Compounds screening/testingOrganic active ingredientsSide effectCost effectiveness
Owner:ENHANCED PHARMA
PTH functional domain conjugate peptides, derivatives thereof and novel tethered ligand-receptor molecules
InactiveUS20060199765A1Good curative effectSugar derivativesPeptide/protein ingredientsAgonist drugsThyroid parathyroid glands
Novel parathyroid hormone (PTH) peptides and analogs thereof of the PTH(1-34) fragment are disclosed that combine the N-terminal signaling domain (residues 1-9) and the C-terminal binding domain (residues 15-31) via a linker. Nucleic acid molecules and peptides for PTH(1-9)-(Gly)5-PTH(15-31) (PG5) and PTH(1-9)-(Gly)7-PTH(15-31) and a novel PTH receptor are disclosed. Additionally, methods of screening for PTH agonists, pharmaceutical compositions and methods of treatment are disclosed.
Owner:THE GENERAL HOSPITAL CORP
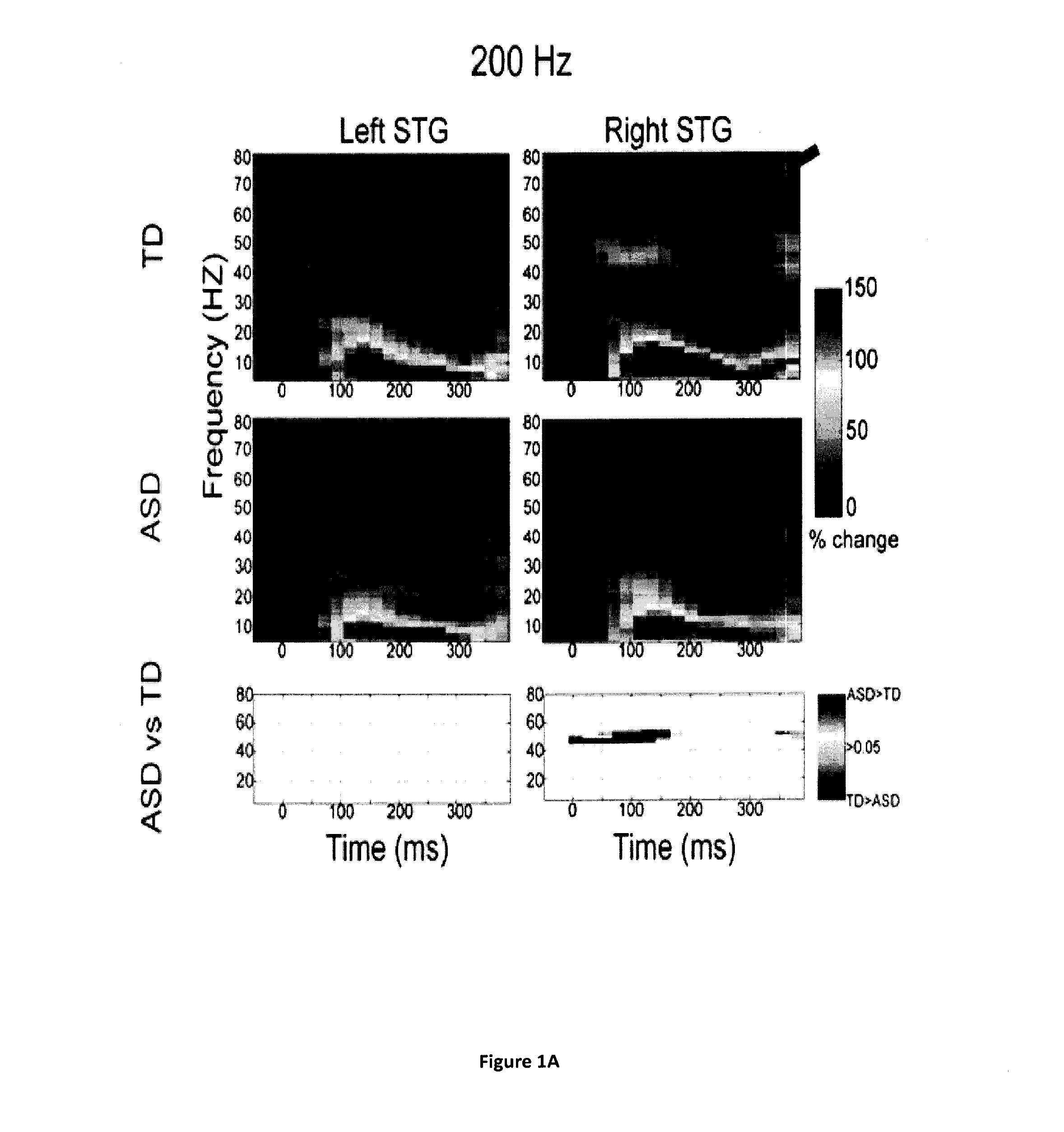
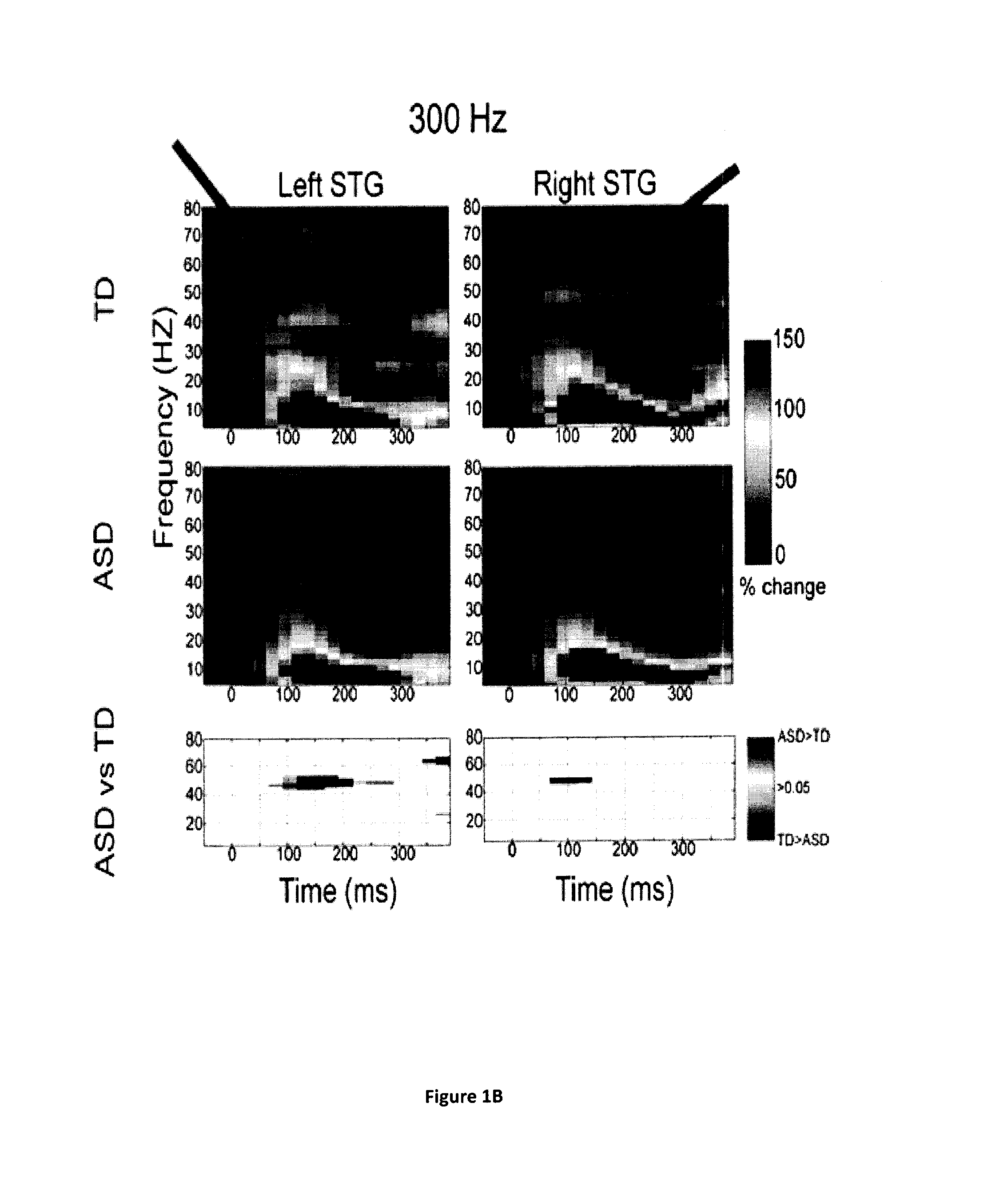
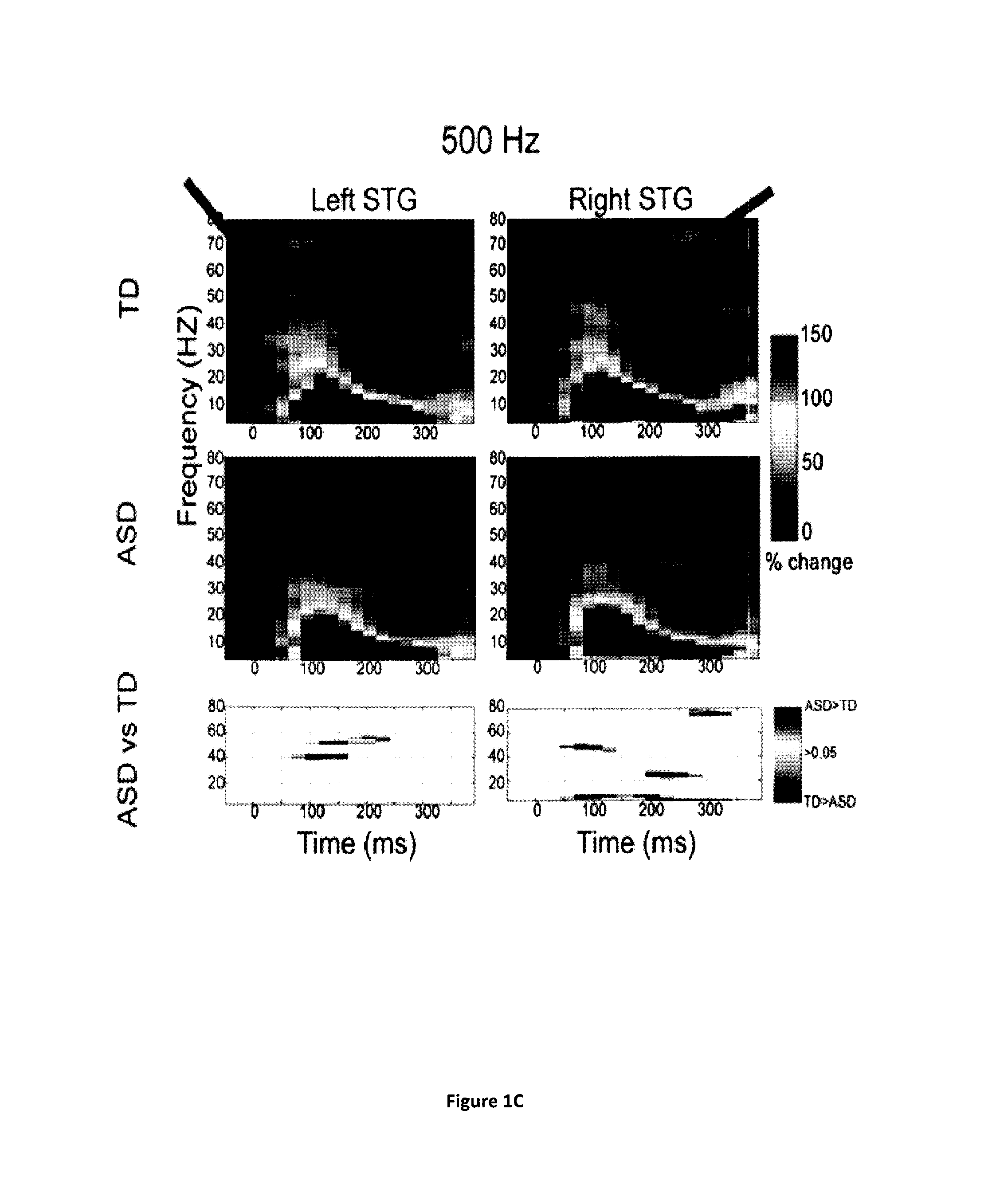
Magnetoencephalography biomarkers of gaba-b agonist drug activity in autism spectrum disorders
ActiveUS20160022207A1Improve response delayReduce delaysDiagnostic recording/measuringSensorsClinical psychologyAgonist drugs
Owner:THE CHILDRENS HOSPITAL OF PHILADELPHIA
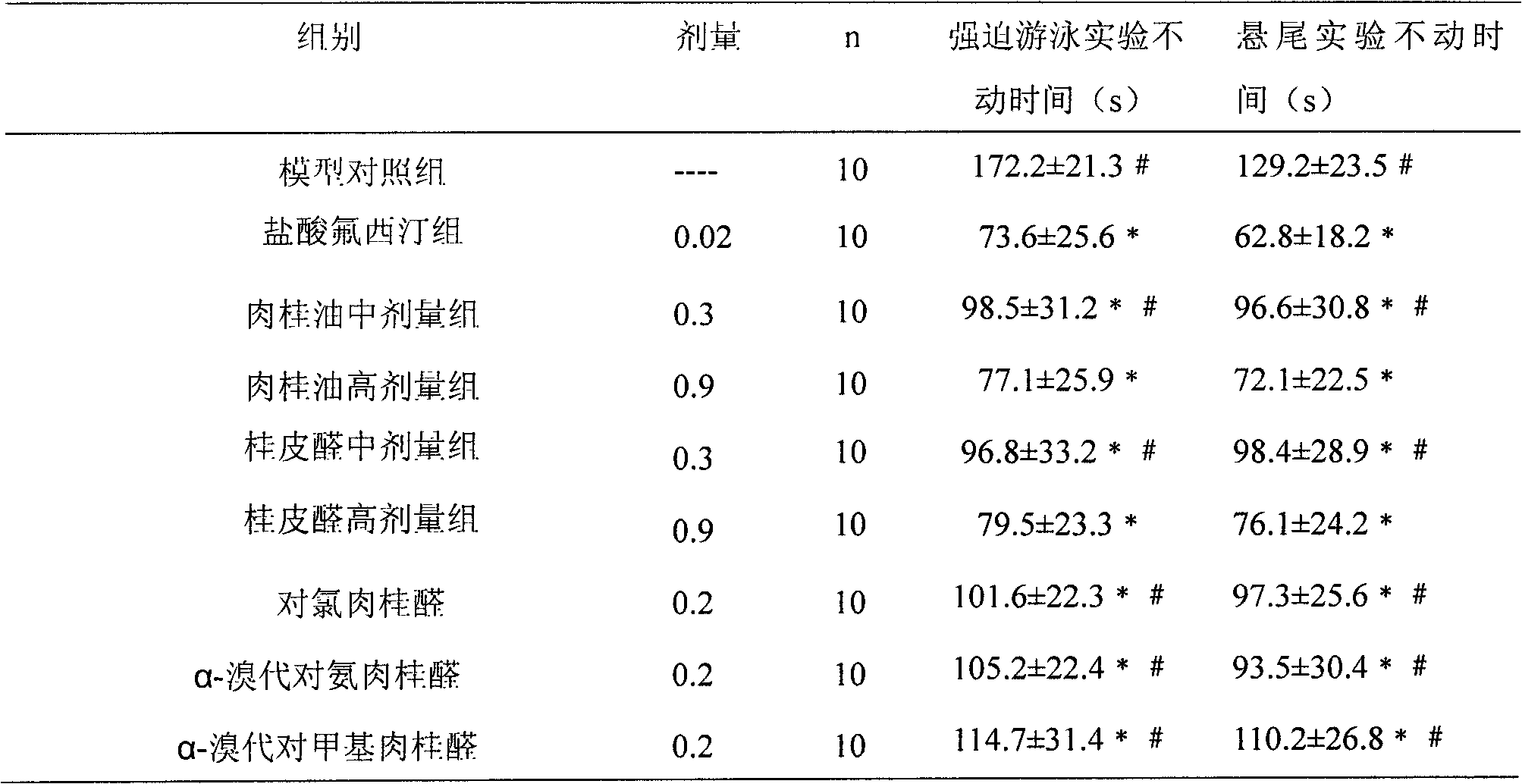
Application of cinnamon oil, cinnamyl aldehyde and derivatives of cinnamyl aldehyde in preparation of histamine H3 acceptor antagonist or inverse agonist medicaments
InactiveCN102218091AClear efficacyQuality is easy to controlNervous disorderAldehyde active ingredientsAgonist drugsCinnamon Oil
The invention provides application of cinnamon oil, cinnamyl aldehyde and derivatives of cinnamyl aldehyde in the preparation of histamine H3 acceptor antagonist or inverse agonist medicaments, and also provides a novel H3 acceptor antagonist or inverse agonist medicament which is formed by using cinnamon oil, cinnamyl aldehyde or derivatives of cinnamyl aldehyde as a main active ingredient and adding other active ingredients. The medicament can be used for preventing and treating senile dementia depression. The H3 acceptor antagonist or inverse agonist medicament provided by the invention has the advantages of clear curing effect, controllable quality and safety, and provides a new choice for clinical work.
Owner:CHENGDU MEDICAL COLLEGE
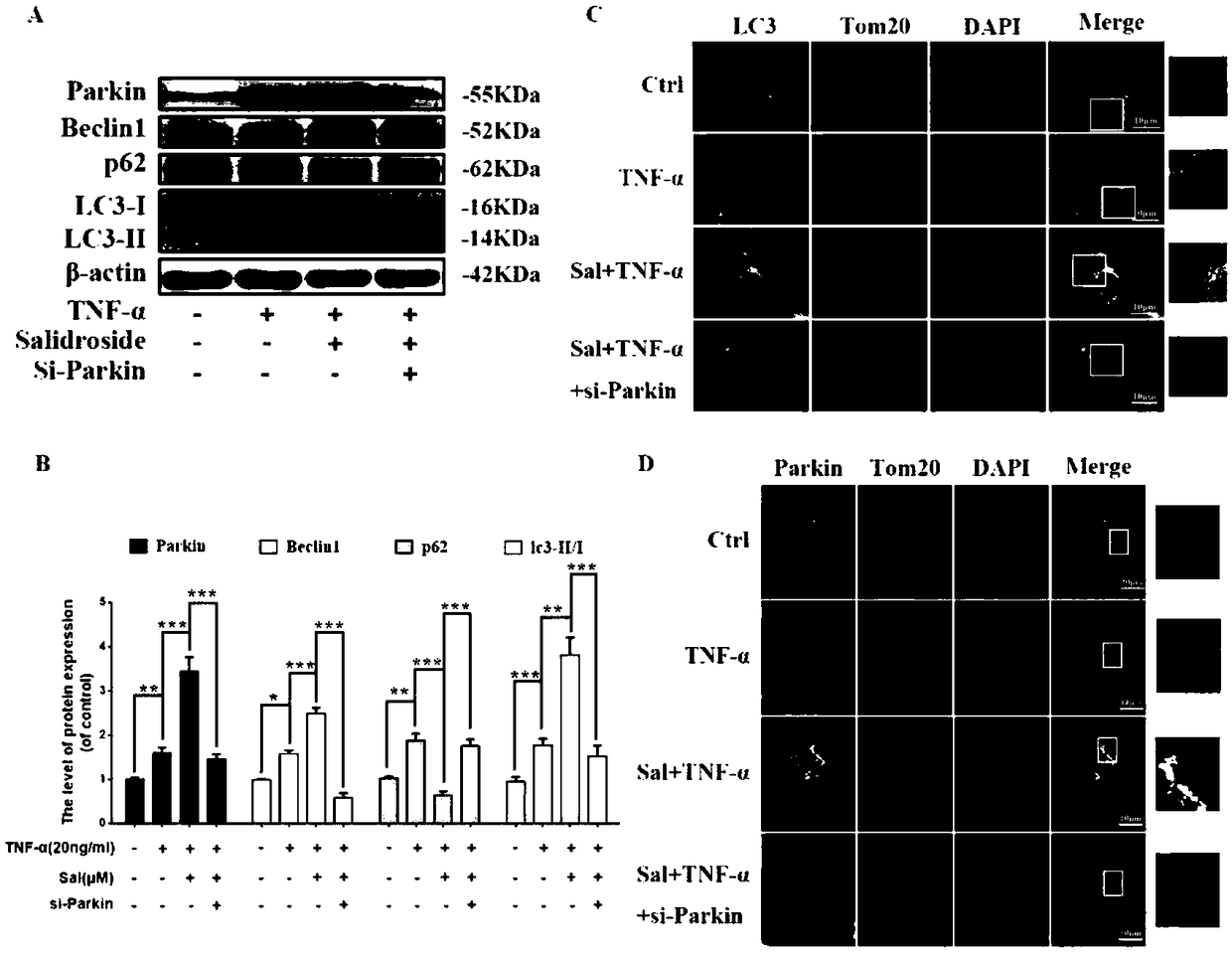
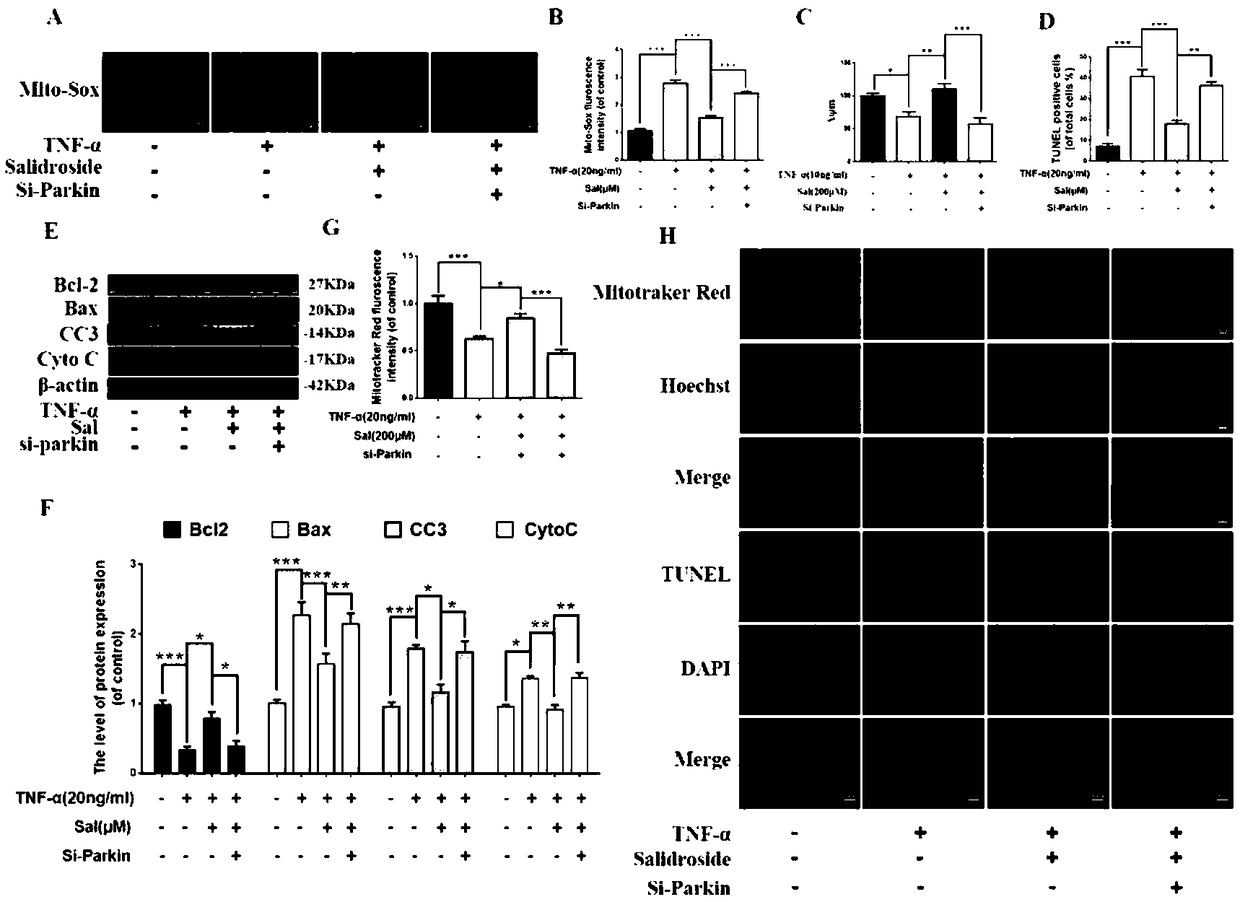
Application of salidroside to preparation of Parkin protein agonist drug
InactiveCN108743597AReduce doseAnti-apoptotic effect is obviousOrganic active ingredientsNervous disorderSalidrosideMedicine
The invention specifically discloses new application of salidroside, in particularly application of salidroside to the preparation of a Parkin protein agonist drug. By promoting the expression of parkin protein, salidroside, as a Parkin protein agonist, promotes mitochondrial autophagy and mitochondrial homeostasis, inhibiting apoptosis, and thereby the salidroside can be used for treating the neurodegenerative disease and the degenerative disease of the musculoskeletal system.
Owner:THE SECOND HOSPITAL AFFILIATED TO WENZHOU MEDICAL COLLEGE
Device and method for detecting beta-agonists drug
InactiveCN102645532AAchieving Simultaneous DetectionReduce testing costsMaterial analysisMedicineAgonist
The invention relates to the field of biological detection and discloses a detection device and a detection method of a large class of beta-agonists drugs by applying an immune chromatography technology. According to the invention, an antibody / receptor which is reacted with a large class of the beta-agonists drugs is used for preparing an immune chromatography test strip to realize the simultaneous detection of a large class of the beta-agonists drugs. Compared with the prior art, the detection on a large class of the beta-agonists drugs for one time can be realized, the detection cost is low and the method is convenient and fast.
Owner:SHENZHEN BIOEASY BIOTECHNOLOGY CO LTD +1
Beta-agonist semi-antigen, and preparation method and application thereof
ActiveCN103508910AGood potencyStrong specificityFibrinogenOrganic compound preparationSite monitoringHapten
The invention discloses a semi-antigen and a preparation method and application thereof and specifically relates to a beta-agonist semi-antigen. The invention further discloses a preparation method and application of the beta-agonist semi-antigen. A kit rapid detection product established on the basis of the beta-agonist semi-antigen has the advantages of convenient usage, low detection cost, usage of highly efficient, accurate and rapid detection methods, capacity of simultaneous detection of large batch samples and applicability to on-site monitoring of residue of beta-agonist drugs in animal-origin samples and screening of considerable samples.
Owner:BEIJING KWINBON BIOTECH
Casr agonist
ActiveCN102239136AExcellent CaSR agonistic activityOrganic active ingredientsGroup 5/15 element organic compoundsGlutamic Acid DerivativesAgonist
Many variations of compounds having CaSR agonistic activity are searched and a CaSR agonist agent, a pharmaceutical composition, a preventive or therapeutic agent for diarrhea, and a kokumi taste-imparting agent containing the compound are provided. The CaSR agonist agent, pharmaceutical composition, preventive or therapeutic agent for diarrhea, and kokumi taste-imparting agent, characterized by containing a glutamic acid derivative or a pharmaceutically acceptable salt thereof having CaSR agonistic activity.
Owner:EA PHARMA CO LTD
Method for controlling non-invasive brain entry of magnetic nano particles on basis of cell drug loading technology
InactiveCN109125728AEasy to operateWeak antigenicityNervous disorderEnergy modified materialsAgonist drugsMagnetite Nanoparticles
The invention discloses a method for controlling non-invasive brain entry of magnetic nano particles on the basis of a cell drug loading technology. The method comprises a preparation technology of cell (such as red blood cells and neutrophile granulocyte) wrapped magnetic nano particles, a blood brain barrier overcoming technology and a gradient magnetic field positioning and treatment technology. The preparation technology of the cell wrapped magnetic nano particles comprises the steps of hypotonic dilution and isotonic sealing; the blood brain barrier overcoming technology relies mainly onA2a adenosine receptor agonist drugs such as regadenoson; and a gradient magnetic field uses a principle of an electromagnetic effect and is generated by supplying current to a plurality of turns of coils located on an iron core. Magnetic cells of the technology have good magnetism and biocompatibility; accurate targeting and long-time retention of the magnetic cells in a body can be achieved through focusability of the gradient magnetic field; the magnetic nano particles can be positioned in a specific brain area by opening a blood brain barrier; a reaction can be generated in the brain through a pulse magnetic field; and accurate loading of the magnetic nano particles is achieved.
Owner:SOUTHEAST UNIV
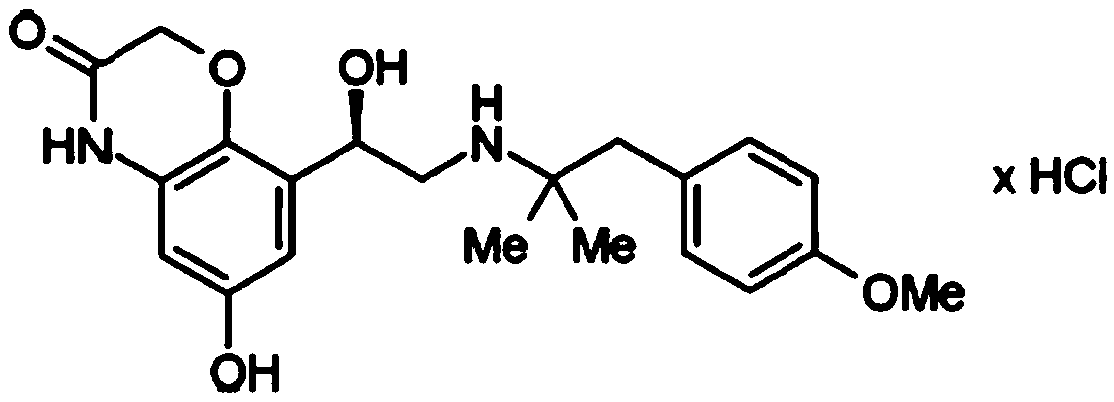
Crystal form C of olodaterol hydrochloride and preparation method thereof
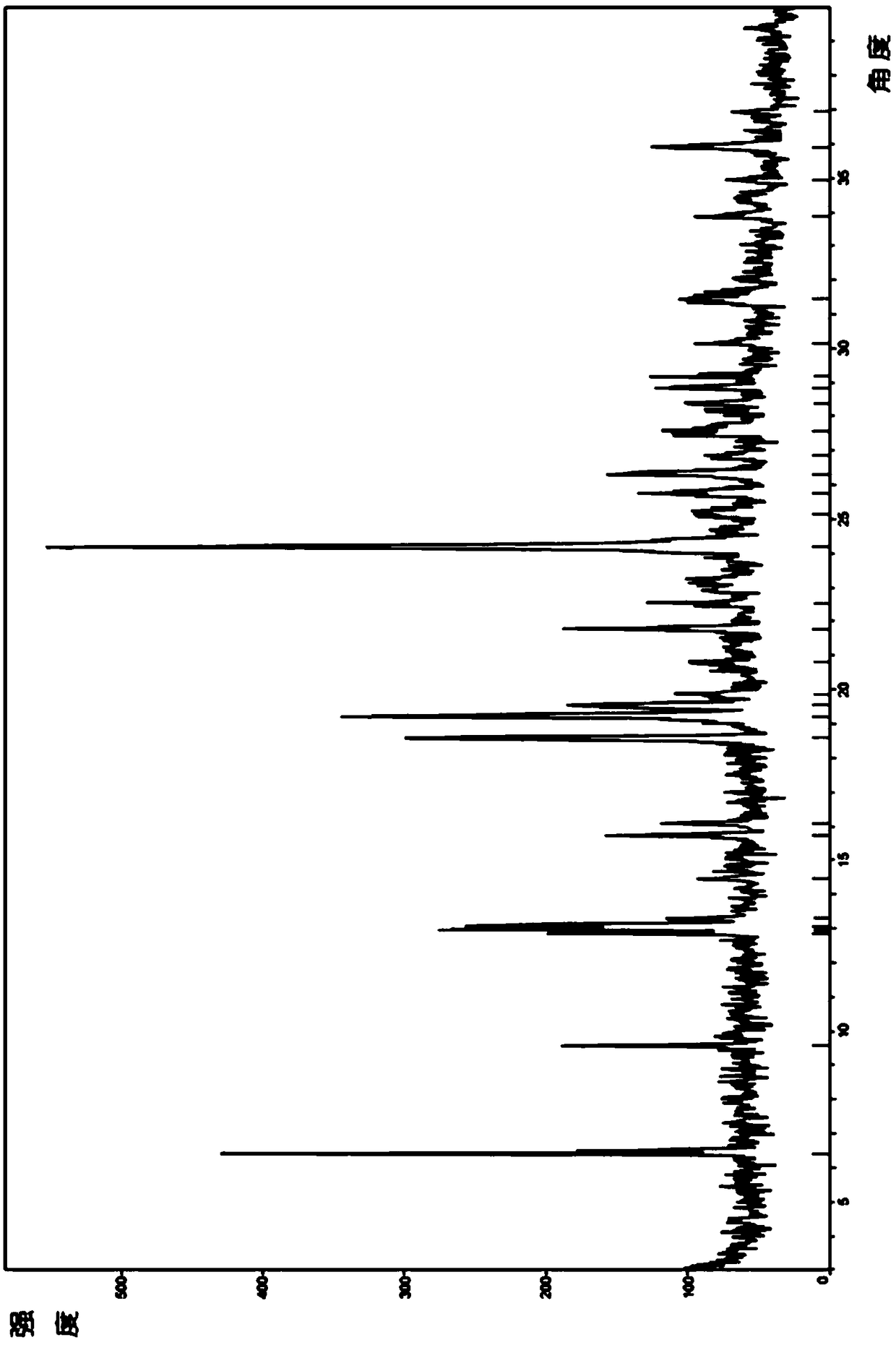
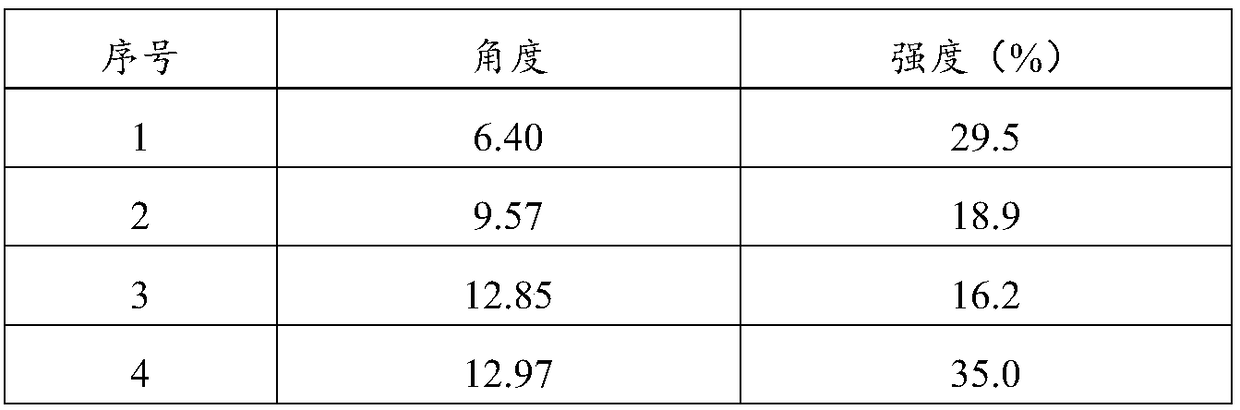
ActiveCN108822054ANot easy to absorb moistureImprove stabilityOrganic active ingredientsOrganic chemistry methodsCost ControlsX-ray
The invention relates to a crystal form C of a long-acting beta2 adrenergic agonist olodaterol hydrochloride and a preparation method thereof and a pharmaceutical composition containing the crystal form C. The crystal form is represented by the characteristic absorption peak of X-ray diffraction pattern. In comparison with the prior art, the crystal form C of olodaterol hydrochloride is not easy to absorb moisture, stability is remarkably raised, and the quality of the product is convenient to control; and the preparation technology is simple, is beneficial to cost control in the industrial production, and has high economic value.
Owner:SHANGHAI FANGYU HEALTH PHARMA TECH CO LTD +1
Recombinant porcine beta 2-adrenergic receptor protein and application thereof
InactiveCN103980359AHigh affinityGuaranteed accuracyCompound screeningApoptosis detectionAgonist drugsBio engineering
The invention belongs to the field of bio-engineering, and particularly relates to a method for preparing recombinant porcine beta 2-adrenergic receptor protein and an application of the recombinant porcine beta 2-adrenergic receptor protein in rapidly detecting beta 2 agonist medicaments. The recombinant porcine beta 2-adrenergic receptor protein obtained by separation and purification has beta 2 agonist binding activity, SDS-PAGE electrophoresis shows that the molecular weight of the recombinant protein is about 48kDa and the purity of the recombinant protein is more than 80%. The recombinant protein can specifically adsorb beta 2 agonist medicaments and three beta 2 agonist medicaments standard products with different concentrations have an inhibition effect on the reaction when detection is carried out by a competitive ELISA method. According to method disclosed by the invention, the beta 2AR receptor capable of specifically binding with beta 2 agonist is efficiently expressed by a molecular biology method and can be used for rapidly screening beta 2 agonist medicaments instead of conventional antibodies to achieve the detection of multi-residue and screening of unknown substance in medicaments, and the method has good application prospects.
Owner:INST OF QUALITY STANDARD & TESTING TECH FOR AGRO PROD OF CAAS
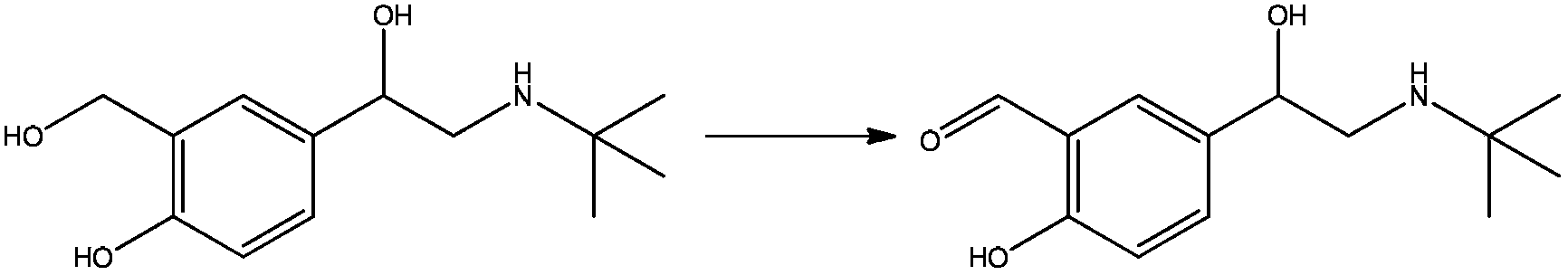
Resolution method for chiral Fenoldopam
ActiveCN104876869AOptically-active compound separationBulk chemical productionAgonist drugsProtecting group
The invention discloses resolution of a D1 dopamine receptor agonist drug and in particular discloses a resolution method of fenoldopam. The resolution method of the D1 dopamine receptor agonist drug comprises the steps of protecting a phenolic hydroxyl group and an amino group in the structure of fenoldopam, then carrying out chemical resolution with a chiral acidic resolution agent to obtain a pure optical isomer, and finally removing a protecting group and salifying to obtain optically pure methanesulfonic acid fenoldopam.
Owner:SICHUAN KELUN PHARMA RES INST CO LTD
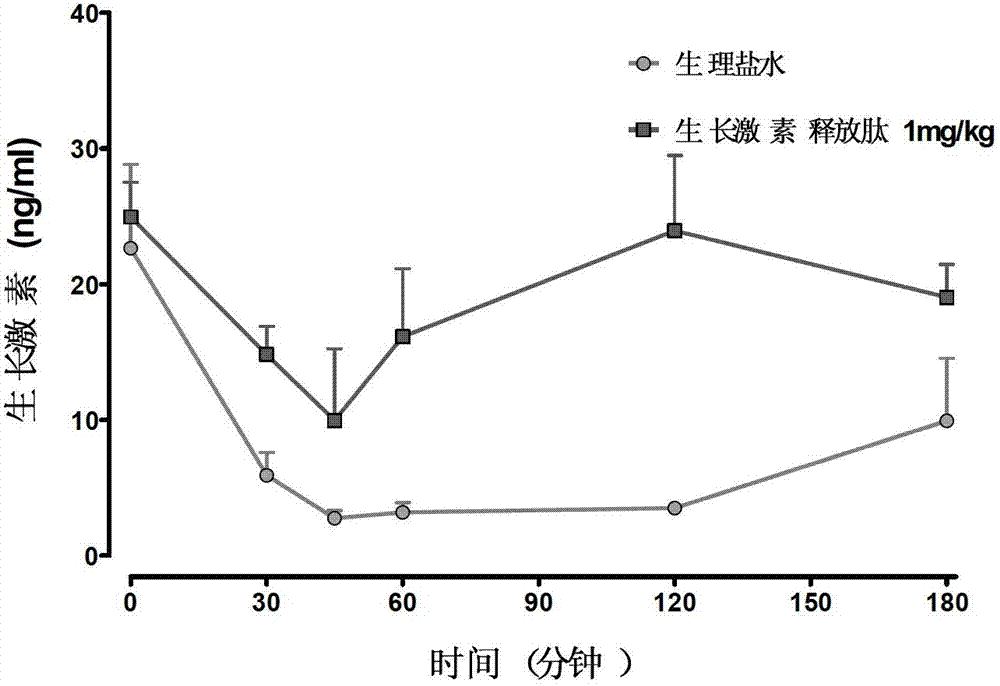
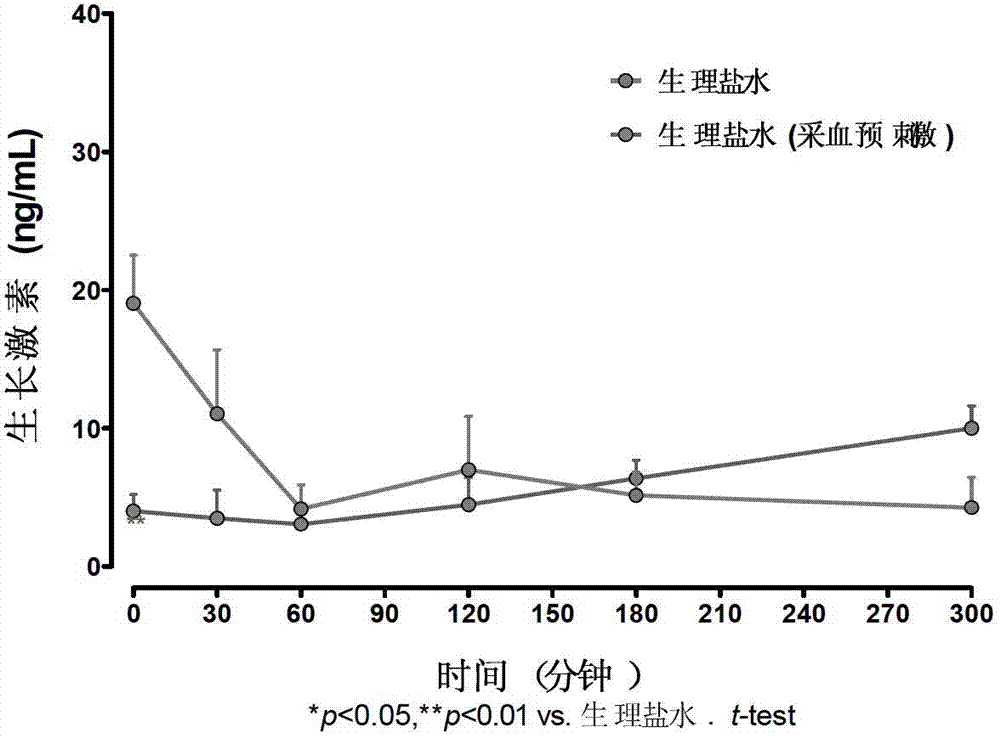
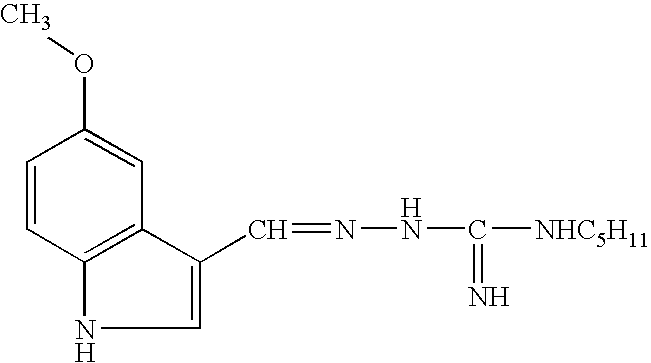
Method for screening GHSR1a agonist drug
A method for screening a GHSR1a agonist drug mainly comprises the following steps of: 1) carrying out prestimulation for multiple times before administration so as to stabilize basic level of growth hormone; 2) respectively drawing 30 microliters of blood 5, 15, 30, 60, 180 and 300 minutes after administration; 3) separating 10 microliters of blood plasma and using a Rat / Mouse GH ELISA kit to determine the content of the growth hormone in the blood plasma; and 4) choosing a drug, which enables the area rising rate under a blood plasma growth hormone concentration-time curve to be greater than 50%. According to the method for screening the GHSR1a agonist drug, a screening model of the stable, sensitive and repeatable GHSR1a agonist drug is established by the means of assumptive blood collection before administration. The invention can provide a reliable and effective method for screening the GHSR1a agonist drug in animal body.
Owner:辉源生物科技(上海)有限公司
5-HT4 partial agonist pharmaceutical compositions
InactiveUS20090104263A1High dissolution rateHigh capillaryOrganic active ingredientsBiocideOral medicationAgonist drugs
A solid pharmaceutical composition for oral administration comprising tegaserod in base or salt form in an amount of up to 10% by weight a bulking agent in an amount of 70 to 90% by weight a disintegrant in an amount of less than 15% by weight a glidant and a lubricant,
Owner:AUBERT JEROME +1
Compositions to enhance the efficacy and safety of bio-pharmaceutical drugs
InactiveUS20010004529A1Prevent desensitizationReducing desensitizationCompounds screening/testingBiocideSide effectCost effectiveness
Optimal ratios of pharmaceutical compositions of beta-1 and beta-2 agonists with their respective antagonists. Safer, more cost-effective drugs for heart and lung therapies are made by combining specific antagonists with their agonists to prevent desensitization of cellular receptors, reducing some of the unwanted side-effects of the agonist drugs alone. Determining the optimal concentration of an antagonist or inhibitor, which is necessary to prevent desensitization, without causing unnecessary and unwanted inhibition, creates a new class of pharmaceuticals. To derive an optimum ratio for a specific composition, a formulative method is provided to detail how competitive antagonists of the receptor should be combined with agonists, in specific proportions, to maximize and maintain receptor response throughout drug administration. The "optimal ratio" methodology used to determine a specific agonist / antagonist composition, to prevent beta-1 or beta-2 receptor desensitization, is experimentally verified and validated for specific compositions. Alteration of a specific ratio is practiced to account for the pharmacokinetic / dynamic differences between animals and humans and within human populations.
Owner:ENHANCED PHARMA
PTH functional domain conjugate peptides, derivatives thereof and novel tethered ligand-receptor molecules
Novel parathyroid hormone (PTH) peptides and analogs thereof of the PTH(1-34) fragment are disclosed that combine the N-terminal signaling domain (residues 1-9) and the C-terminal binding domain (residues 15-31) via a linker. Nucleic acid molecules and peptides for PTH(1-9)-(Gly)5-PTH(15-31) (PG5) and PTH(1-9)-(Gly)7-PTH(15-31) and a novel PTH receptor are disclosed. Additionally, methods of screening for PTH agonists, pharmaceutical compositions and methods of treatment are disclosed.
Owner:GARDELLA THOMAS J +3
Extraction method and application of indole monoterpene compound
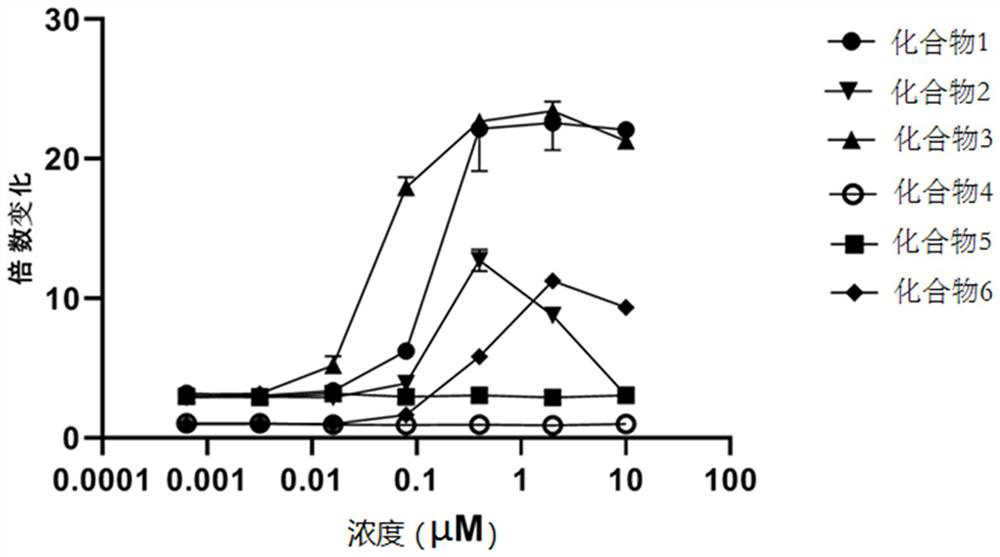
ActiveCN110746421APotent agonismOrganic active ingredientsNervous disorderAgonist drugsPharmaceutical drug
The invention discloses an extraction method and application of an indole monoterpene compound, and belongs to the technical field of traditional Chinese medicine extraction. A pharmacodynamic substance in uncaria rhynchophylla that has a significant agitation effect on a 5-HT1A receptor is clarified, it is found that a compound 1 and a compound 2 have the strong agitation effect on the 5-HT1A receptor. The indole monoterpene compound obtained by extracting can effectively agitate the 5-HT1A receptor, and it is indicated that the compound can be used as an agonist drug for the 5-HT1A receptor.
Owner:DALIAN MEDICAL UNIVERSITY
Olodaterol hydrochloride crystalline form B and preparation method thereof
PendingCN108997248ANot easy to absorb moistureImprove stabilityOrganic active ingredientsOrganic chemistry methodsAdrenergic receptor agonistsAgonist drugs
The invention relates to a crystalline form B of a long-acting beta 2 adrenergic agonist drug olodaterol hydrochloride and a preparation method thereof, and a pharmaceutical composition containing thecrystalline form B, wherein the crystalline form is characterized by an X-ray diffraction pattern characteristic absorption peak thereof. Compared with the prior art, the crystalline form B of olodaterol hydrochloride provided by the invention is not easy to absorb moisture, remarkably improves stability and is convenient for product quality control; the preparation process is simple, which is beneficial to the cost control in industrial production and has high economic value.
Owner:SHANGHAI FANGYU HEALTH PHARMA TECH CO LTD
Beta-agonist hapten, artificial antibody as well as preparation methods and applications of beta-agonist hapten and beta-agonist artificial antibody
ActiveCN108181463AHigh sensitivityGood broad-spectrum specificityOvalbuminSerum albuminChemical reactionSalbutamol
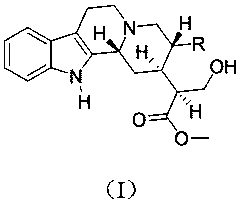
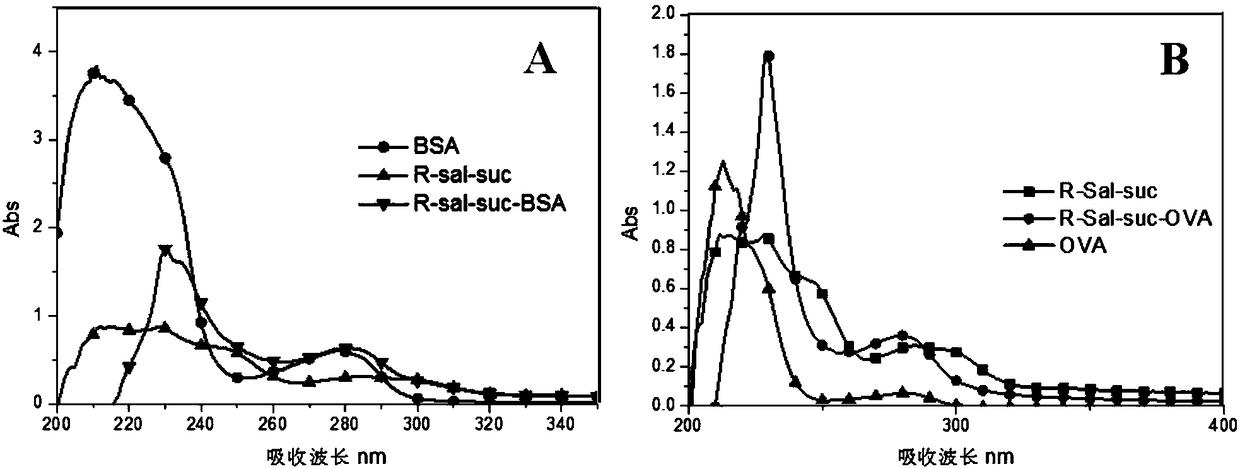
The invention discloses a beta-agonist hapten. The molecular structure of the beta-agonist hapten is shown in the following formula (I) in the description. The beta-agonist hapten is successfully synthesized from R-(-)-salbutamol as a raw material by one-step chemical reaction. The hapten is coupled with carrier protein, an artificial antigen is prepared, and the broad-spectrum specific beta-agonist artificial antibody is obtained after immunization. The antibody can recognize 31 kinds of beta-agonist drugs and analogs of the beta-agonist drugs, the semi-inhibitory concentration for R-(-)-salbutamol is 0.5 ng / mL, the linear range is 0.11-40.86 ng / mL, and the minimum detection limit is 0.04 ng / mL. The antibody can detect multiple beta-agonist drugs simultaneously, can be used for on-site rapid detection of food safety and has broad application prospect.
Owner:SOUTH CHINA AGRI UNIV
Method of screening atomization device suitable for beta2 receptor agonist
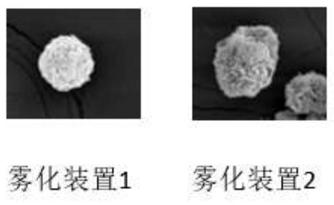
PendingCN111610261AOptimal drug therapyImprove accuracyComponent separationParticle size analysisPhysical chemistryAgonist drugs
The invention relates to a method for screening an atomization device suitable for a beta2 receptor agonist. The method comprises the following steps: (1) detecting the particle size ratio, the volumeaverage particle size and the surface area average particle size of 1-5[mu]m atomization particles in an atomization device to be screened; (2) detecting the concentration of the electrolyte solution; (3) selecting an atomization device which simultaneously meets the following conditions a) and b): a) the ratio of the particle size of the 1-5[mu]m atomization particles is 50-70%, the ratio of thevolume average particle size to the surface area average particle size is less than and / or equal to 3, and b) the concentration of the electrolyte solution is 0.2-1% w / v.
Owner:SHANGHAI FANGYU HEALTH PHARMA TECH CO LTD
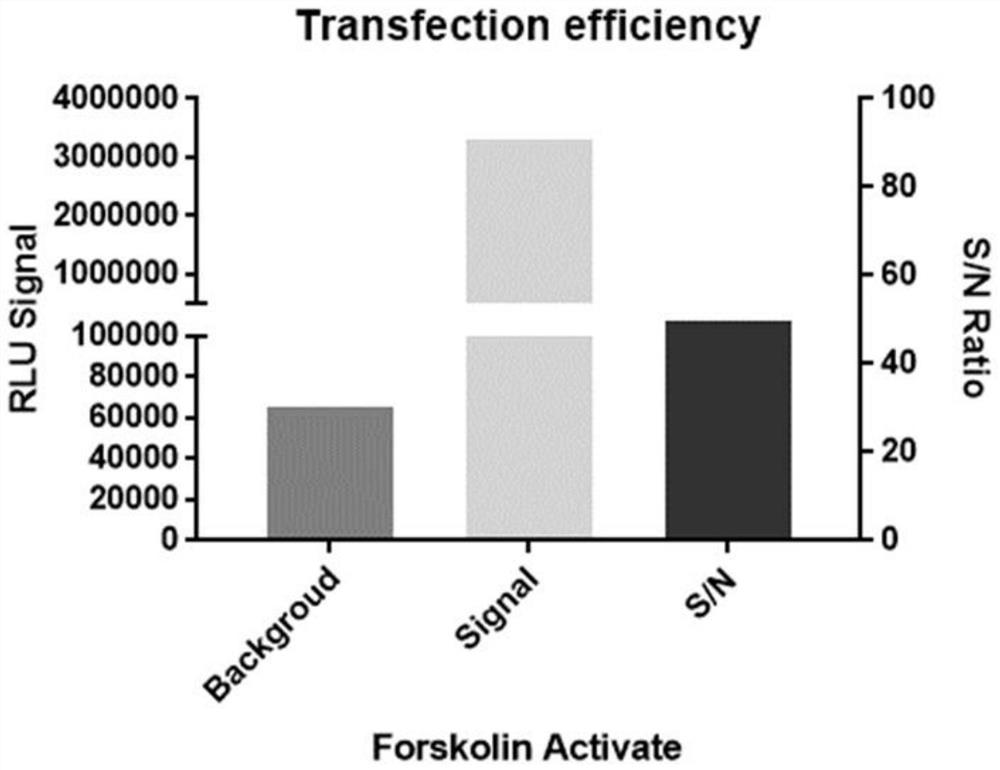
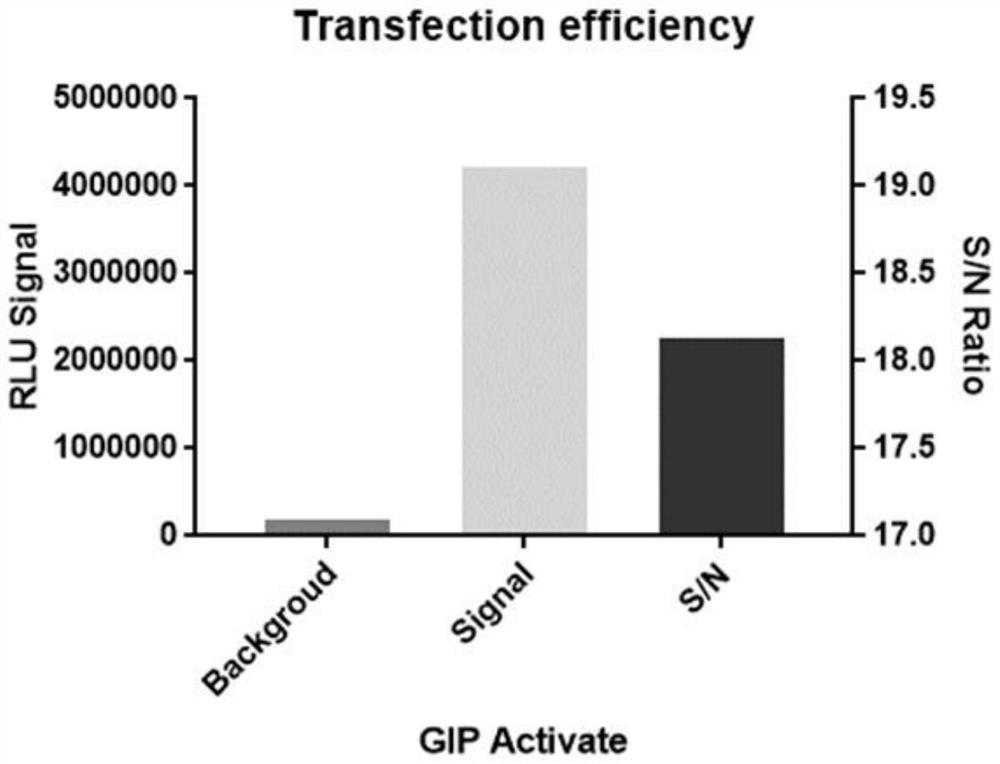
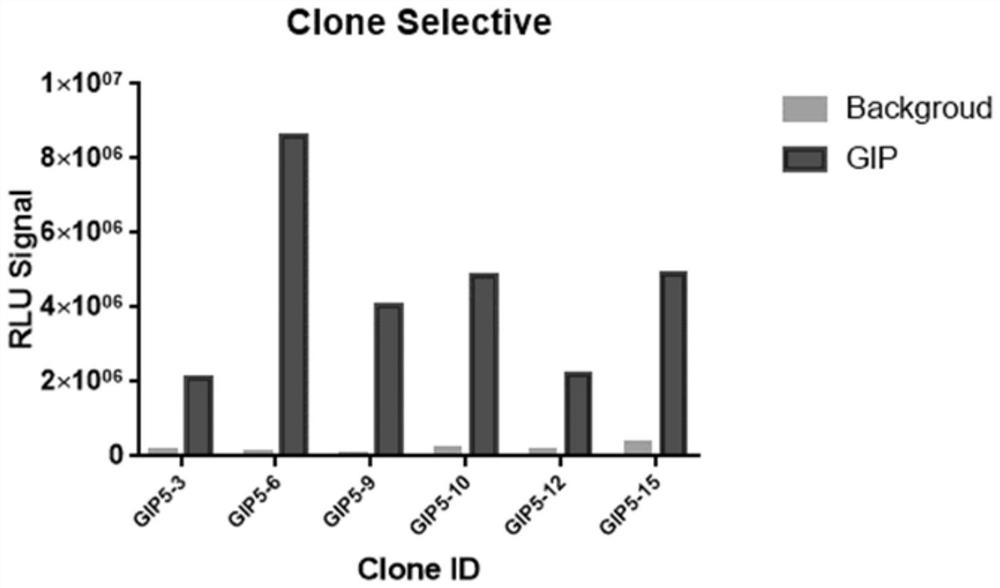
Construction and application of GIPR reporter gene stably transfected cell strain
PendingCN114231493AIncrease sensitivityIncreased sensitivityCompound screeningApoptosis detectionAntagonismAgonist drugs
The invention discloses construction of a GIPR reporter gene stably transfected cell strain, which comprises the following steps: transfecting 293T cells by using CRE reporter gene plasmids, adding eukaryotic antibiotics for screening and monoclonal selection, and performing functional evaluation and selection to obtain an appropriate CRE reporter gene stably transfected cell strain monoclonal; gIPR lentivirus is used for infecting CRE reporter gene stably transfected cell strain monoclonal cells, eukaryotic antibiotics are added for screening and monoclonal selection, and the appropriate GIPR reporter gene stably transfected cell strain is obtained through functional evaluation. A biological activity determination method, a biological analysis quantitative method and a neutralizing antibody determination method which are not limited to GIPR agonist drugs are developed by utilizing the cell strain. The problems that the detection steps are tedious, the time cost is high, the reagent consumable cost is high, experimental data interpretation is complex, the method is not sensitive enough, the anti-interference capacity is poor, the GIPR activation / antagonism mechanism and effect cannot be completely represented, and universality is not achieved are solved.
Owner:宁波熙宁检测技术有限公司 +1
Compound blood pressure lowering pharmaceutical composition and uses thereof
ActiveCN108686214AReduce constipationReduce tachycardiaOrganic active ingredientsCardiovascular disorderSide effectAgonist drugs
The present invention provides uses of combined application of a calcium antagonist drug and a 5-HT1A receptor agonist drug in preparation of drugs for treatment and prevention of high blood pressure,and a blood pressure lowering pharmaceutical composition, which comprises a therapeutically effective amount of a calcium antagonist and an active metabolite thereof, a therapeutically effective amount of a 5-HT1A receptor agonist and an active metabolite thereof, and a pharmaceutically acceptable carrier. According to the present invention, through the combined application of the calcium antagonist drug and the 5-HT1A receptor agonist drug, the blood pressure lowering effect can be significantly enhanced, and the side effect of the calcium antagonist can be reduced; and particularly comparedto the combined medication, the application of the compound preparation made of the calcium antagonist and the 5-HT1A receptor agonist as the blood pressure lowering drug can achieve the unexpected blood pressure lowering effect and reduce calcium antagonist induced side effects including constipation and tachycardia.
Owner:SICHUAN CREDIT PHARMA
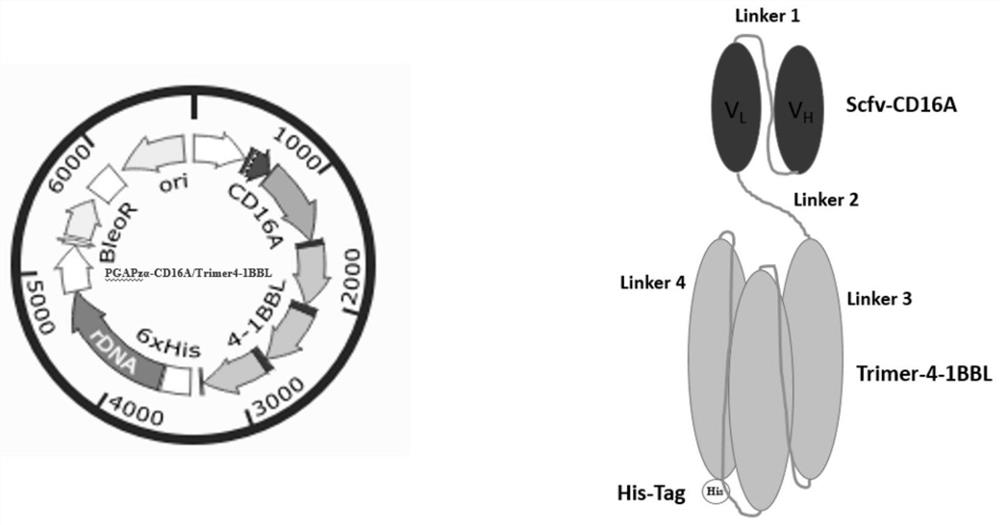
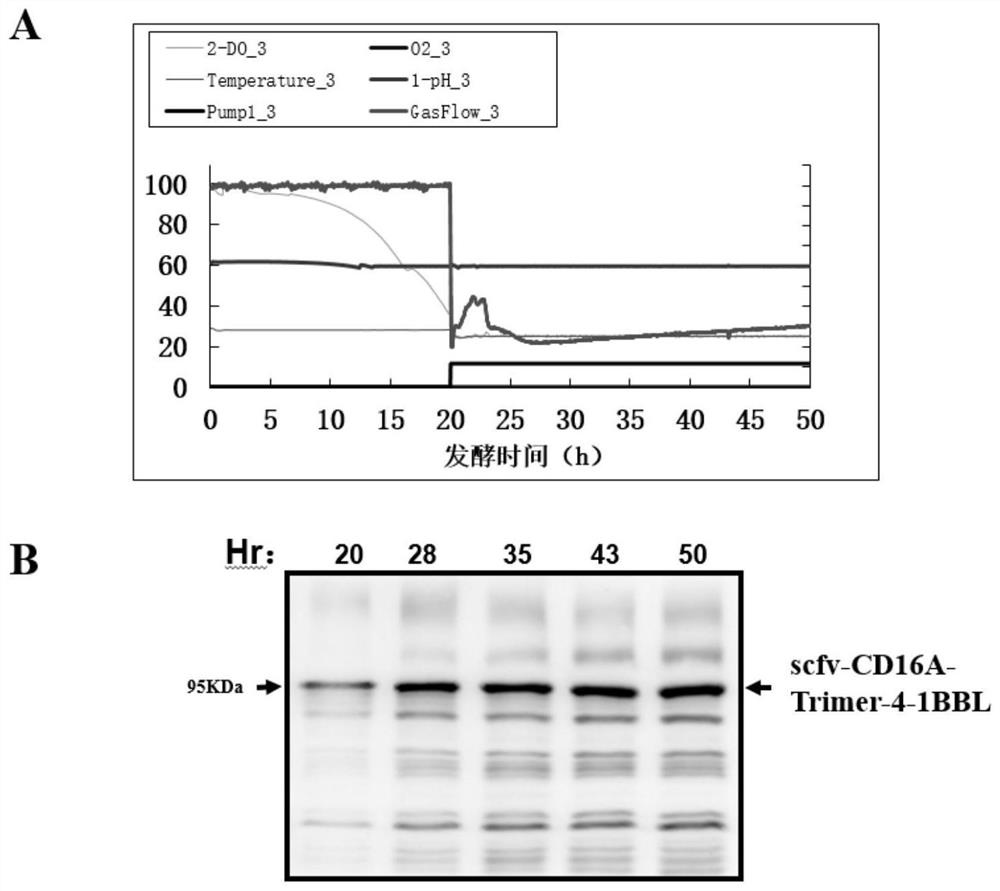
Bispecific NK cell agonist as well as preparation method and application thereof
The invention relates to the field of biological medicine, in particular to a bispecific NK cell agonist as well as a preparation method and application thereof. The invention provides a fusion protein, which takes a CD16A receptor and a 4-1BB receptor as targets to obtain a novel bispecific NK cell agonist drug, namely scfv-CD16A-Trimer-4-1BBL, which has the activity of activating and amplifying NK cells in vitro, enhances the antiviral and anti-tumor capabilities of the NK cells, has potential effects of promoting NK cell proliferation and enhancing T cell functions, and can be used for preparing a novel bispecific NK cell agonist drug. The application potential of in-vitro culture and amplification of the NK cells is achieved, and the amplified NK cells have good cytotoxicity.
Owner:SHANGHAI NK CELLTECH CO LTD
Magnetoencephalography biomarkers of GABA-B agonist drug activity in autism spectrum disorders
Methods for screening for therapies against autism spectrum disorders and methods for determining whether a subject would be responsive to a therapy against an autism spectrum disorder are disclosed.
Owner:THE CHILDRENS HOSPITAL OF PHILADELPHIA
Treatment of neuropathic pain
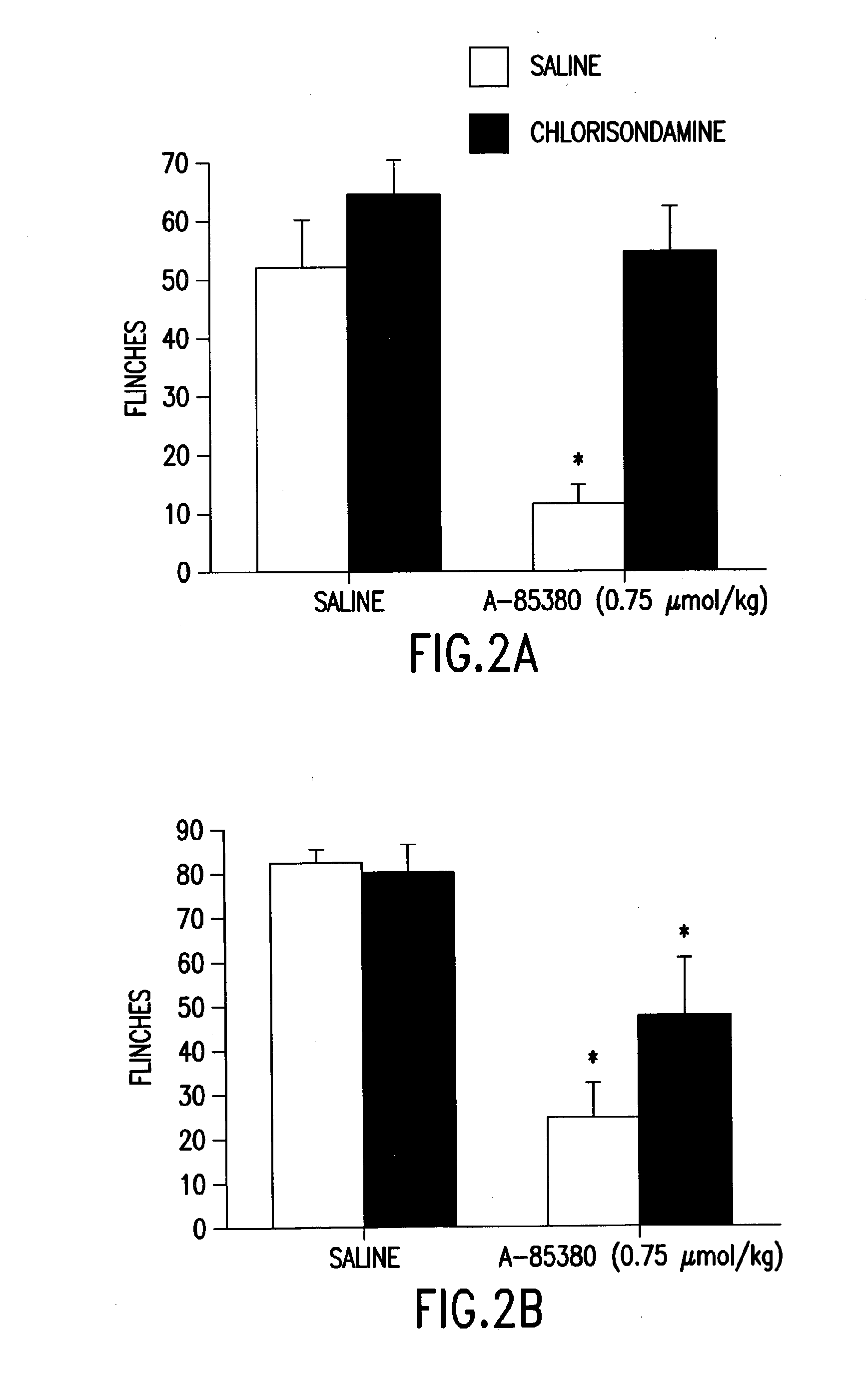
A method for treating a patient suffering from neuropathic pain, comprising administering to a patient in need of such treatment an effective amount of an agonist drug capable of binding to the neuronal nicotinic receptor (NNR) but which does not readily cross the blood-brain barrier.
Owner:RUETER LYNNE E +1
A kind of cell line and detection method for detecting the activity of antibody-immune agonist conjugated drug
ActiveCN114395532BHigh sensitivityLarge detection dynamic rangeCompound screeningApoptosis detectionAgonist drugsEfficacy
Owner:GENEQUANTUM HEALTHCARE SUZHOU
A method for broad-spectrum identification of β-receptor agonist drugs
ActiveCN104297406BEnsure safetyEffective supervision meansComponent separationChemical structureInformation repository
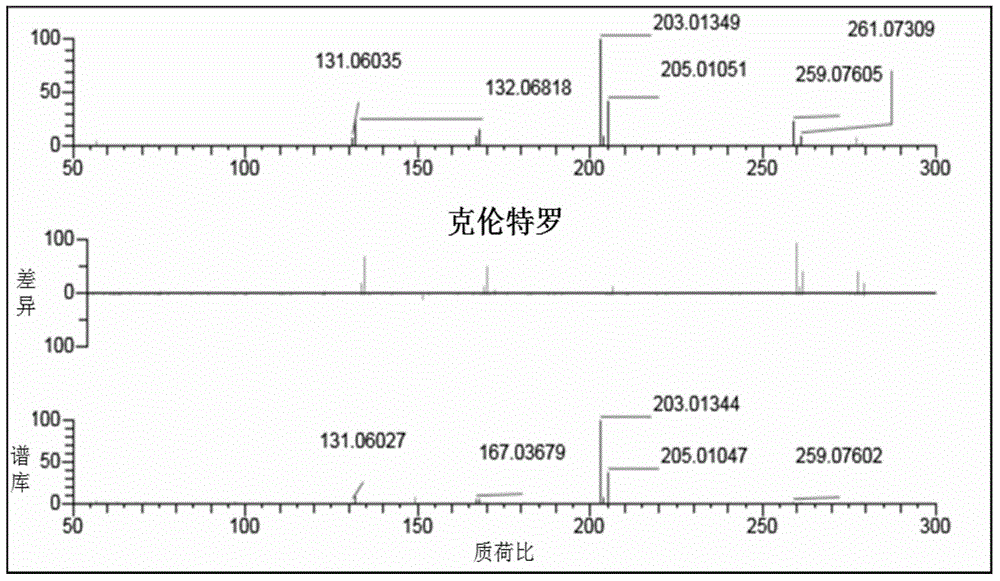
The invention provides a method for broad spectrum identification of beta-receptor stimulant medicines, and relates to the field of medicine testing. According to the method, mass spectrum signals associated with the characteristic chemical structures of the beta-receptor stimulant medicines can be obtained by virtue of an ultra-high-performance liquid chromatography (HPLC)-quadrupole-orbit trap high-resolution mass spectrum device; a characteristic spectrum information base of the beta-receptor stimulant medicines can be built according to the characteristics of mass spectrum signals of the beta-receptor stimulant and the dissociation law of the beta-receptor stimulant. Whether known medicines exist in a sample or not can be identified by comparing high-flux data acquired by the mass spectrum and data in the characteristic spectrum information base; furthermore, whether a novel unknown medicine possibly exists in the sample or not can be predicted by indentifying the modes of the mass spectrum signals; finally, the method for the broad spectrum identification of the beta-receptor stimulant medicines can be formed, so that the beta-receptor stimulant medicines in unknown samples can be rapidly identified. The method provided by the invention has good social benefit and economic benefit.
Owner:CHINA AGRI UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com