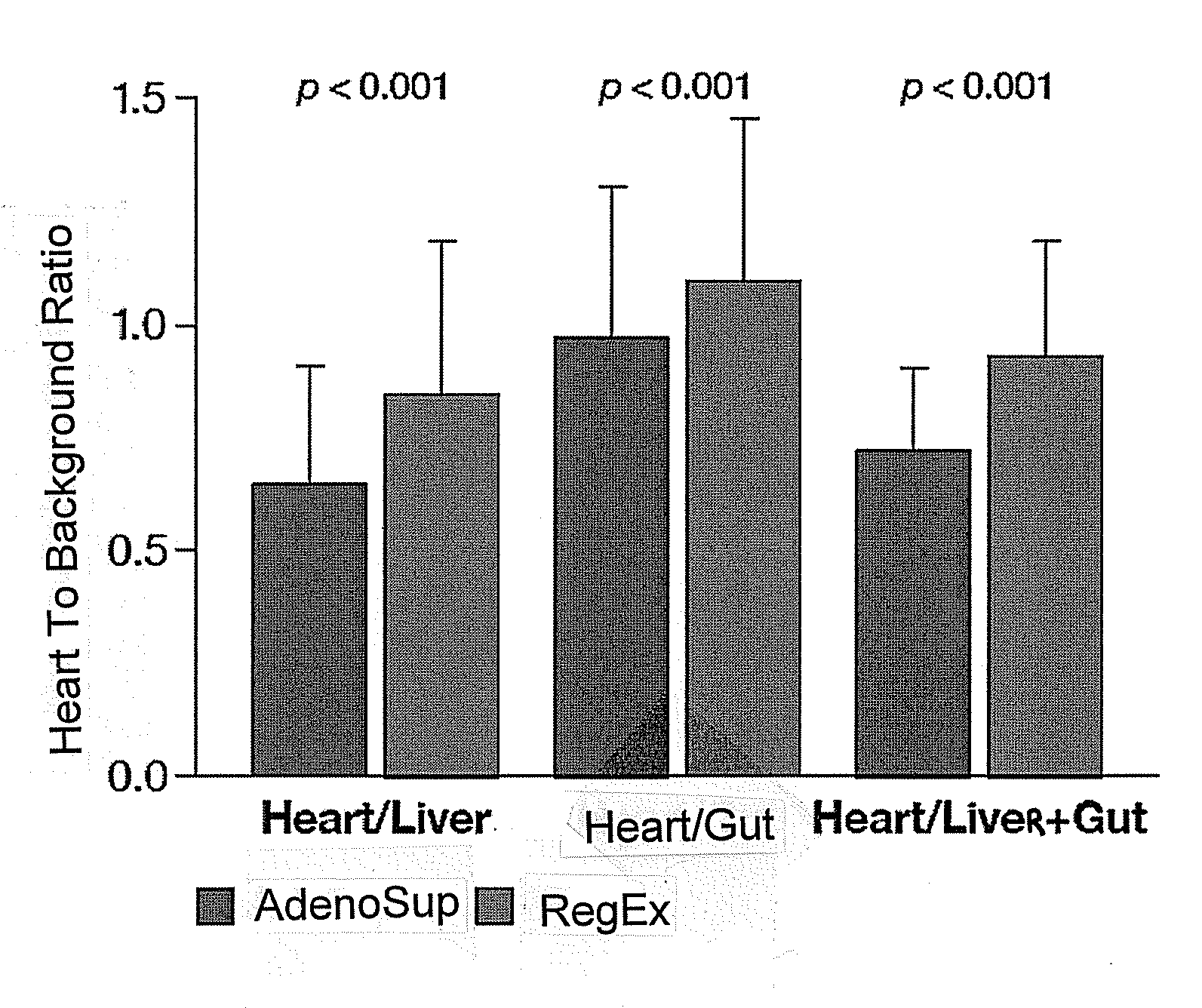
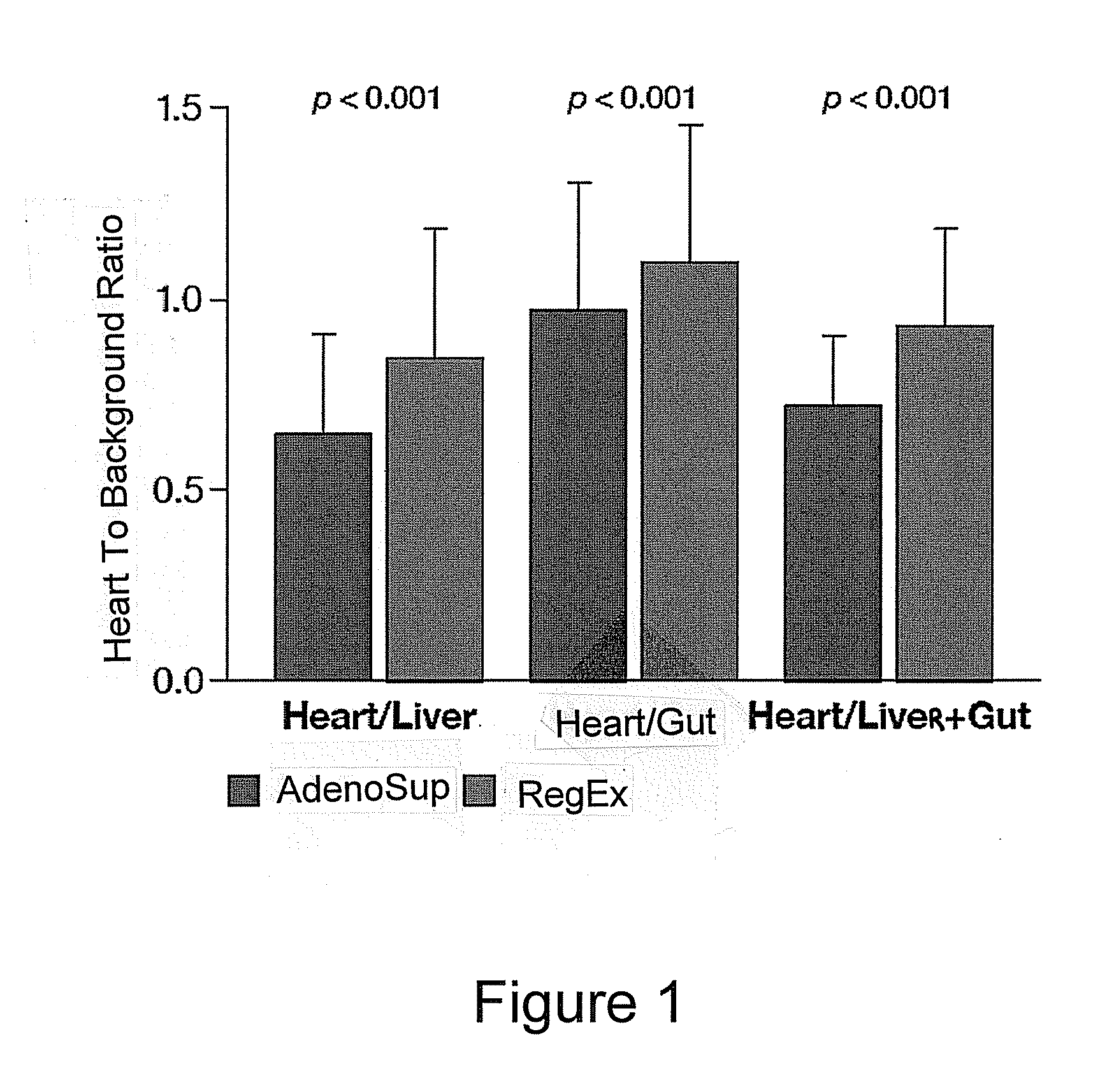
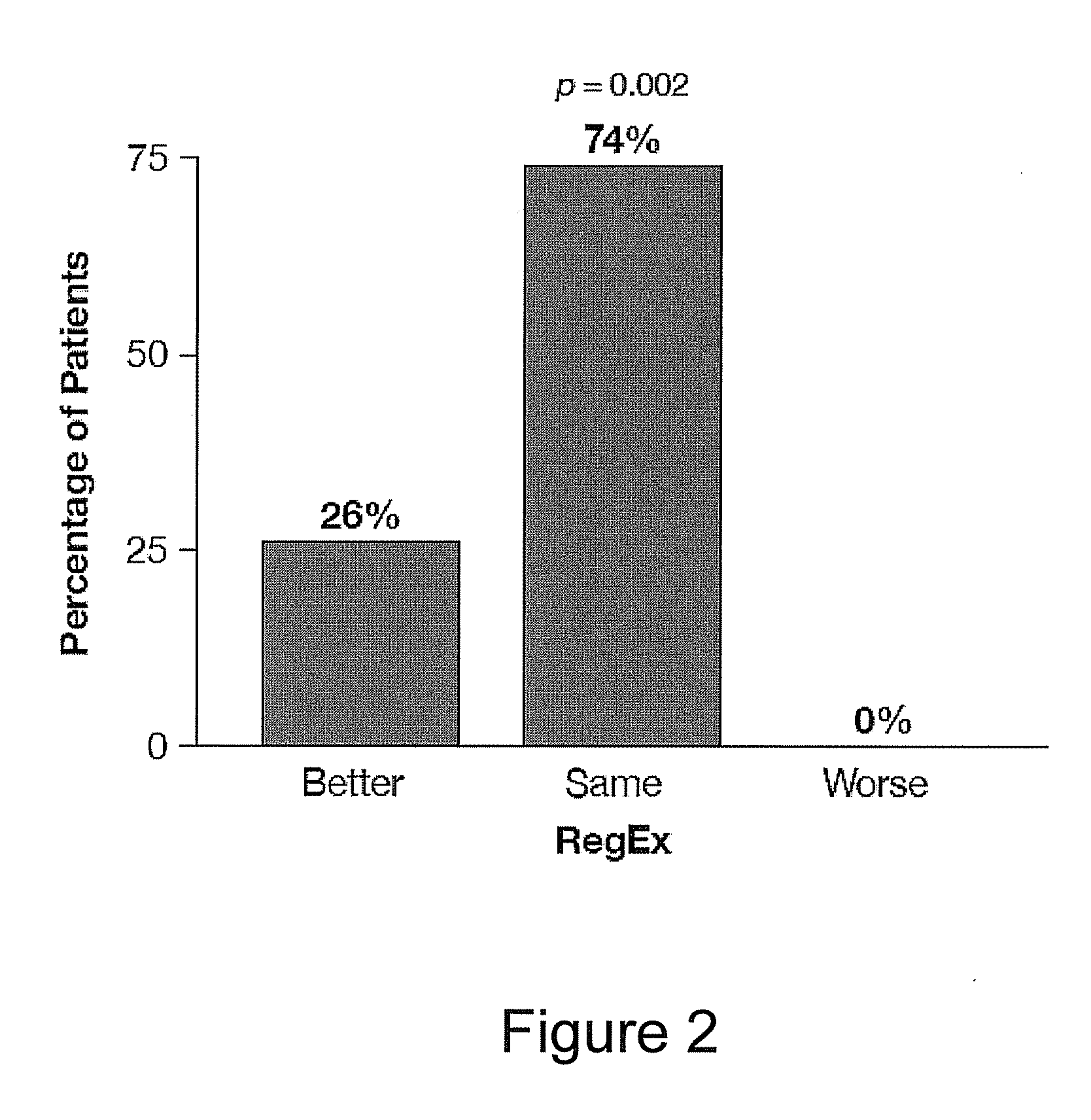
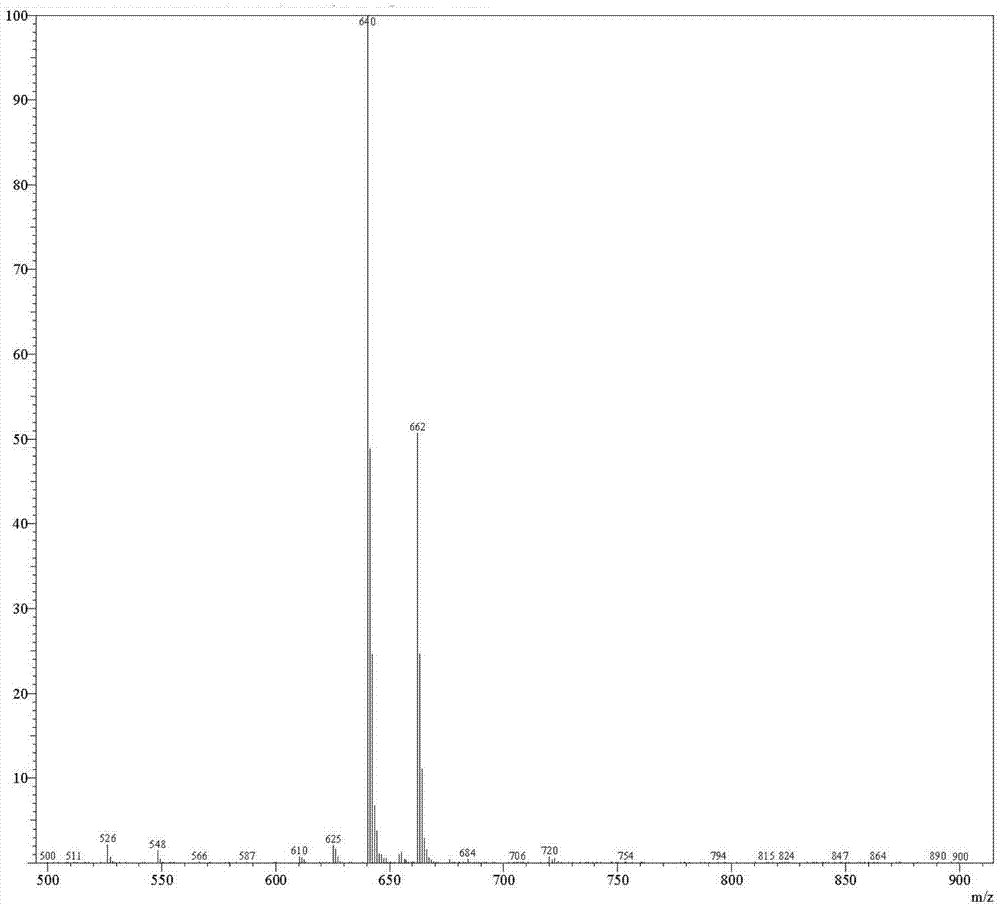
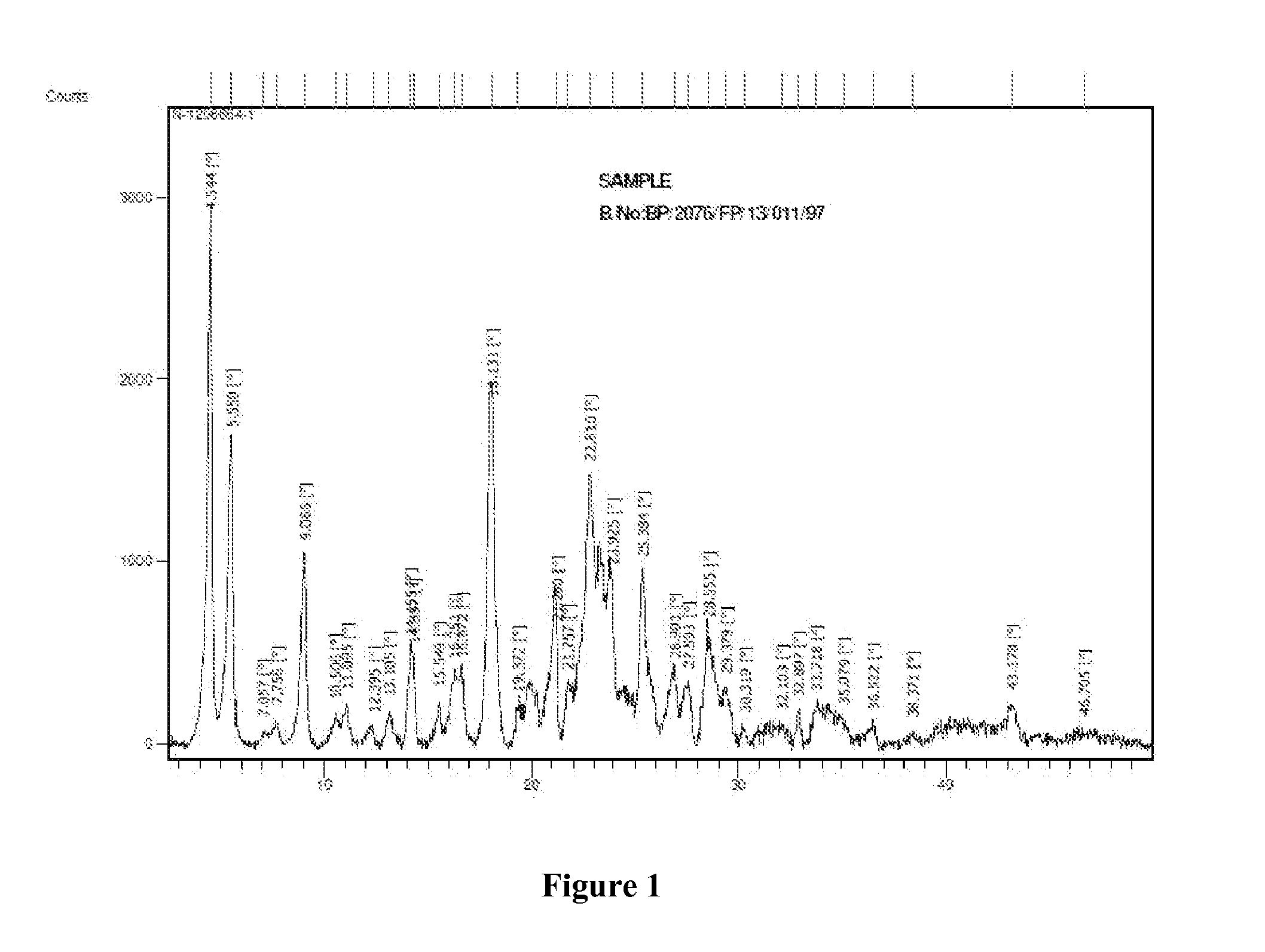
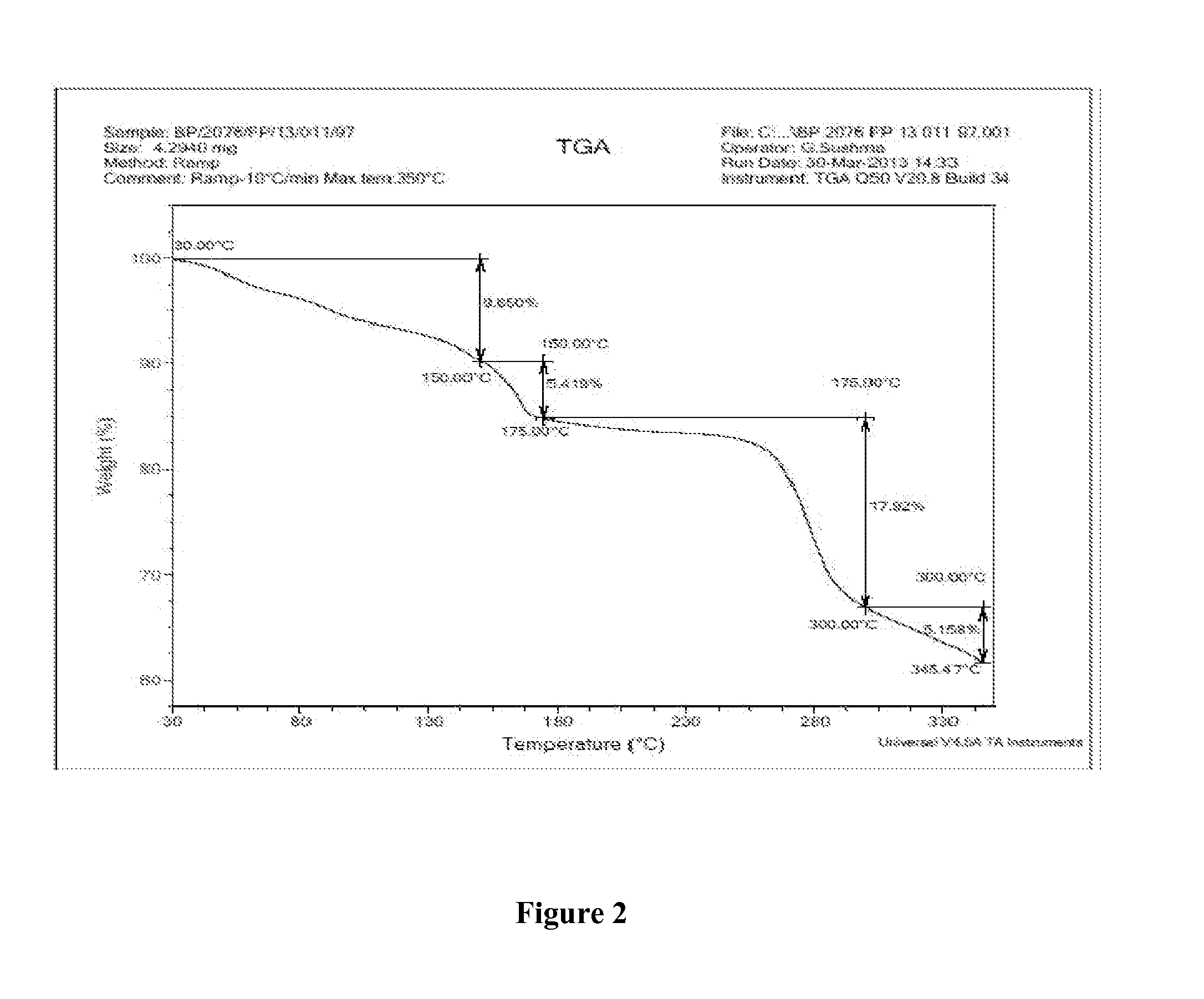
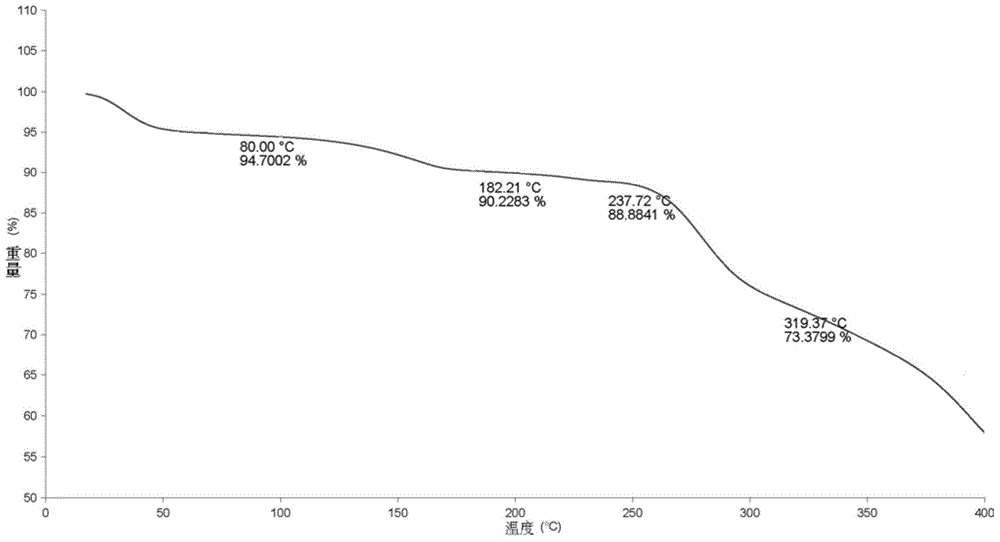
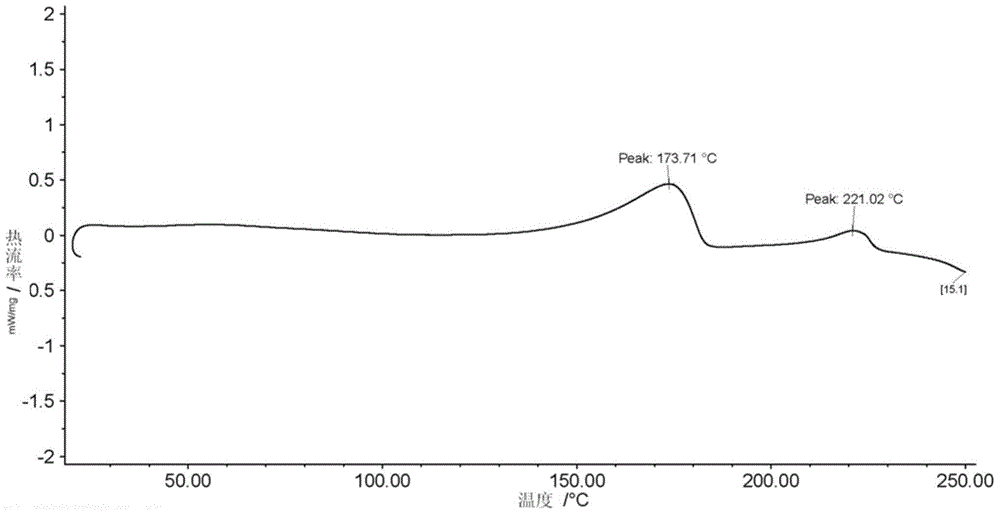
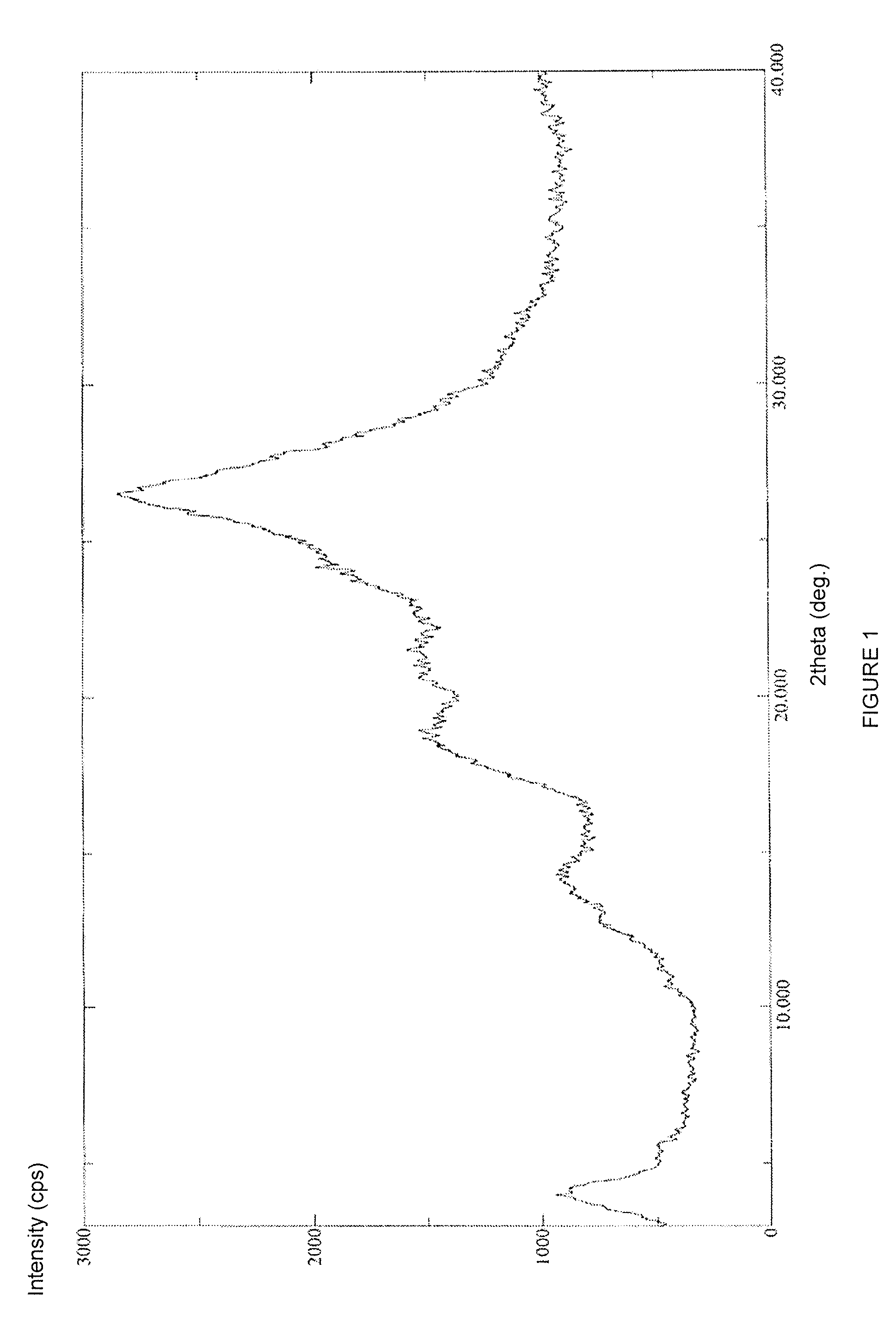
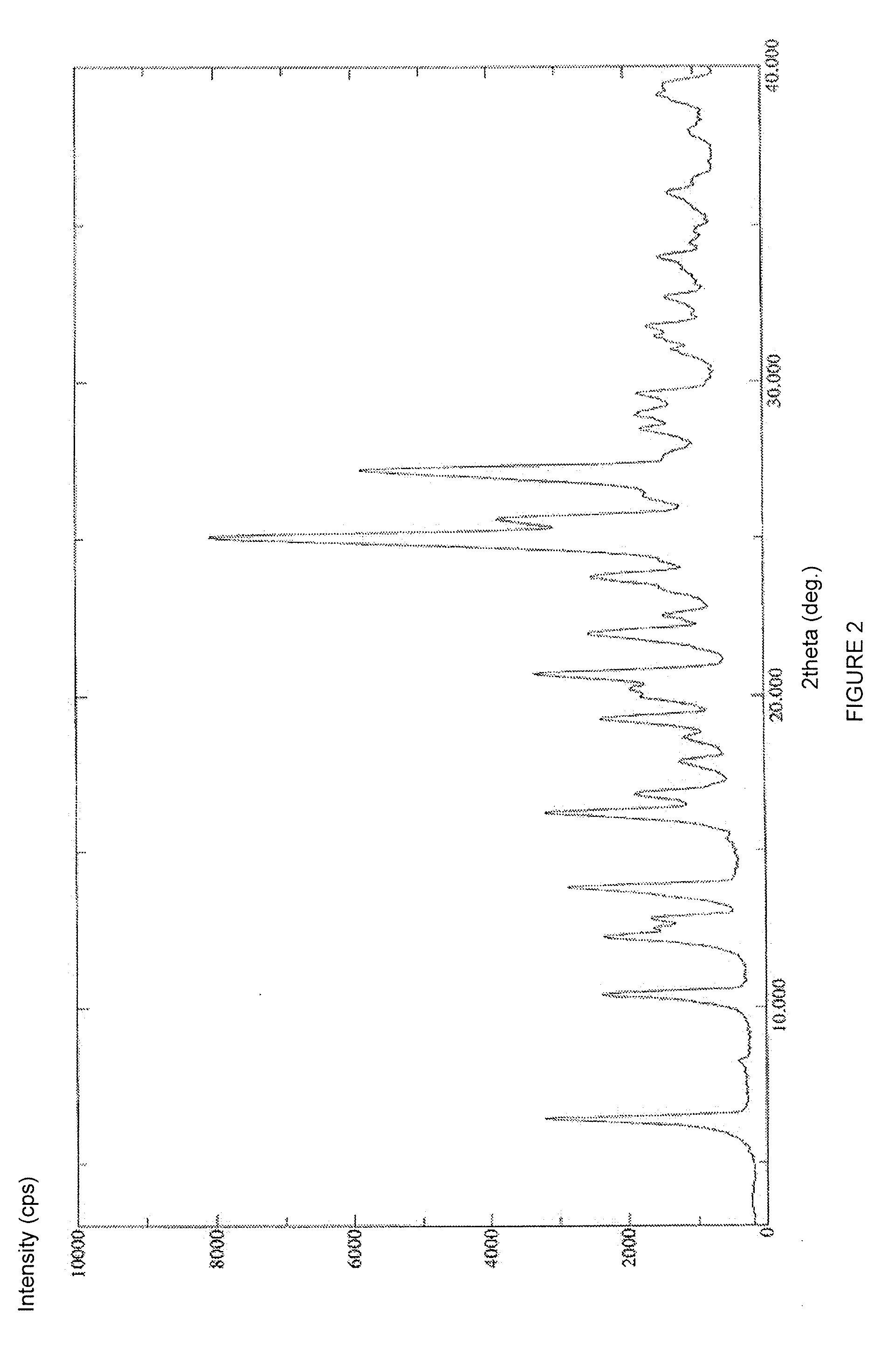
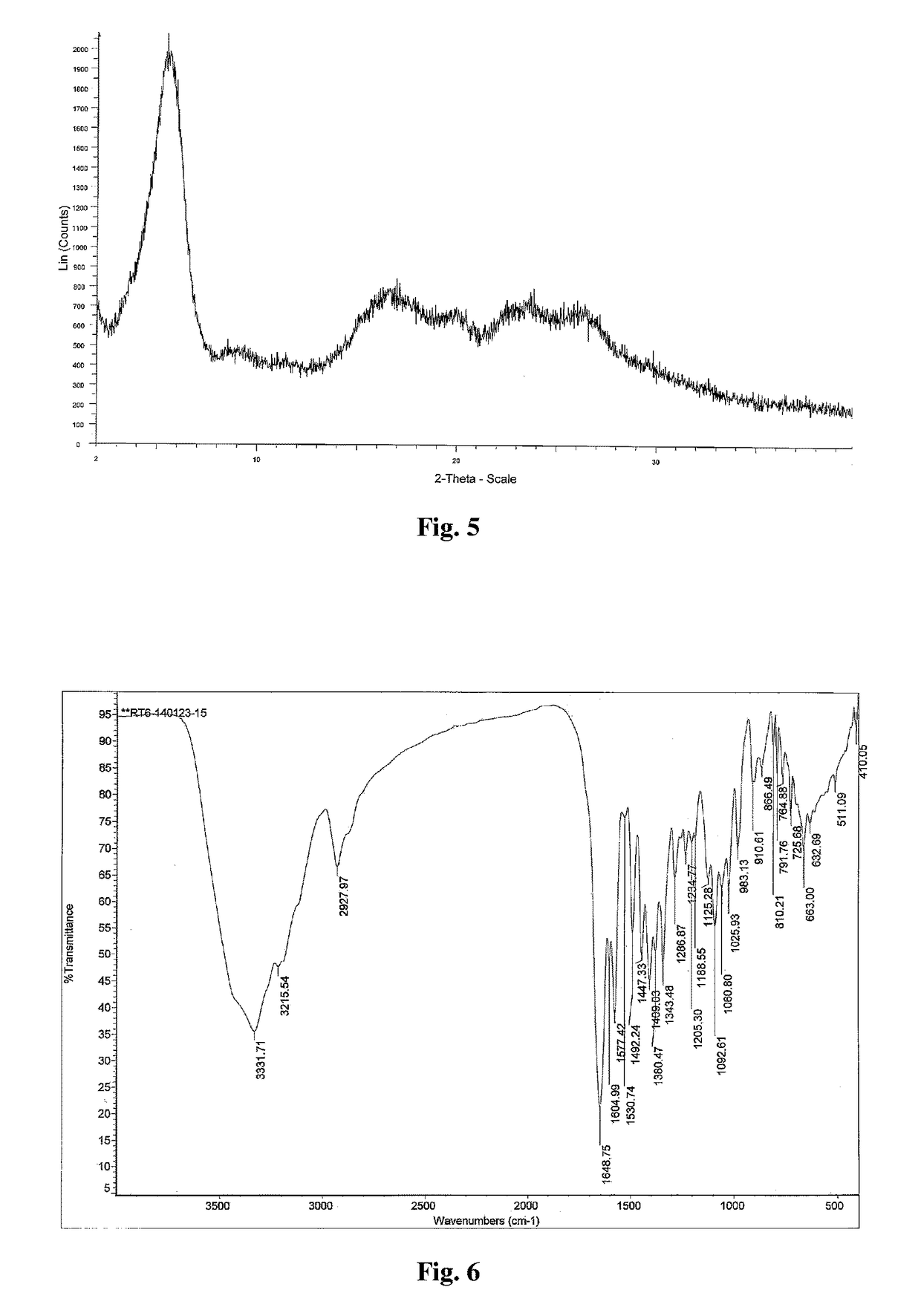
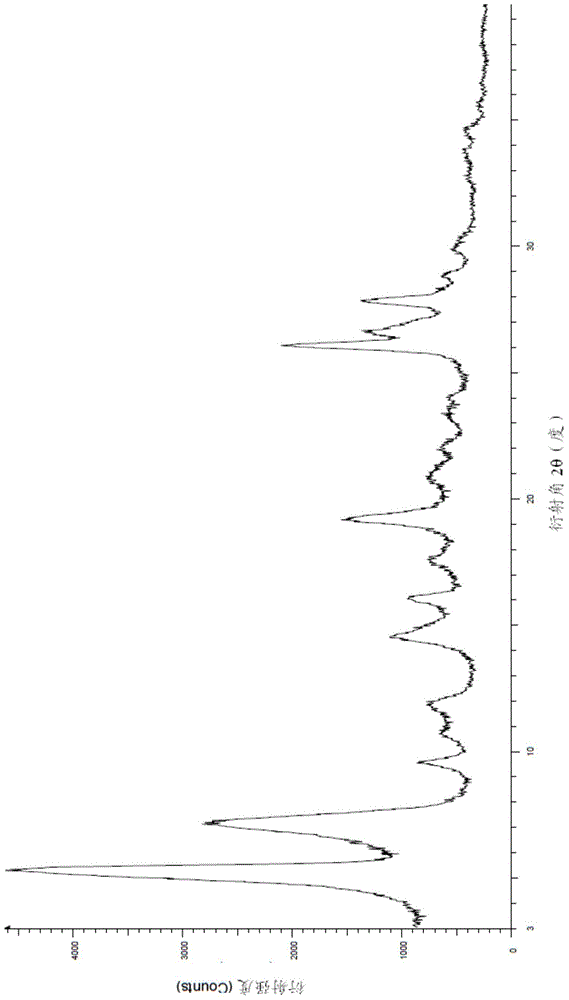
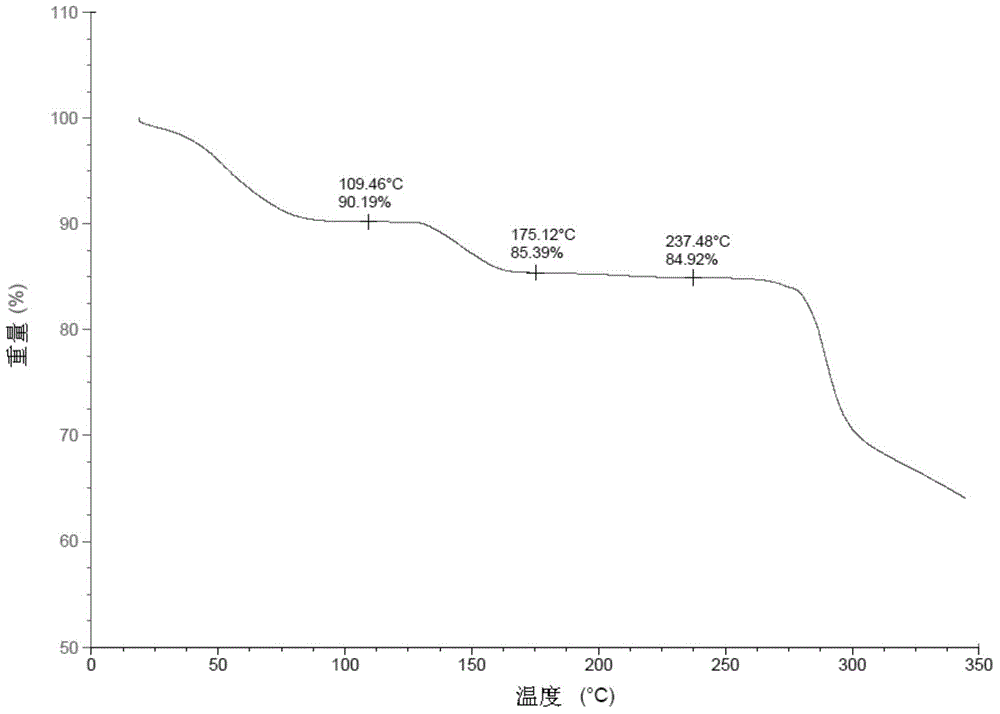
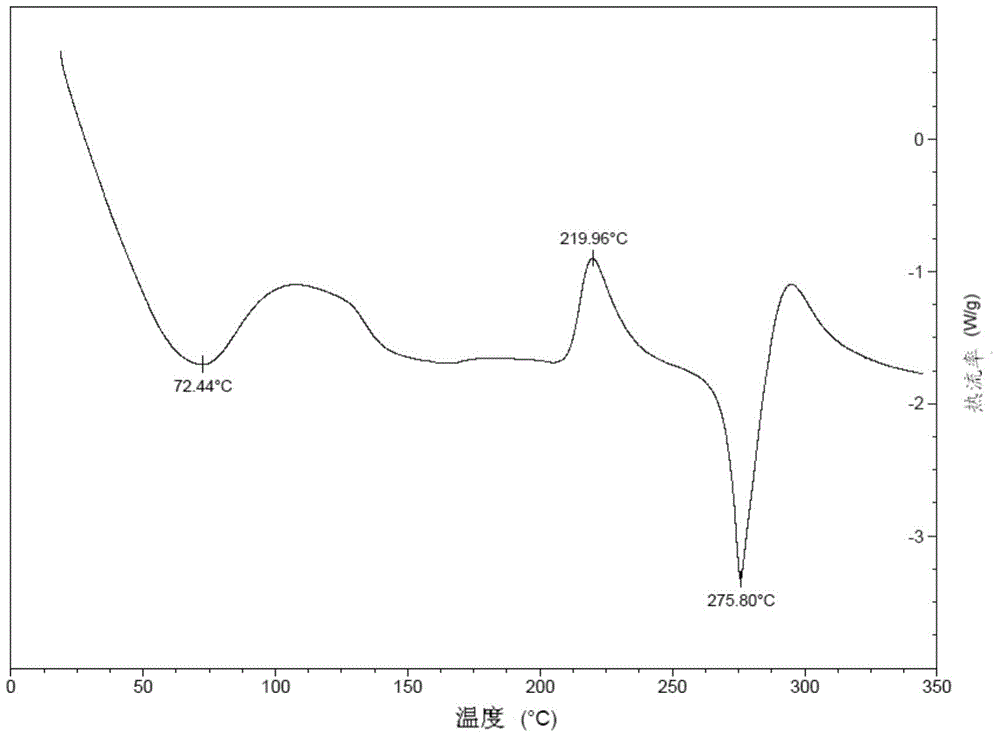
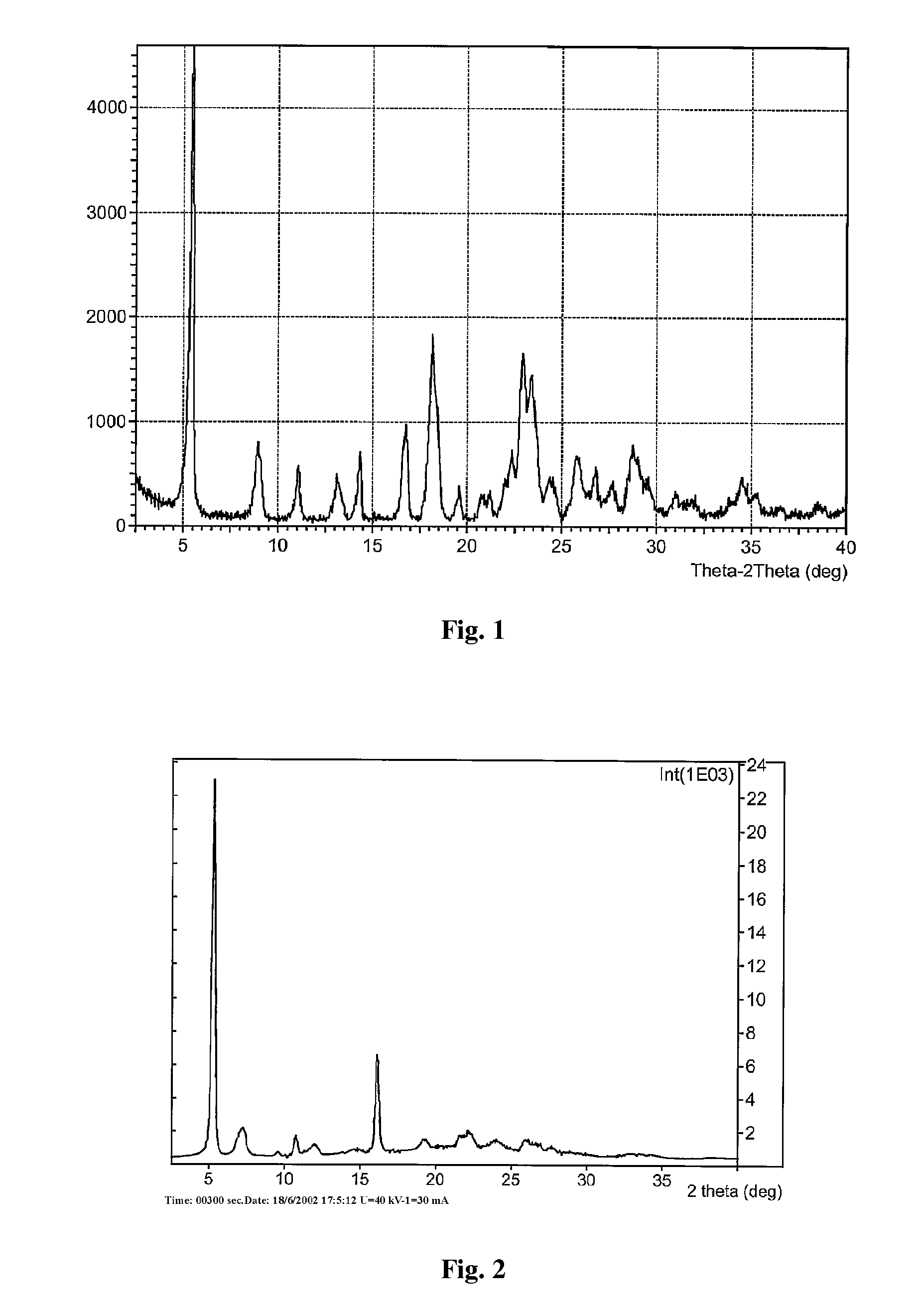
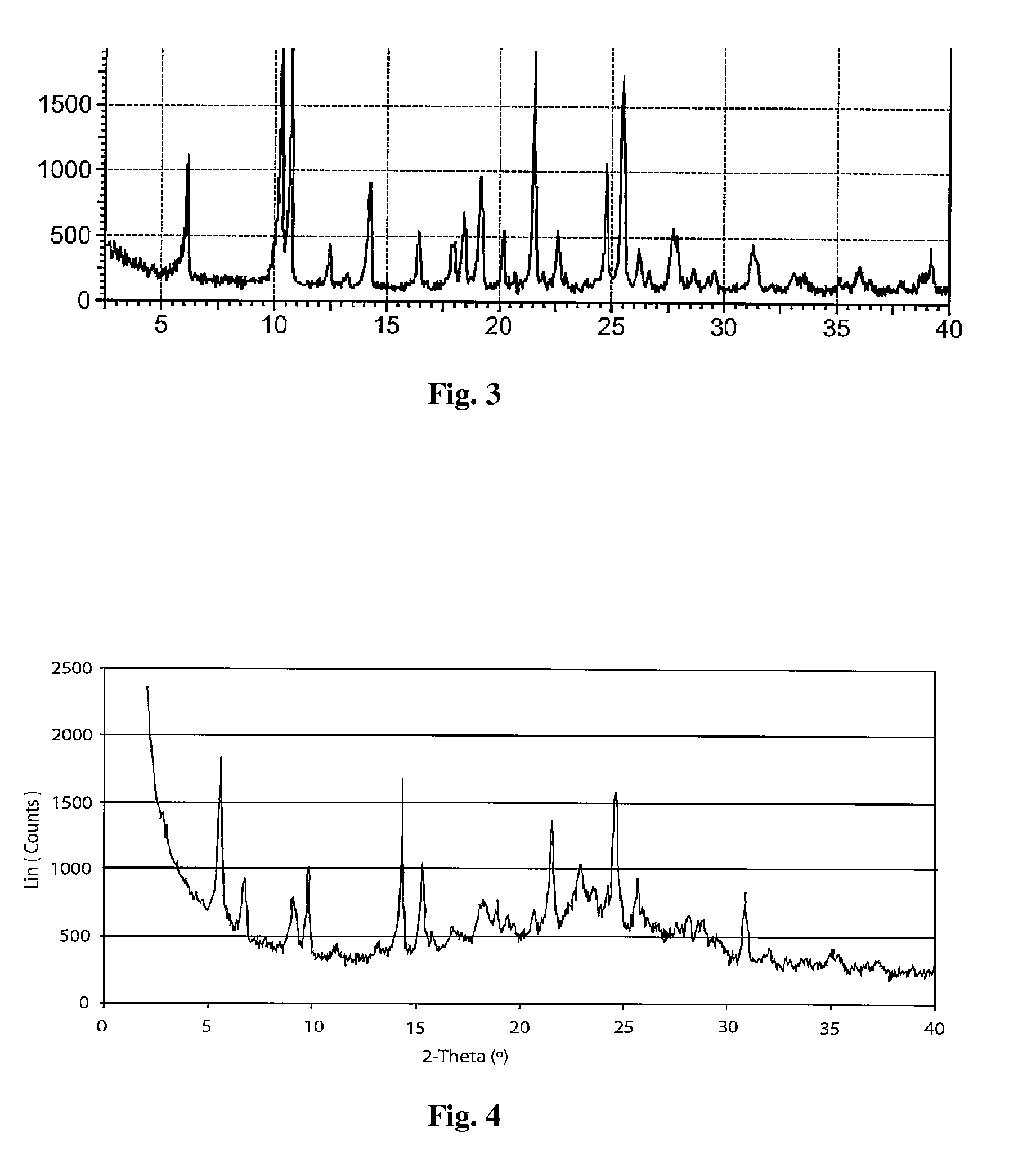
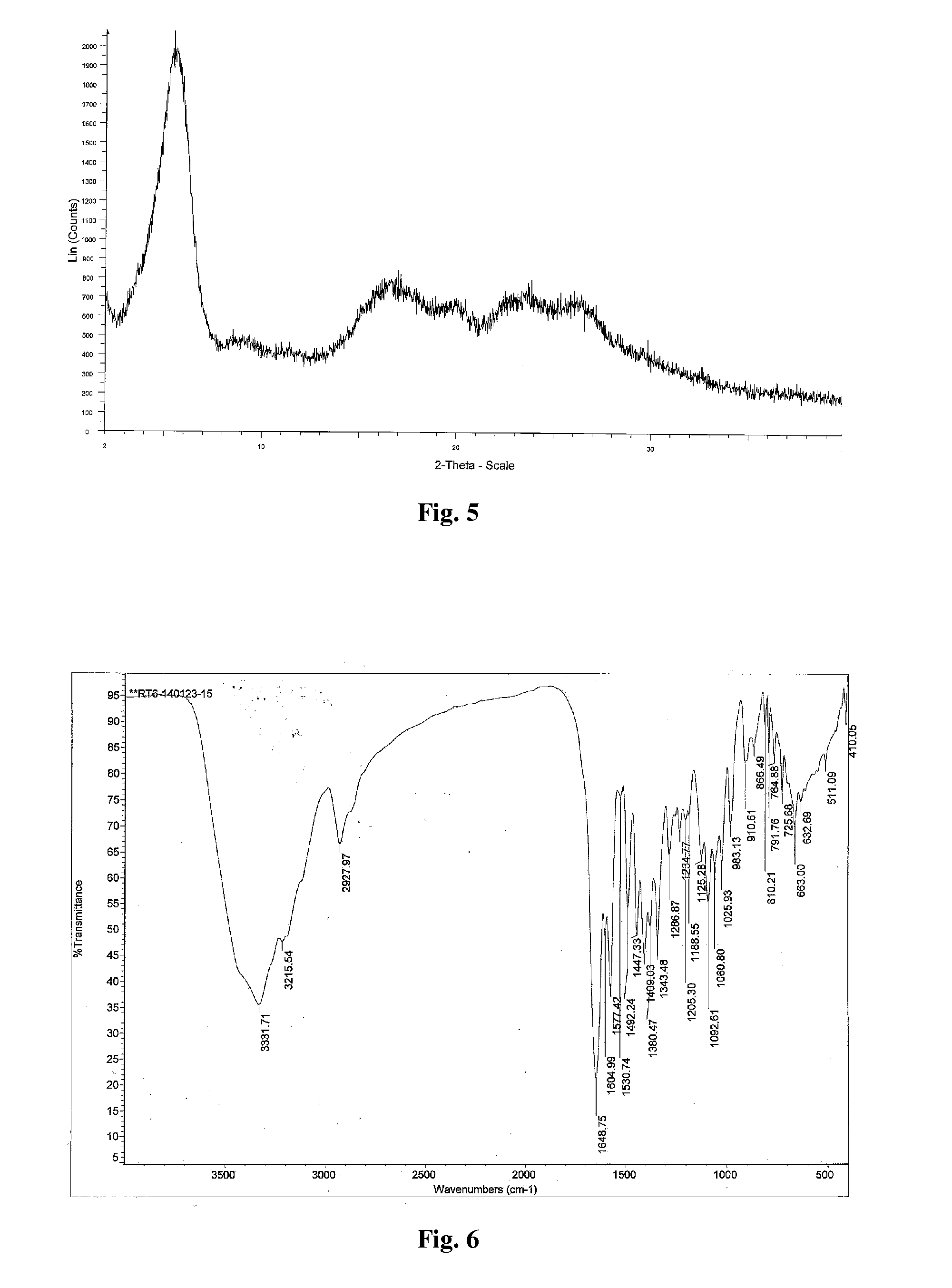
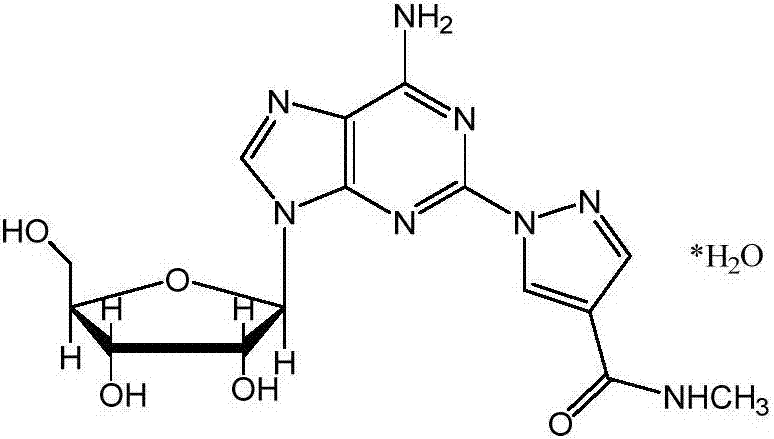
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
38 results about "Regadenoson" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
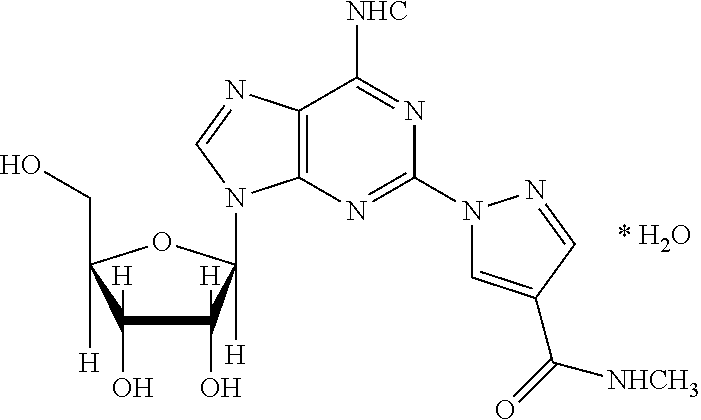
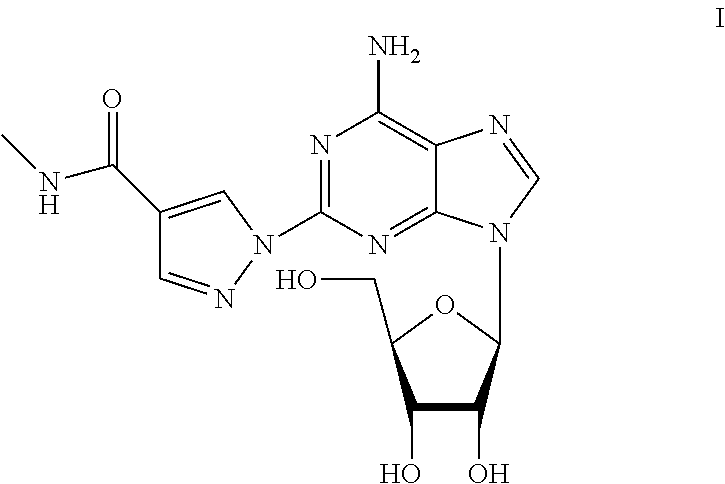
Regadenoson (CVT-3146, Lexiscan) is an A2A adenosine receptor agonist that is a coronary vasodilator that is commonly used in pharmacologic stress testing. It produces hyperemia quickly and maintains it for a duration that is useful for radionuclide myocardial perfusion imaging. The selective nature of the drug makes it preferable to other stress agents such as adenosine, which are less selective and therefore cause more side-effects.
Myocardial Perfusion Imaging
This invention relates to methods for performing myocardial perfusion imaging for diagnosing and characterizing coronary artery disease using an intravenous (IV) bolus injection of regadenoson while the patient is undergoing sub-maximal exercise.
Owner:TPG AXON LEX SUB TRUST +1
Use of A2A adenosine receptor agonists
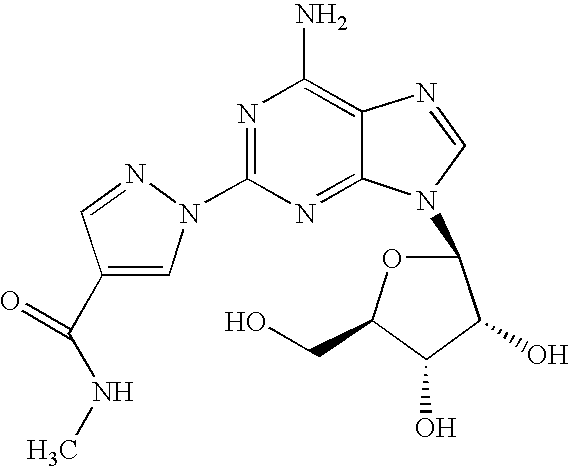
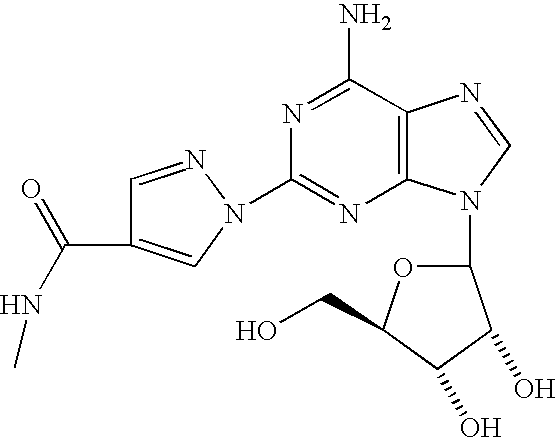
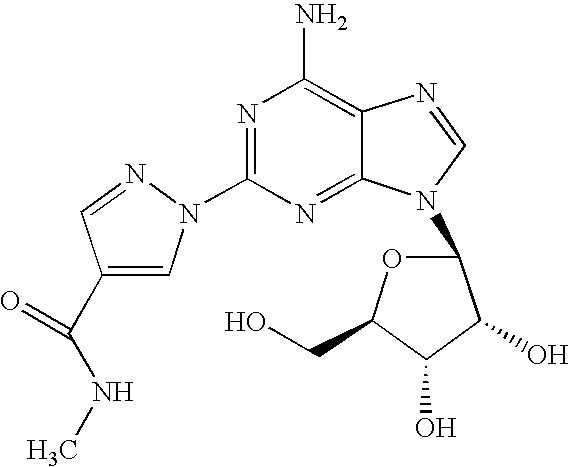
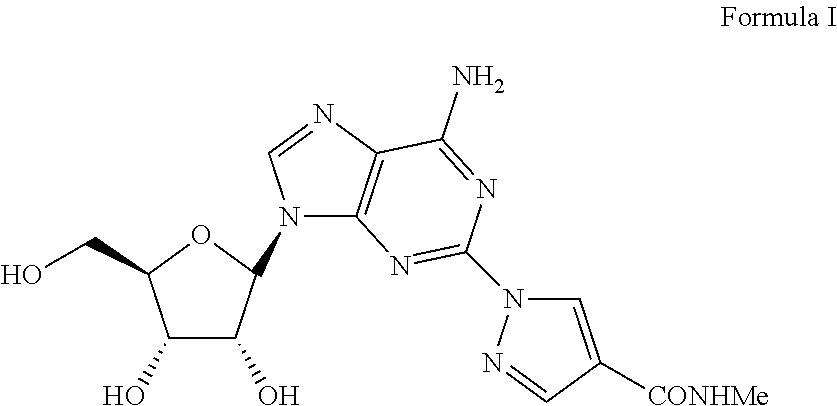
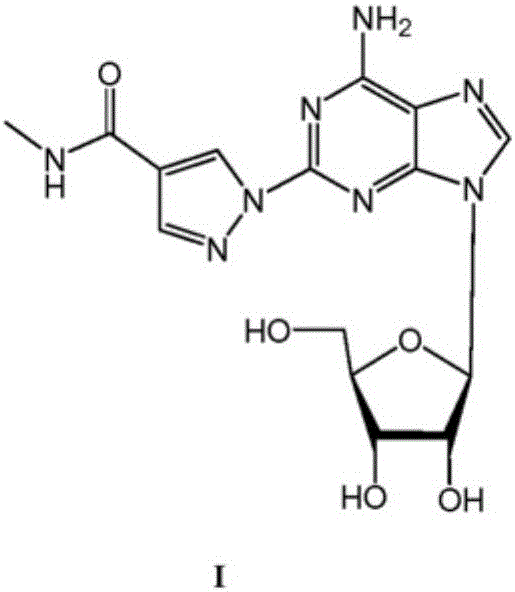
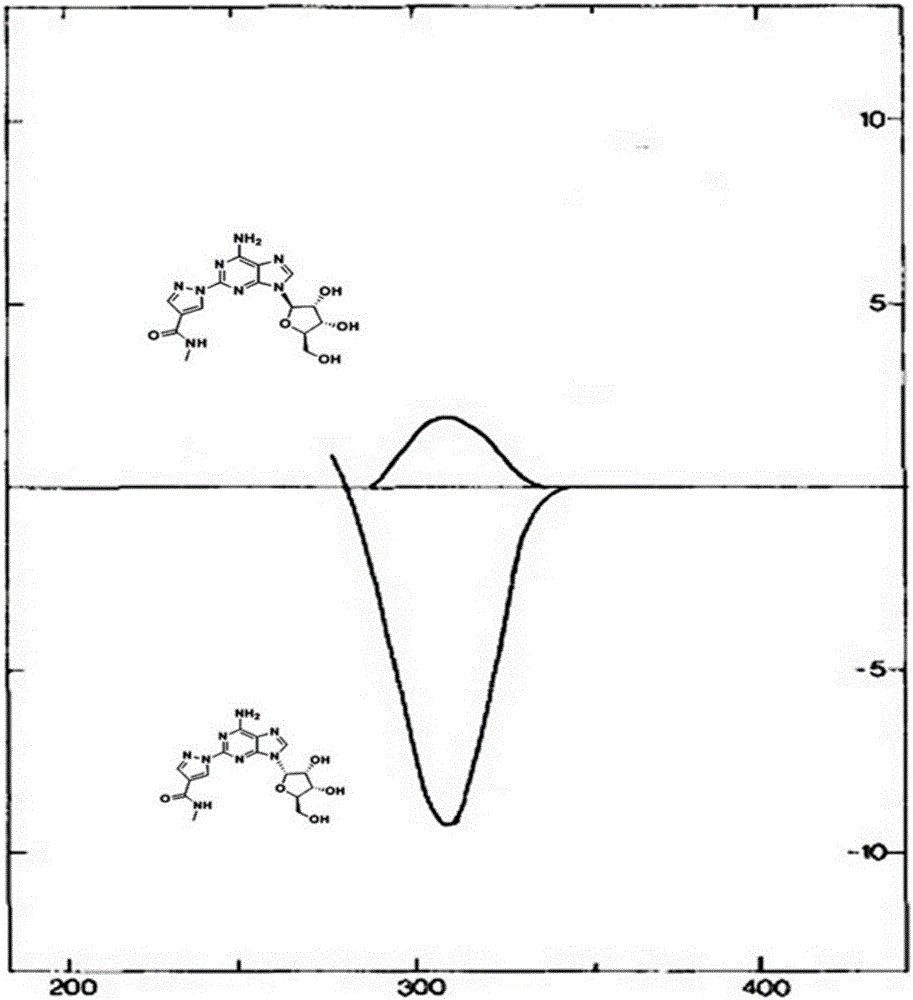
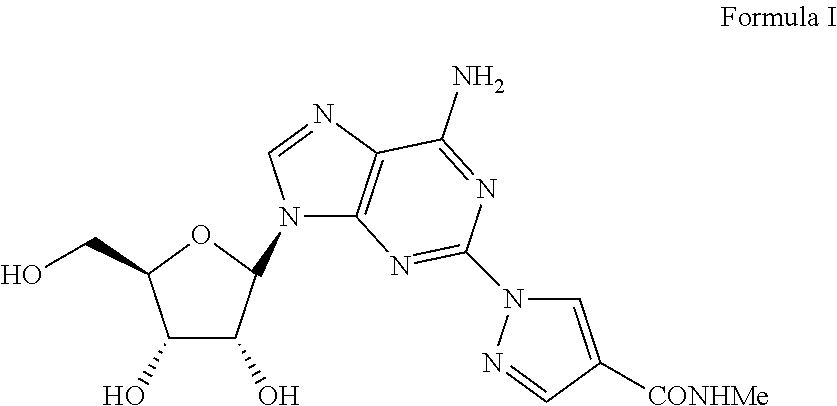
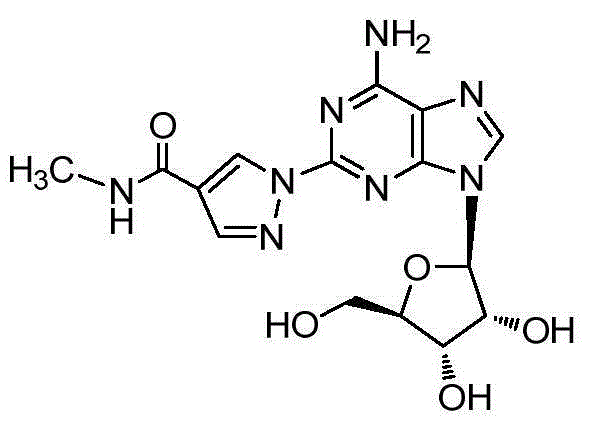
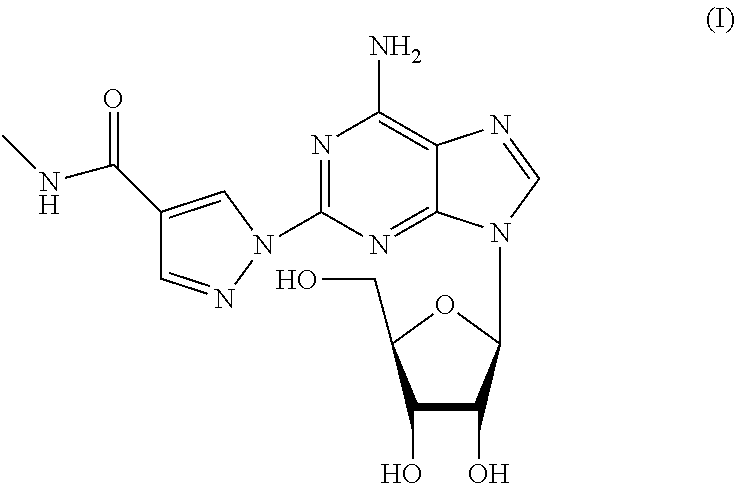
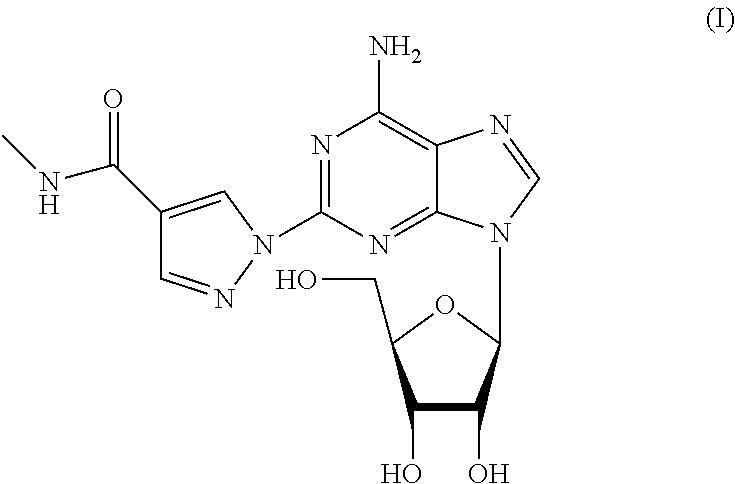
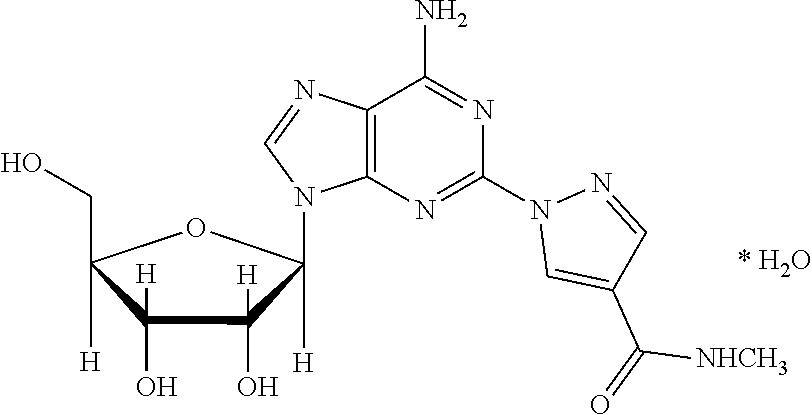
The present invention relates to methods for producing coronary vasodilation with little peripheral vasodilation by administering doses of a pharmaceutical composition including regadenoson, named (1-{9-[(4S,2R,3R,5R)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]-6-aminopurin-2-yl}pyrazol-4-yl)-N-methylcarboxamide, — an adenosine A2A receptor agonist — to a human in an amount sufficient to increase the average coronary peak flow velocity by at least about 16.5 cm / sec.
Owner:TPG AXON LEX SUB TRUST +1
Use of A2A adenosine receptor agonists
InactiveUS20060084625A1Increase peak flow velocityBiocideEchographic/ultrasound-imaging preparationsRegadenosonCardiac muscle
Myocardial imaging methods that are accomplished by administering doses of a pharmaceutical composition including regadenoson—an adenosine A2A receptor agonist—to a human undergoing myocardial imaging in an amount sufficient to achieve at least a minimal increase in average coronary peak flow velocity.
Owner:TPG AXON LEX SUB TRUST +1
Preparation method for regadenoson
InactiveCN104744540AHigh purityFew reaction stepsSugar derivativesSugar derivatives preparationCarboxylic acidOxygen
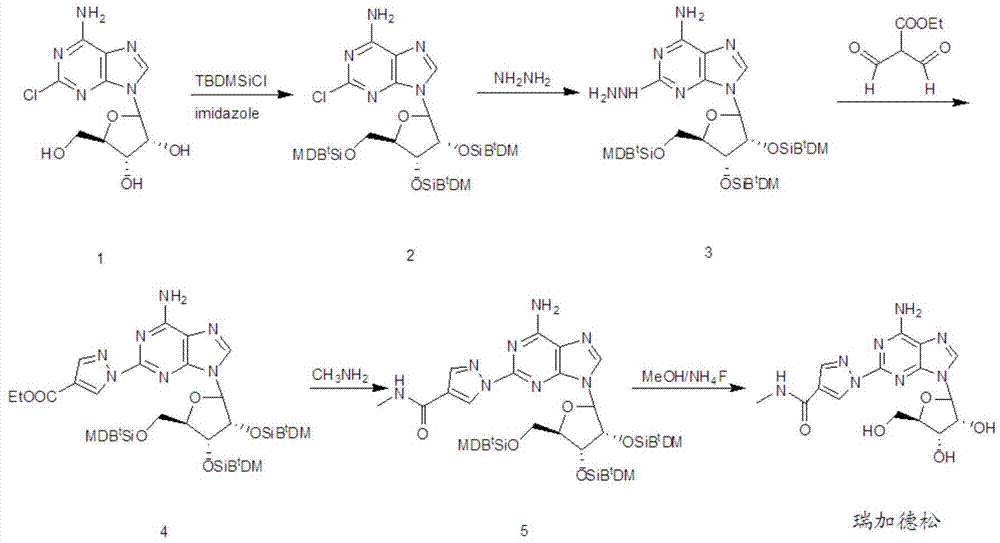
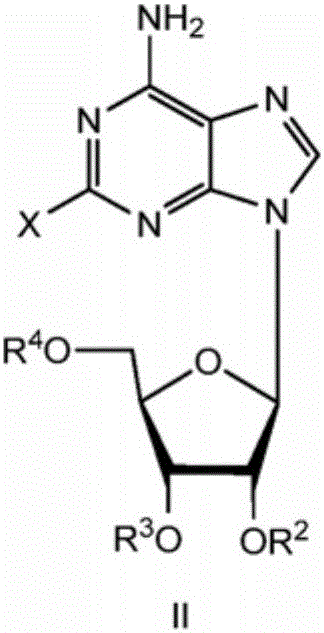
The invention discloses a preparation method for regadenoson, belonging to the field of pharmaceutical chemistry. The preparation method for regadenoson comprises the following steps: with a compound as shown in a formula III as a reaction raw material, subjecting the compound and 2-formyl-3-oxoethyl propanoate to a cyclization reaction in isopropanol so as to produce a compound as shown in a formula IV; then with the compound as shown in the formula IV as a substrate, subjecting the substrate and a methanol solution of methylamine to an acylation reaction so as to produce a compound as shown in a formula V; and reacting the compound as shown in the formula V with tetrabutyl ammonium fluoride in a methanol solution to remove hydroxyl protection so as to prepare regadenoson. According to the preparation method in the invention, the methanol solution of methylamine is used as a reaction medium and reagent, a methanamide compound is produced through one-step reaction under normal pressure, and the acylation reaction is carried out without hydrolysis for formation of a carboxylic acid derivative; thus, reaction steps are reduced, high pressure reaction equipment is not used, cost for production input is lowered, the safety factor of production is increased, and the method is more applicable to large scale production.
Owner:SHANGHAI ZIYUAN PHARMA
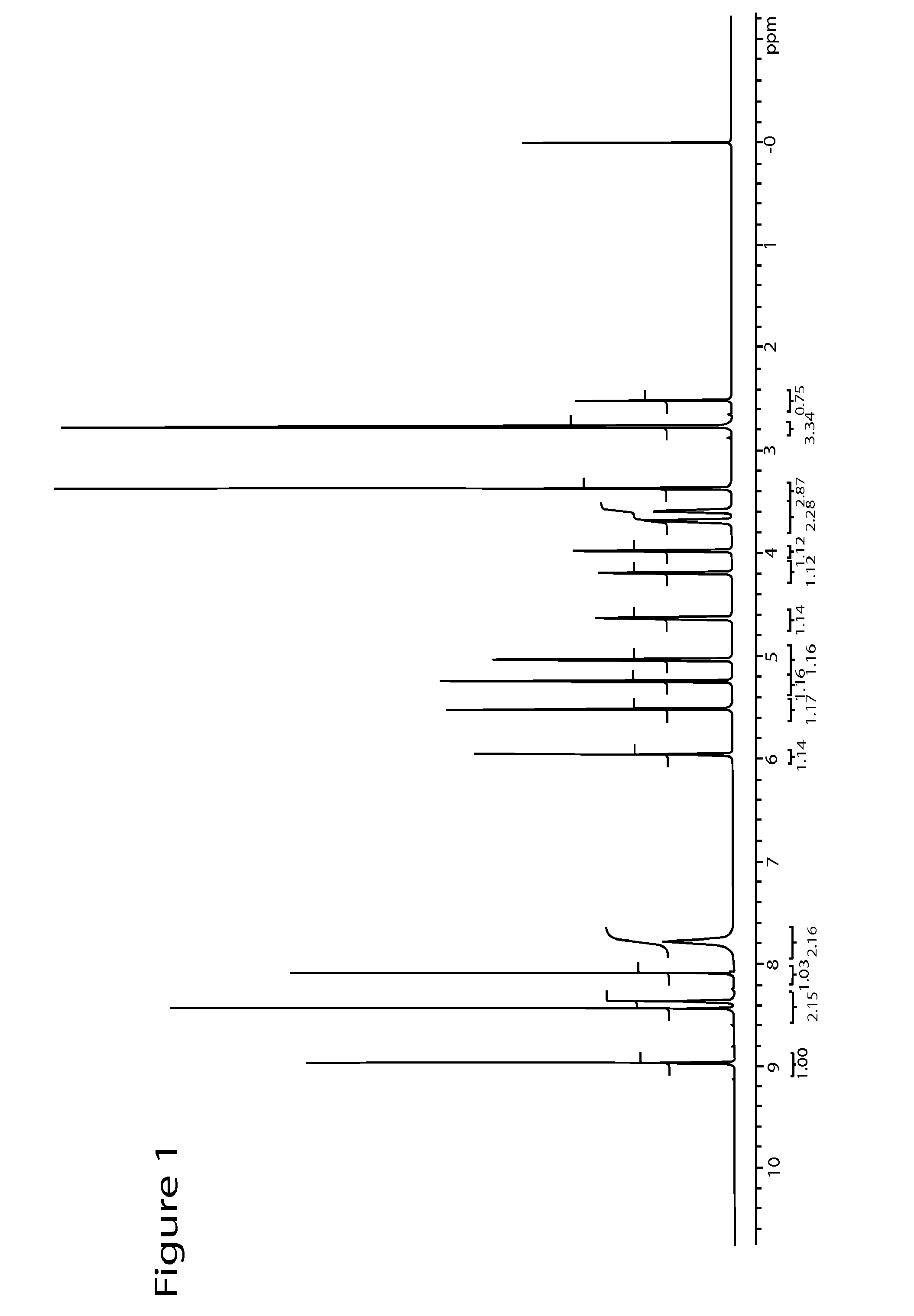
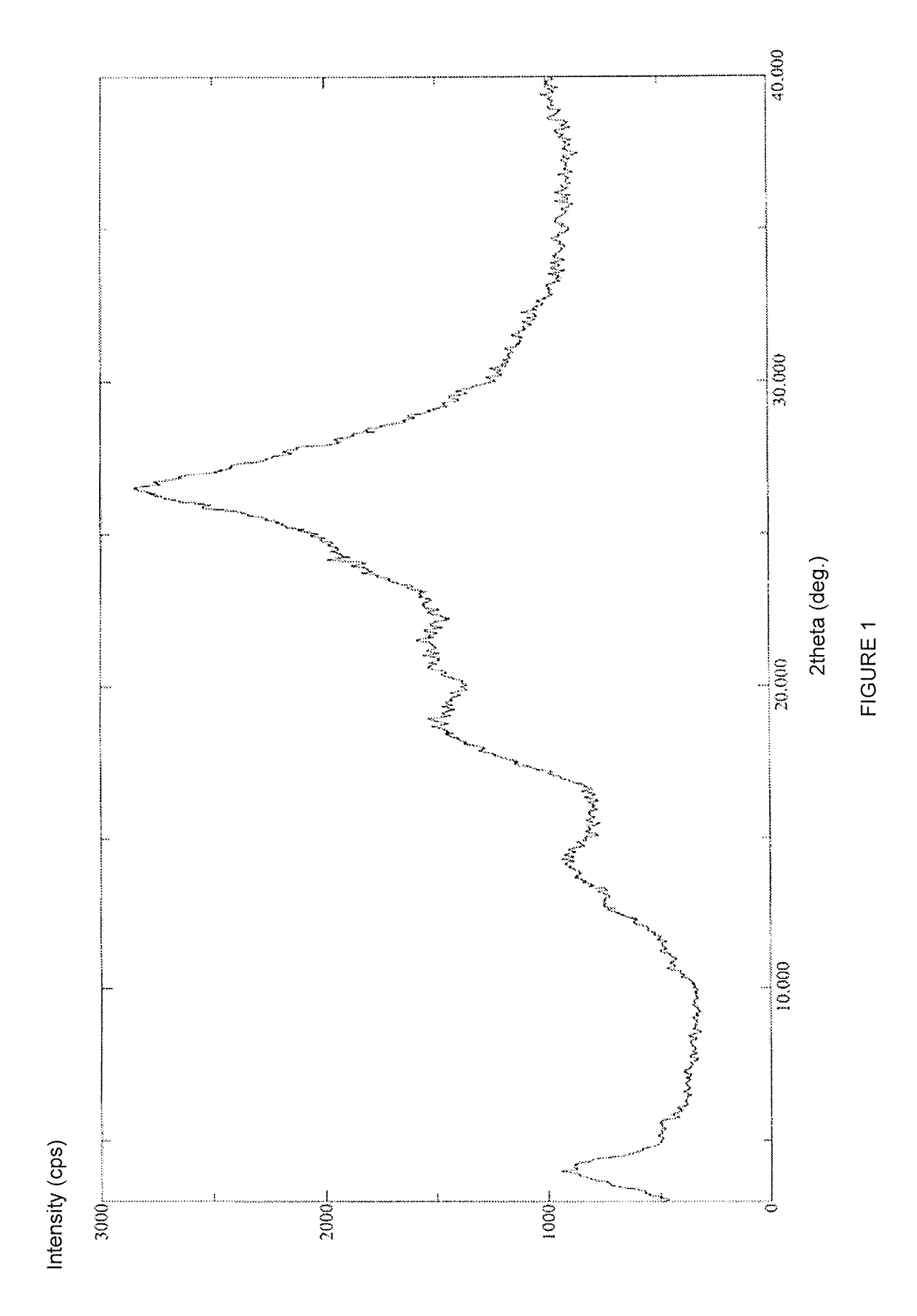
Novel Polymorph of Regadenoson
The invention provides a novel polymorph of Regadenoson. More particularly, the invention provides propylene glycol solvate of Regadenoson. The invention also provides a process for the preparation of propylene glycol solvate of Regadenoson.
Owner:BIOPHORE INDIA PHARMA PVT LTD
Processes for the preparation of regadenoson and a new crystalline form thereof
This disclosure relates to an improved process for the preparation of regadenoson, pharmaceutically acceptable salts thereof, and hydrates thereof, and for the preparation of intermediates useful in the synthesis of regadenoson. The disclosure also relates to a new crystalline form of regadenoson. Processes for the preparation of the crystalline form, compositions containing the crystalline form, and methods of use thereof are also described.
Owner:RELIABLE BIOPHARM LLC
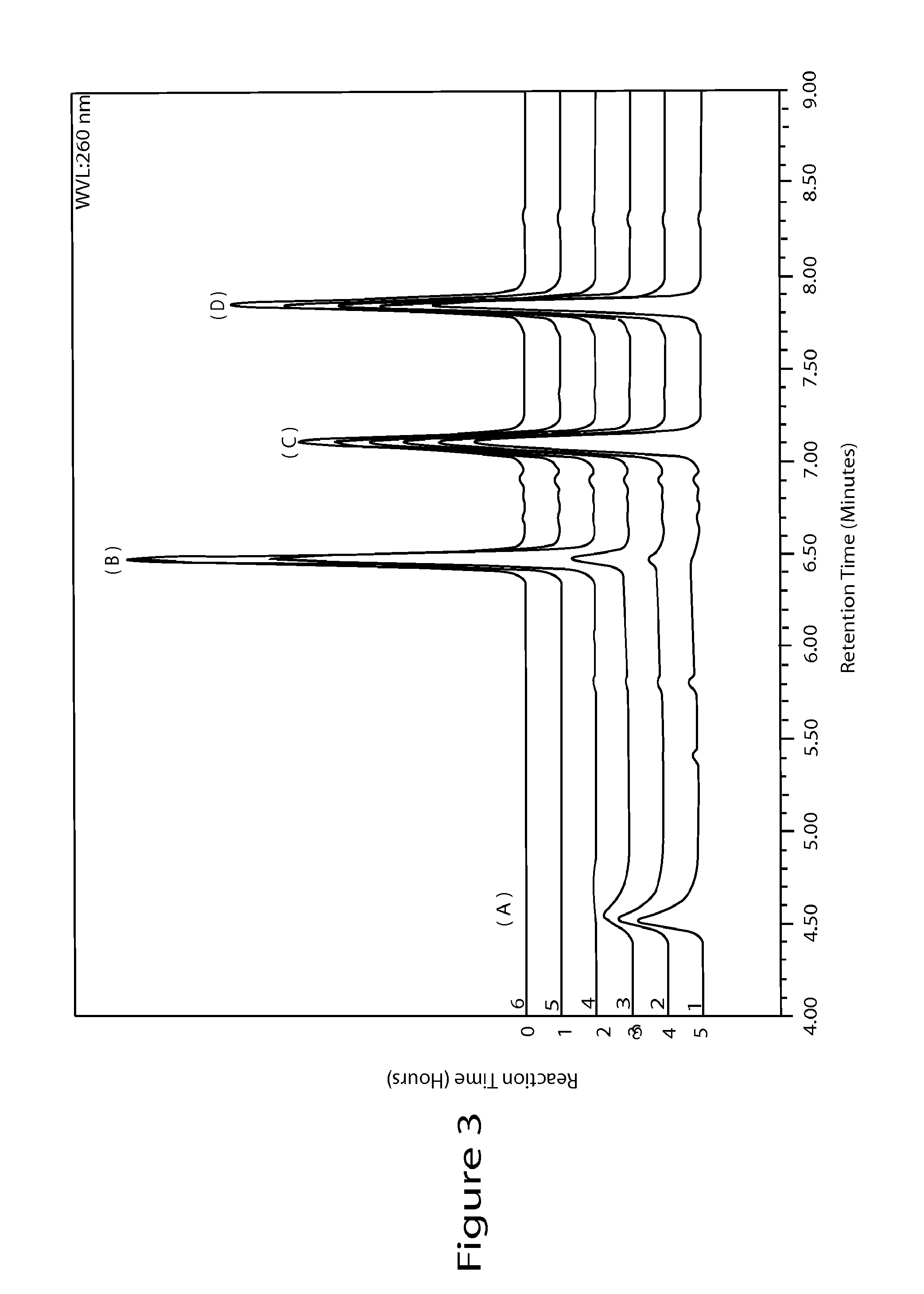
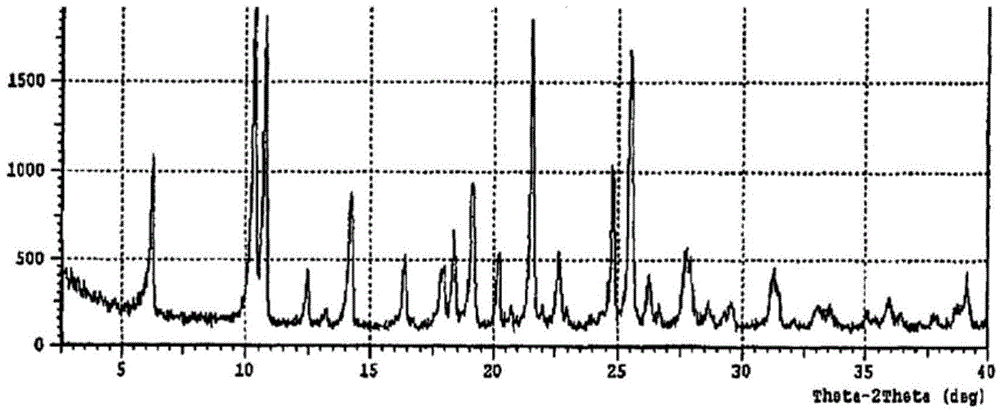
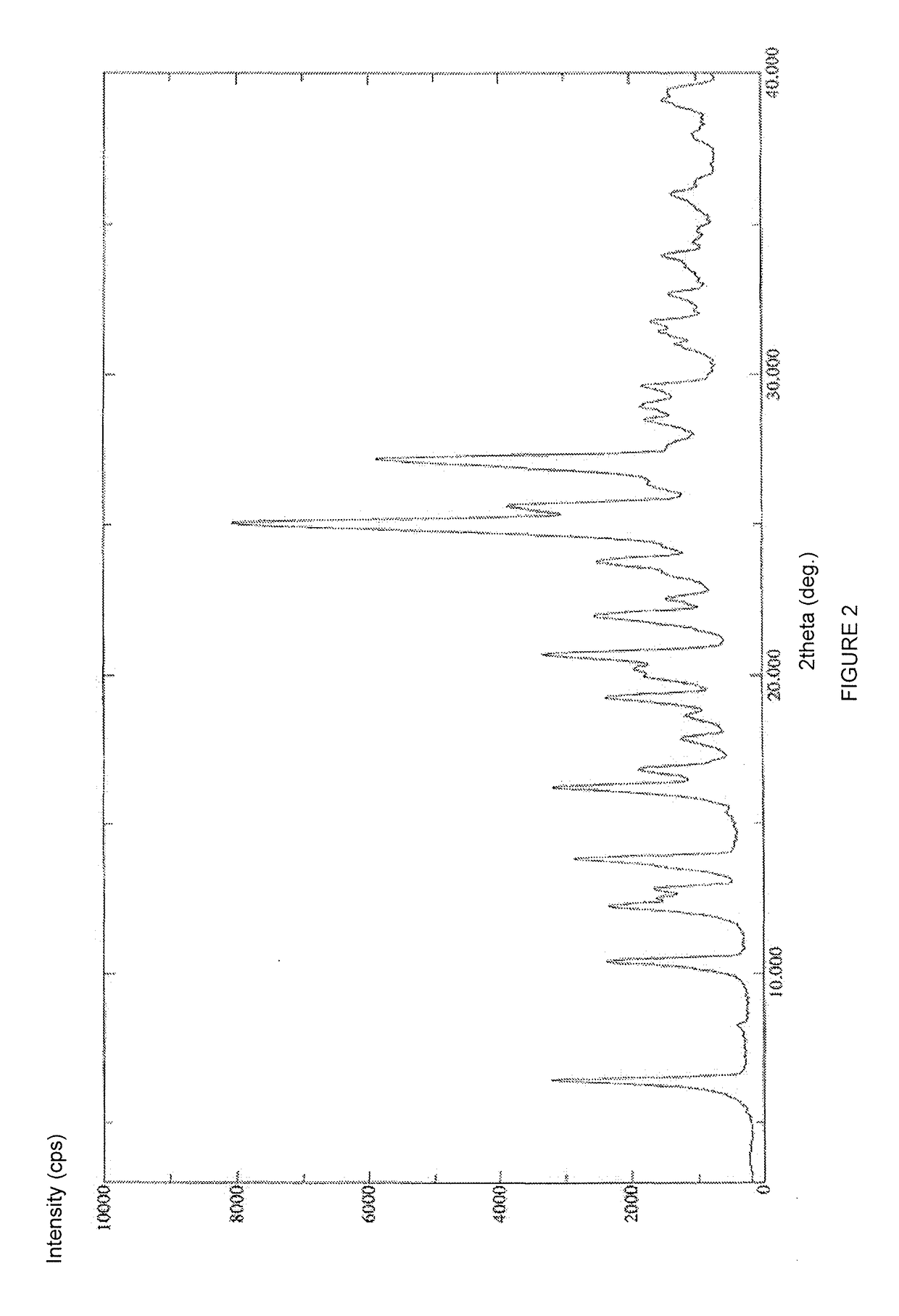
Preparing method for regadenoson crystal form E
ActiveCN105198950AHigh puritySugar derivativesSugar derivatives preparationOrganic solventRegadenoson
The invention relates to the field of crystal form preparation, in particular to a preparing method for a regadenoson crystal form E. The preparing method includes the steps that regadenoson and first organic solvent are mixed and purified through reversed-phase high-performance liquid chromatography, and the component with the retention time of 30 min is collected to obtain a first intermediate product; the first intermediate product is mixed with second organic solvent to obtain an organic phase, and a second intermediate product is obtained through concentration; the second intermediate product and DMF are mixed and crystallized to obtain the regadenoson crystal form E. The purity of the regadenoson crystal form E can be improved greatly by means of the preparing method.
Owner:SHANGHAI ZIYUAN PHARMA
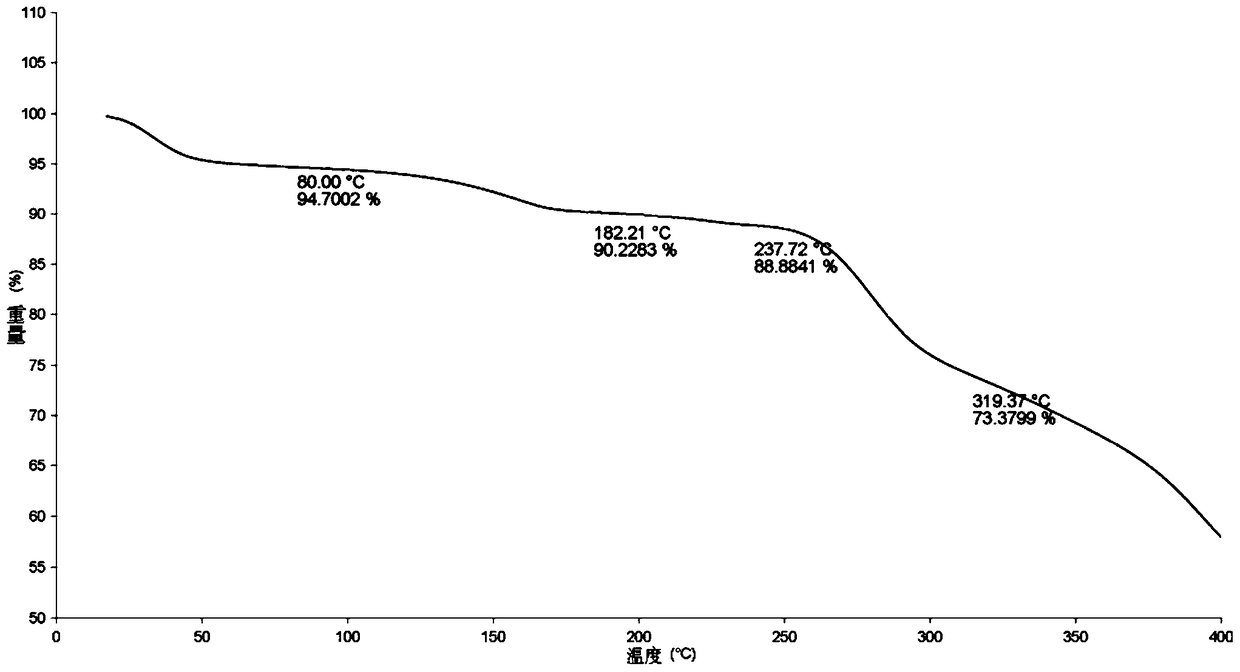
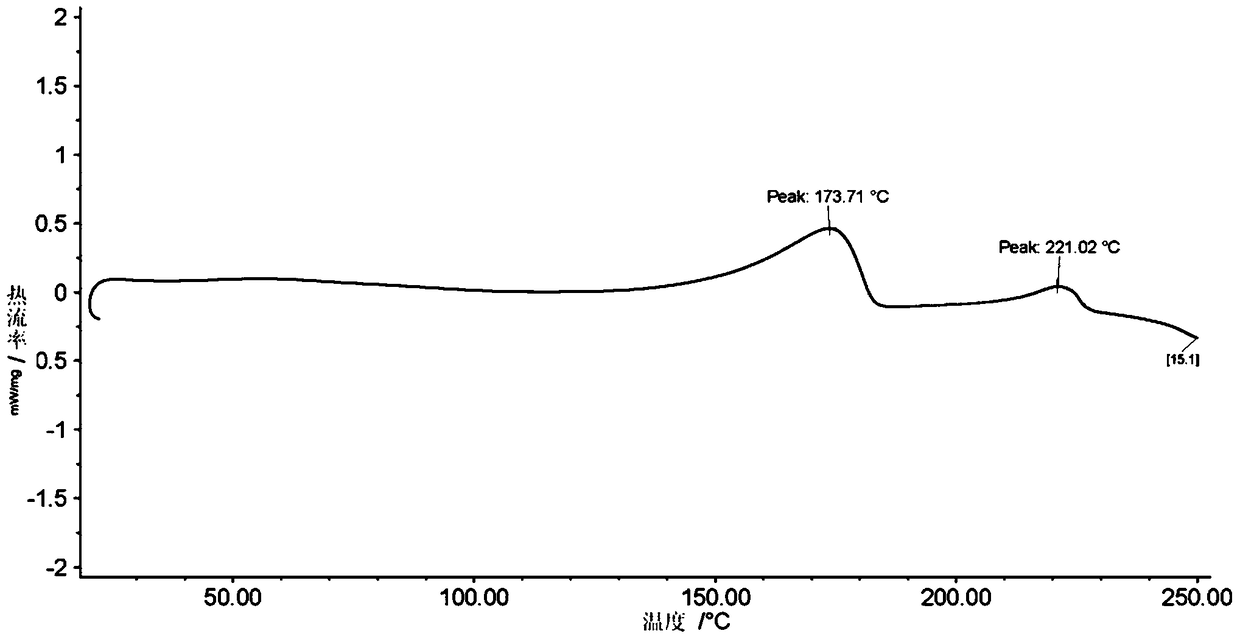
Stable solid forms of regadenoson
A process for the preparation of the amorphous form of Regadenoson of formulais disclosed together with new crystalline polymorphic forms E, F and G and methods for their preparation. Regadenoson amorphous form can be prepared in mild reaction conditions with high chemical purity (>99.6%) and high stability to the heating. A particularly thermodynamically stable anhydrous crystalline form of Regadenoson (form G) is also disclosed, provided with high stability not when exposed to 90% RH at 25° C. for 96 hour, but also to the heating up to 200° C.
Owner:AMRI ITAL SRL
Processes for the preparation of regadenoson and a new crystalline form thereof
This disclosure relates to an improved process for the preparation of regadenoson, pharmaceutically acceptable salts thereof, and hydrates thereof, and for the preparation of intermediates useful in the synthesis of regadenoson. The disclosure also relates to a new crystalline form of regadenoson. Processes for the preparation of the crystalline form, compositions containing the crystalline form, and methods of use thereof are also described.
Owner:RELIABLE BIOPHARM LLC
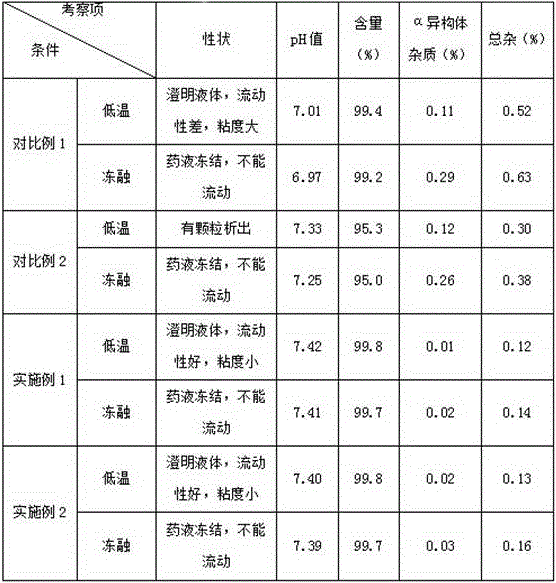
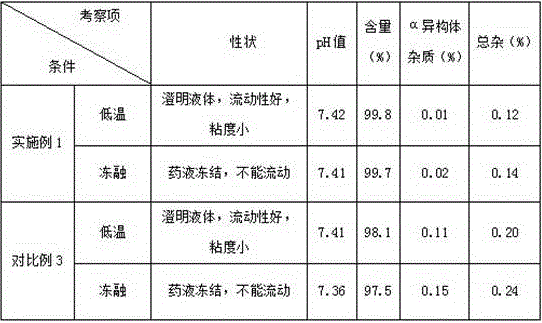
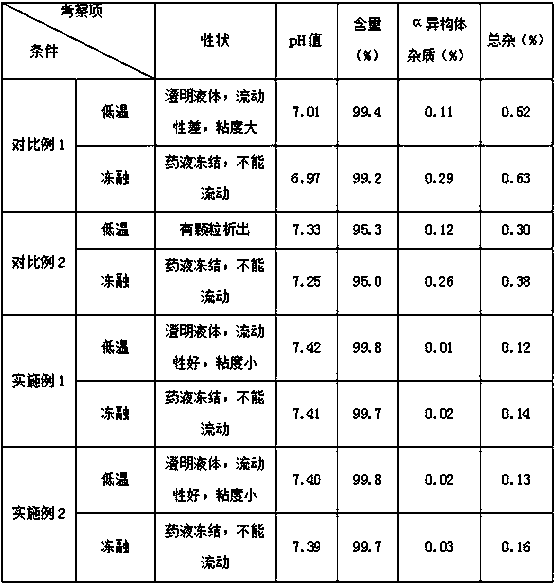
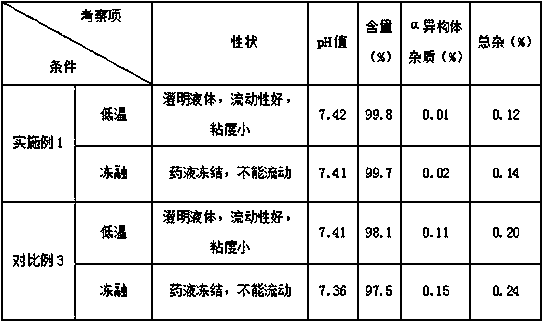
Regadenoson injection and preparation method thereof
ActiveCN105997852AImprove solubilityIncrease dosageComponent separationInorganic non-active ingredientsPhosphateActive agent
The invention belongs to the field of pharmaceutical preparations, and particularly relates to a novel injection of an adenosine A2A receptor agonist Regadenoson and a preparation method of the injection. The Regadenoson injection is prepared from the main drug Regadenoson, a cosolvent, an antioxidant, a stabilizer and an isoosmotic adjusting agent, wherein the cosolvent is selected from L-histidine or a combination of L-histidine and other amino acid, the stabilizer is selected from a combination of tris-hydroxymethyl aminomethane and hydrochloric acid, and sodium chloride is preferably selected as the isoosmotic adjusting agent. According to the injection, propylene glycol and phosphate buffer salt are prevented from being used, a surfactant solubilizer is not adopted, the formula and the process are simple, the injection is suitable for industrialized large-scale production, and the stability is significantly improved after high-temperature sterilization and long-term storage.
Owner:NANJING HERON PHARMA SCI & TECH CO LTD +1
A process for the preparation of regadenoson
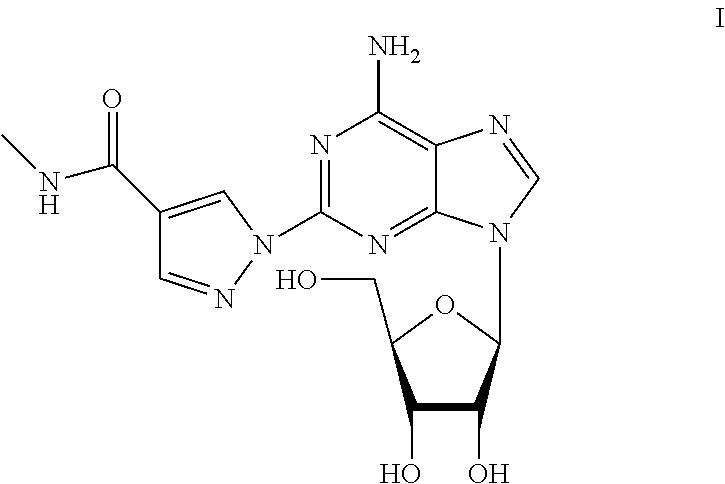
The present invention provides novel processes for the preparation of regadenoson having the formula (I). In some embodiments, the intermediates for the synthesis of regadenoson are also provided.
Owner:SCINOPHARM TAIWAN LTD
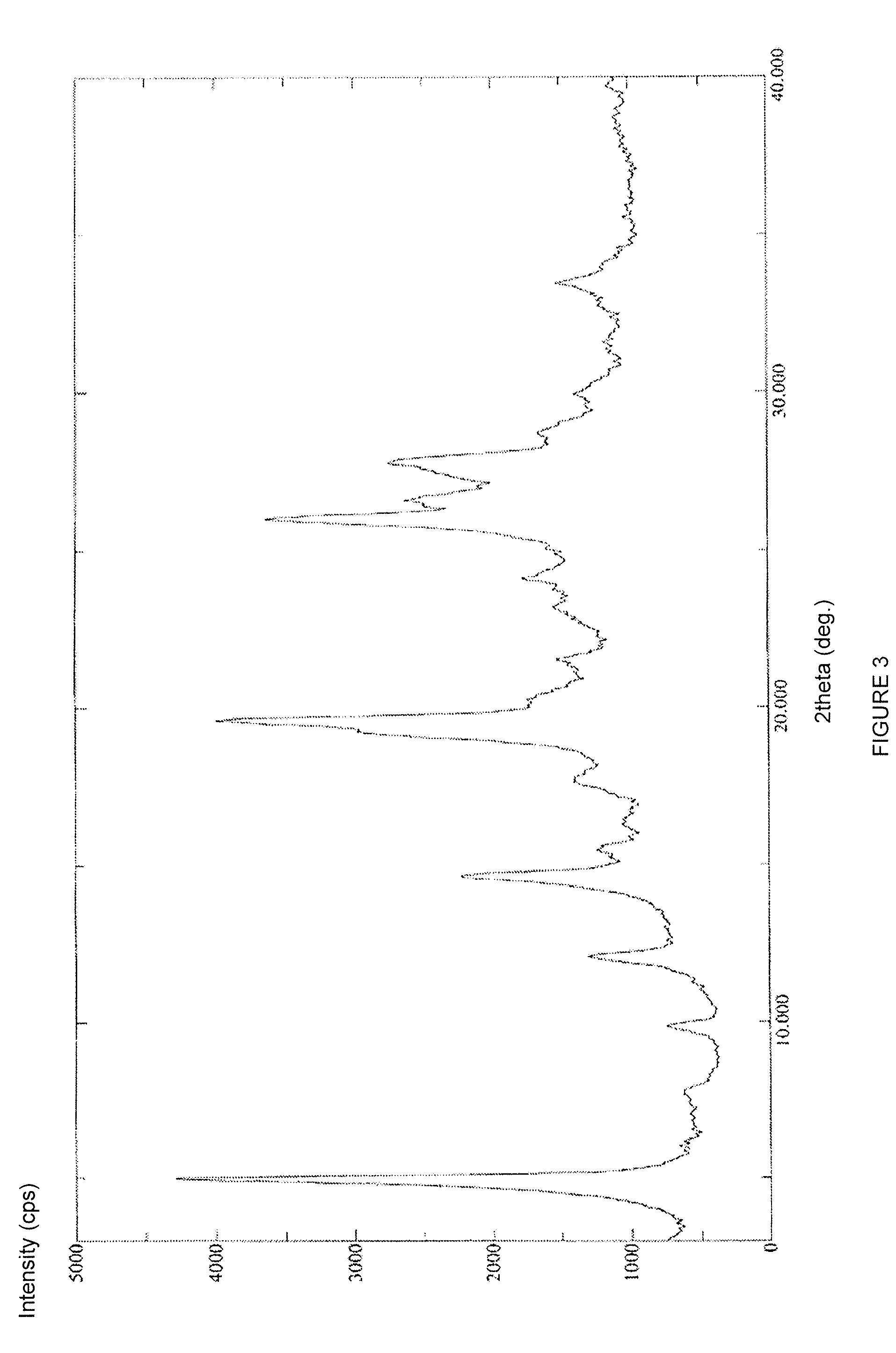
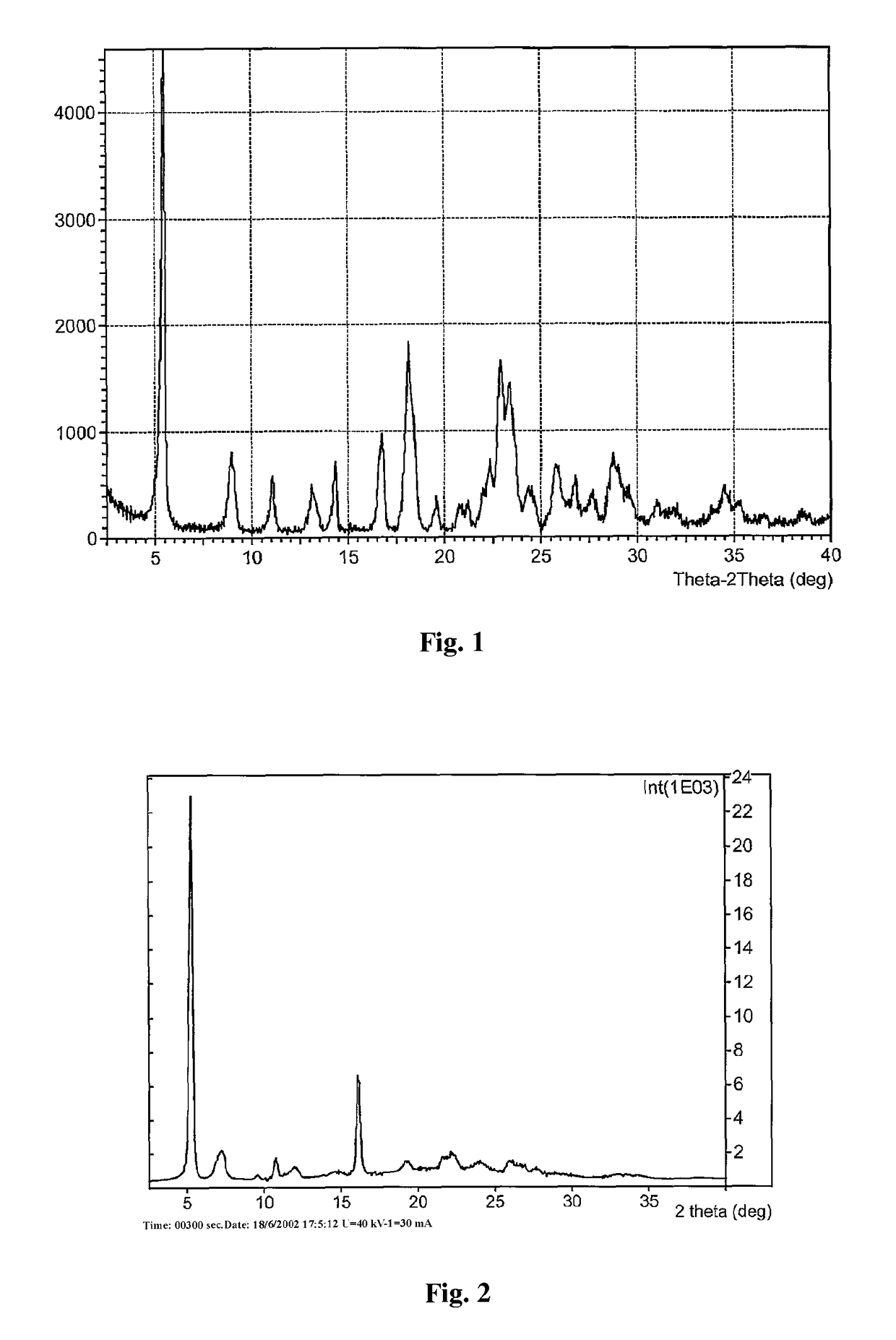
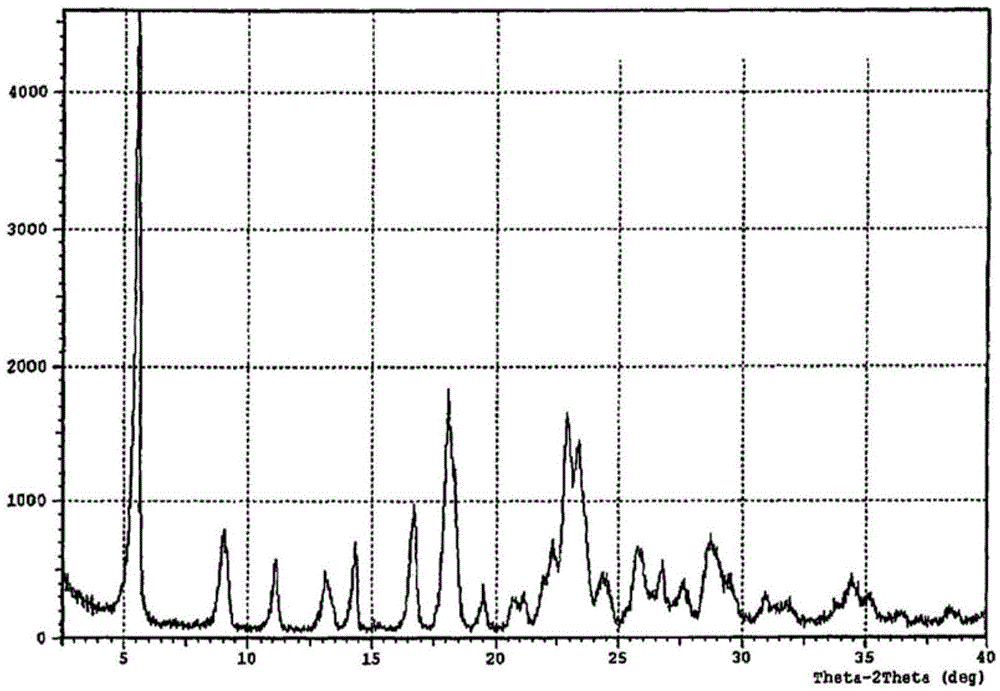
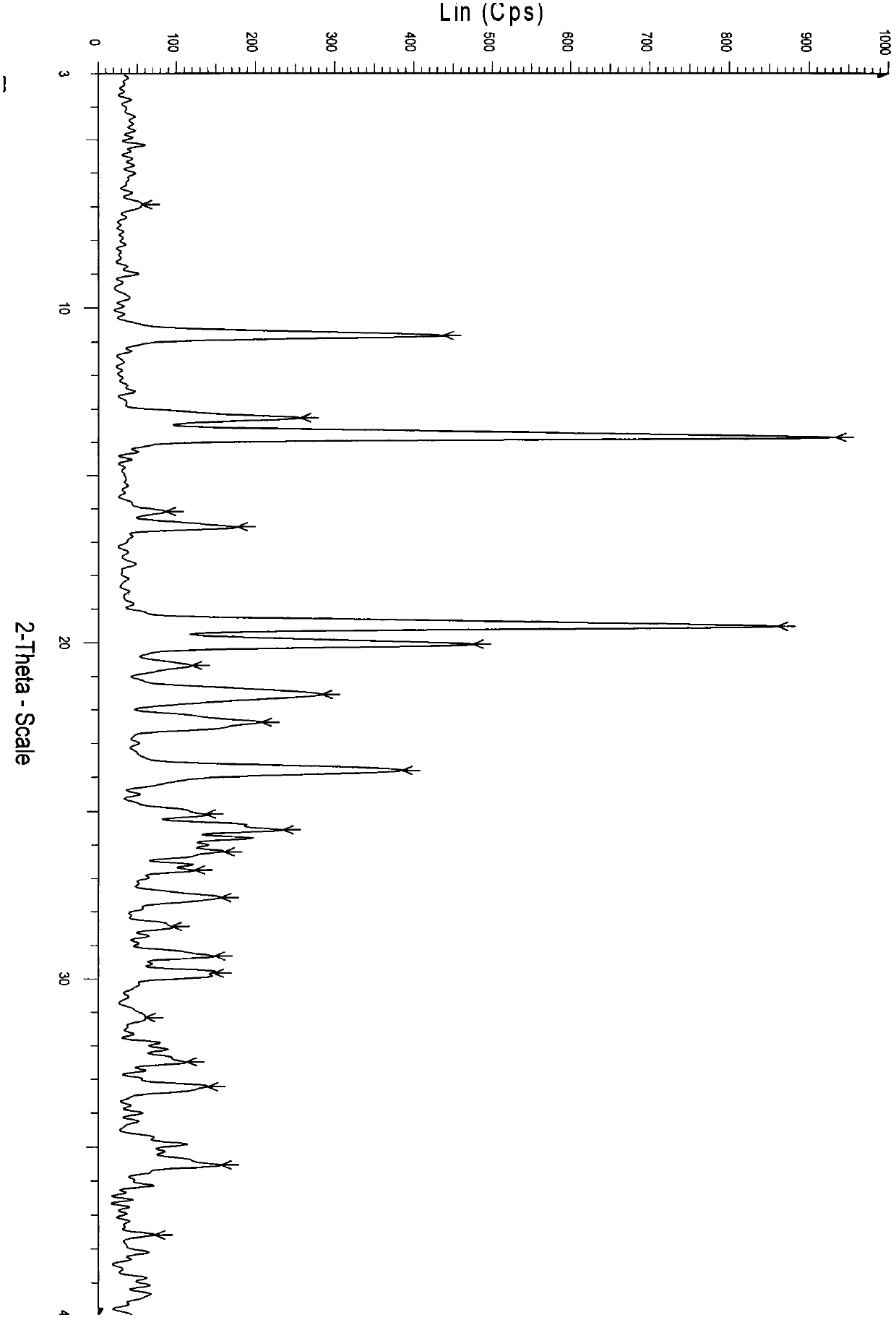
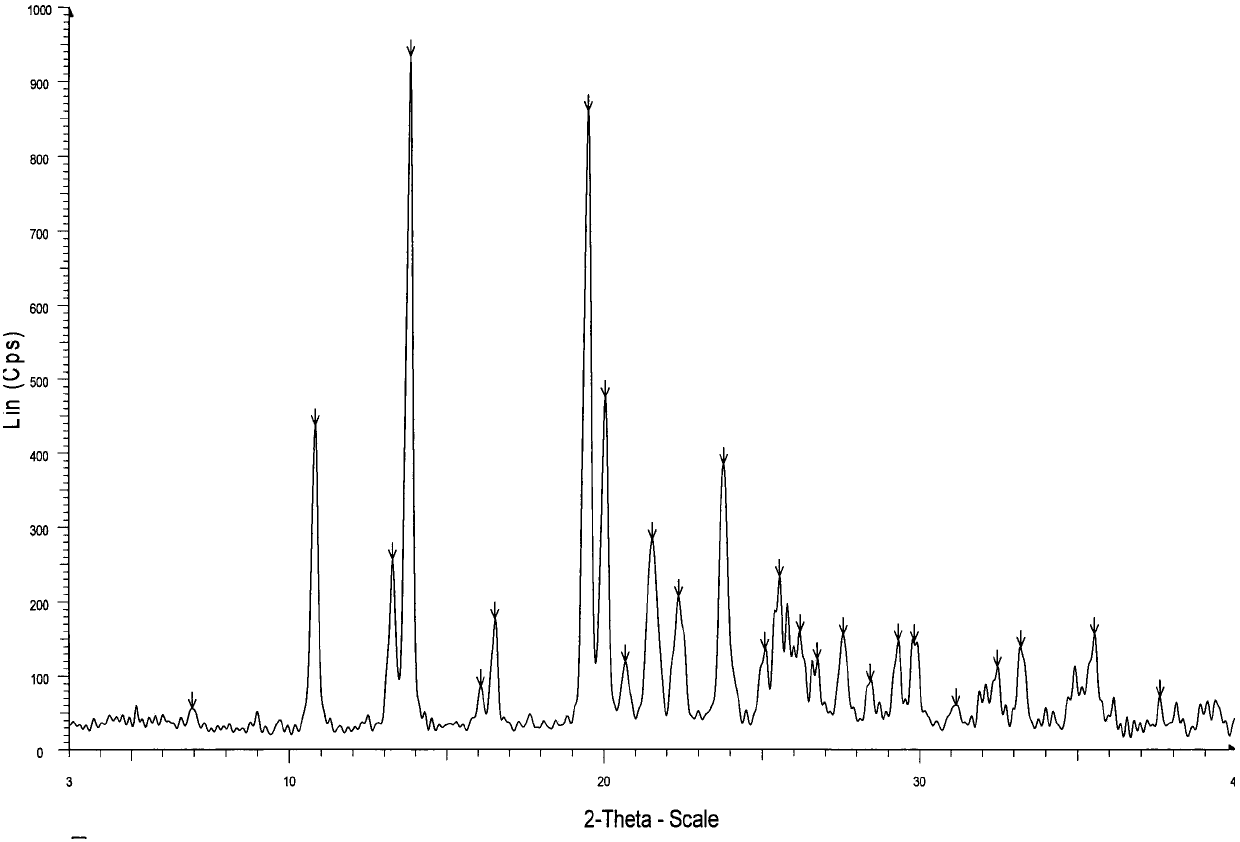
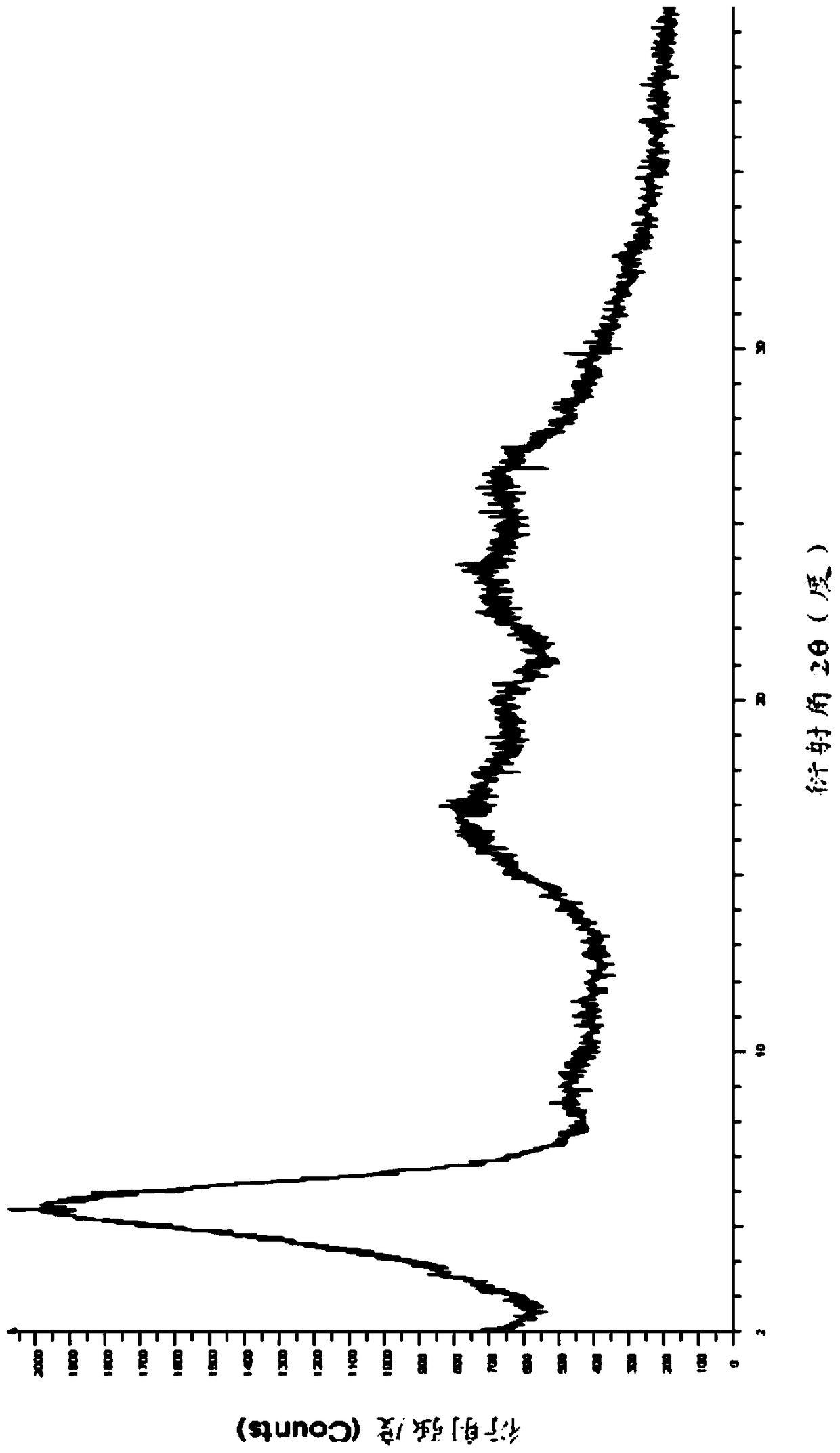
Crystal form of regadenoson and preparation method thereof
The present invention relates to the field of medicinal chemistry, and discloses a new crystal form of regadenoson, i.e., a crystal form E of regadenoson, as well as a method for preparing the new crystal form of regadenoson. The crystal form E of regadenoson according to the present invention has excellent performances in terms of radionuclide myocardial perfusion imaging, and has a poor toxicity, good storage stability, and can be used in the preparation of a medicament used as a stress agent for radionuclide myocardial perfusion imaging.
Owner:SHANGHAI ZIYUAN PHARMA
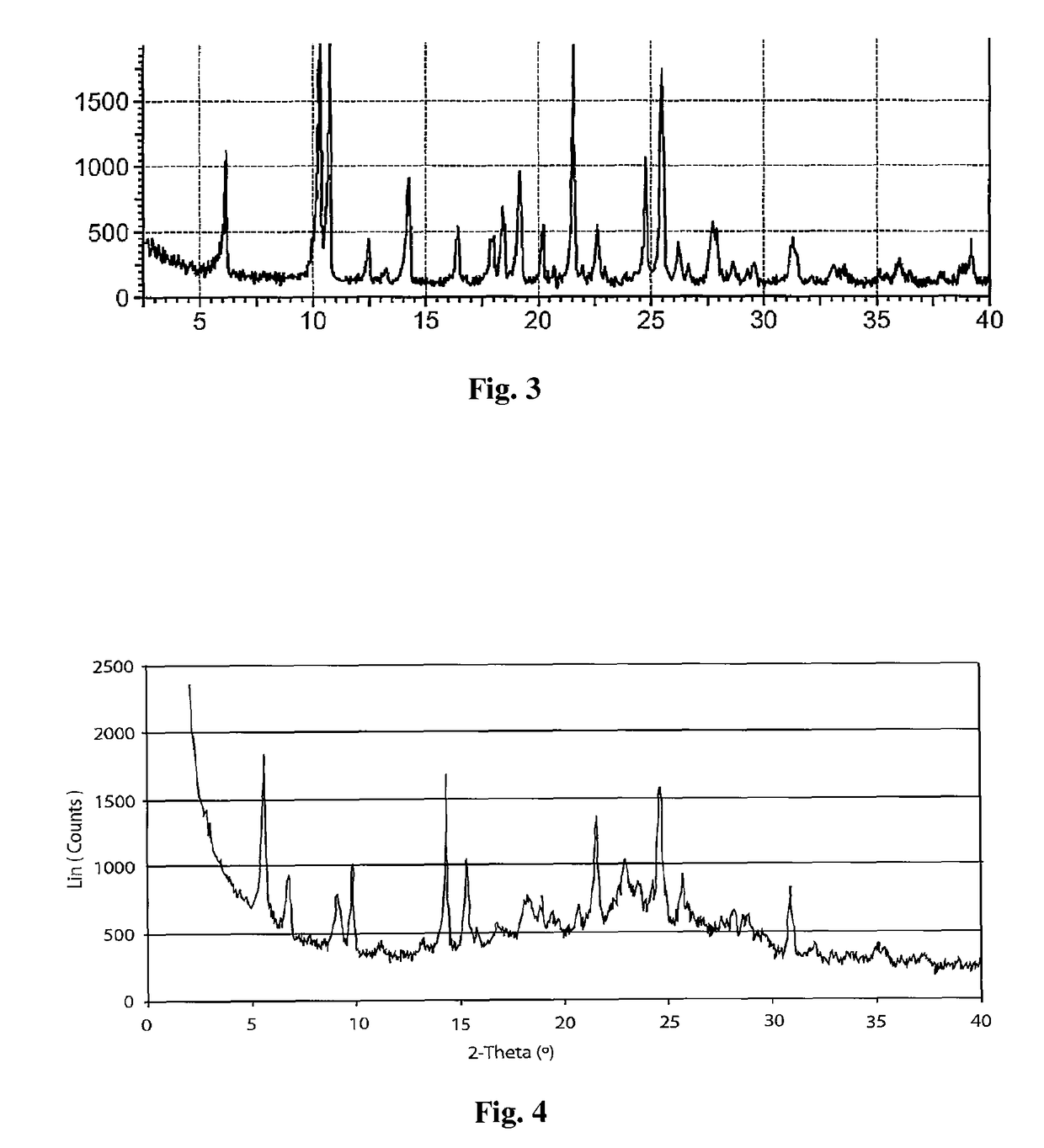
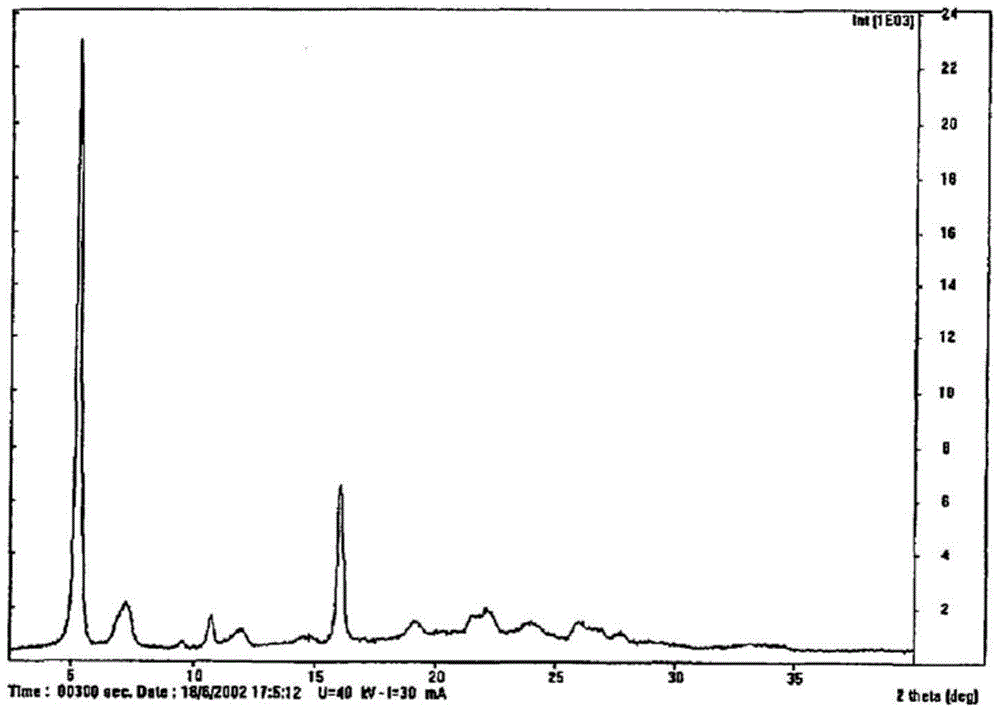
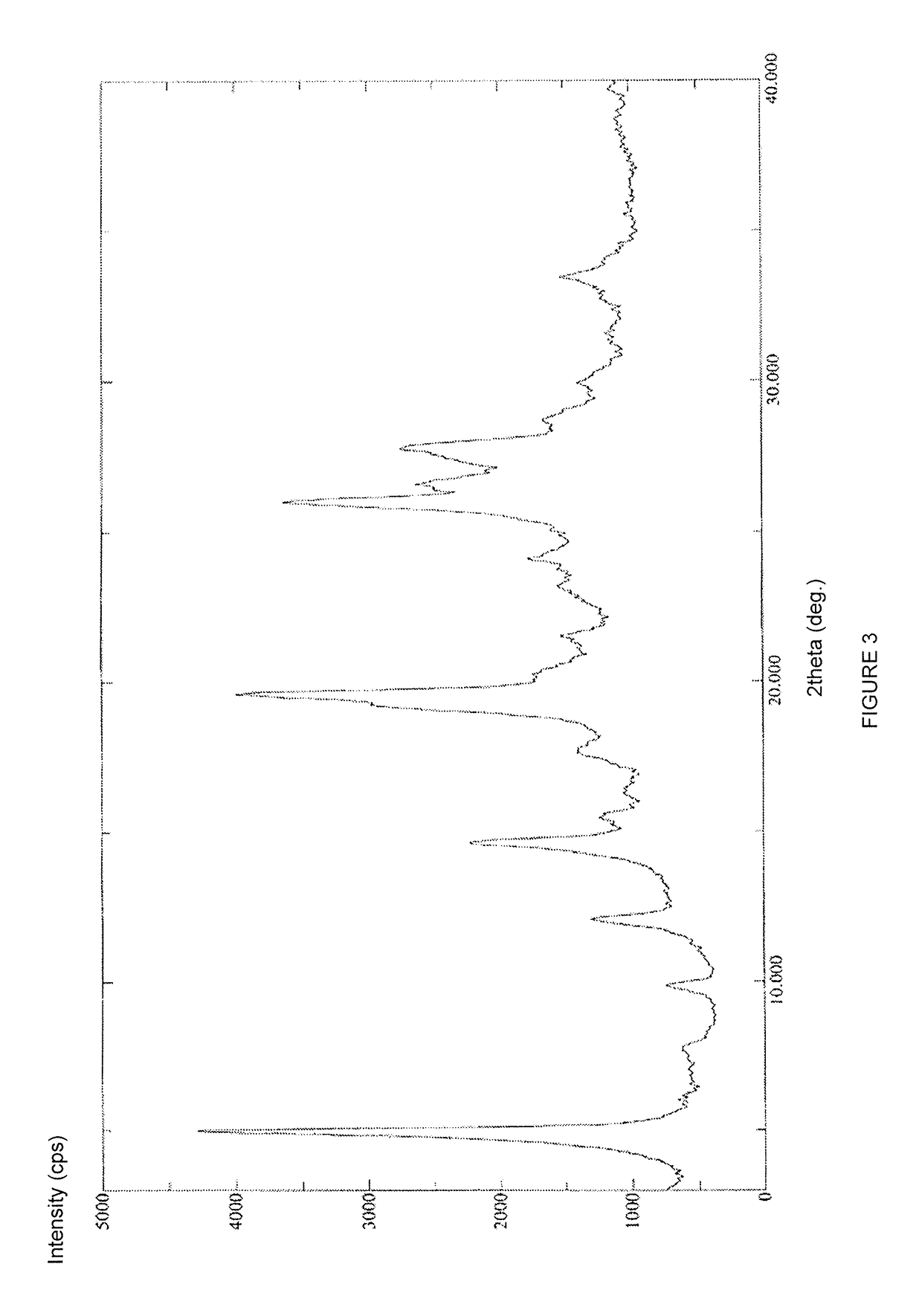
Preparation method of Regadenoson of crystal form B
The invention relates to the field of crystal form preparation, and especially relates to a preparation method of Regadenoson of crystal form B. The preparation method comprises the following steps: taking Regadenoson, mixing Regadenoson with a first organic solvent, carrying out reversed-phase high performance liquid chromatography purification, and collecting a component with the retention time of 15min to obtain a first intermediate; mixing the first intermediate with a second organic solvent to obtain an organic phase, and concentrating to obtain a second intermediate; and mixing the second intermediate with trifluoroethyl alcohol, and crystallizing to obtain the Regadenoson of crystal form B. The preparation method can greatly improve the purity of the Regadenoson of crystal form B.
Owner:SHANGHAI ZIYUAN PHARMA
Crystal form of regadenoson and preparation method thereof
ActiveUS20170002036A1Improve stabilityImprove performanceSugar derivativesOrganic chemistry methodsMedicineRegadenoson
The present invention relates to the field of medicinal chemistry, and discloses a new crystal form of regadenoson, i.e., a crystal form E of regadenoson, as well as a method for preparing the new crystal form of regadenoson. The crystal form E of regadenoson according to the present invention has excellent performances in terms of radionuclide myocardial perfusion imaging, and has a poor toxicity, good storage stability, and can be used in the preparation of a medicament used as a stress agent for radionuclide myocardial perfusion imaging.
Owner:SHANGHAI ZIYUAN PHARMA
Amplified production method of Regadenoson injection
ActiveCN106943347AAvoid the problem of prolonged exposure to the environmentStable in natureOrganic active ingredientsInorganic non-active ingredientsRegadenosonDisodium Edetate
The invention discloses an amplified production method of a Regadenoson injection. The method comprises the following steps: (1) preparing a 15%-35% by mass propylene glycol aqueous solution and heating the solution to 60-75 DEG C or adding propylene glycol into water for injection at 60-75 DEG C to be dissolved to prepare a 15%-35% by mass propylene glycol aqueous solution, then adding Regadenoson to be mixed and dissolved at 60-75 DEG C, then successively adding and dissolving disodium hydrogen phosphate, sodium dihydrogen phosphate and edetate disodium, mixing till disodium hydrogen phosphate, sodium dihydrogen phosphate and edetate disodium are fully dissolved, replenishing water for injection to a constant volume to obtain a medicine liquid; and (2) filtering and canning the prepared medicine liquid, and then sterilizing the medicine liquid to obtain the Regadenoson injection. The process disclosed by the invention solves the problem that the medicine liquid is exposed in a naked environment for a relatively long time. The medicine liquid with high quality is prepared according to a correct feeding sequence, the laboratory experiment is transferred to amplified production in a workshop, and the sterile level of the product is improved; the preparation process has the advantages of being mild in condition, simple and rapid to operate, low in cost, stable in property of medicine liquid, high in quality standard and suitable for industrial production.
Owner:NANJING KING FRIEND BIOCHEM PHARMA CO LTD
Regadenoson crystal form and preparation method thereof
InactiveCN105085593AImprove performanceWeak toxicityOrganic active ingredientsSugar derivativesRegadenosonMedicinal chemistry
The present invention relates to the field of pharmaceutical chemistry, and discloses a new regadenoson crystal form, ie., regadenoson crystal form E, and a preparation method of the new regadenoson crystal form. According to the present invention, the regadenoson crystal form E has excellent performances in the field of radionuclide myocardial perfusion imaging, has characteristics of weak toxicity and good storage stability, and can be used for preparing drugs for radionuclide myocardial perfusion imaging stress agents.
Owner:SHANGHAI ZIYUAN PHARMA
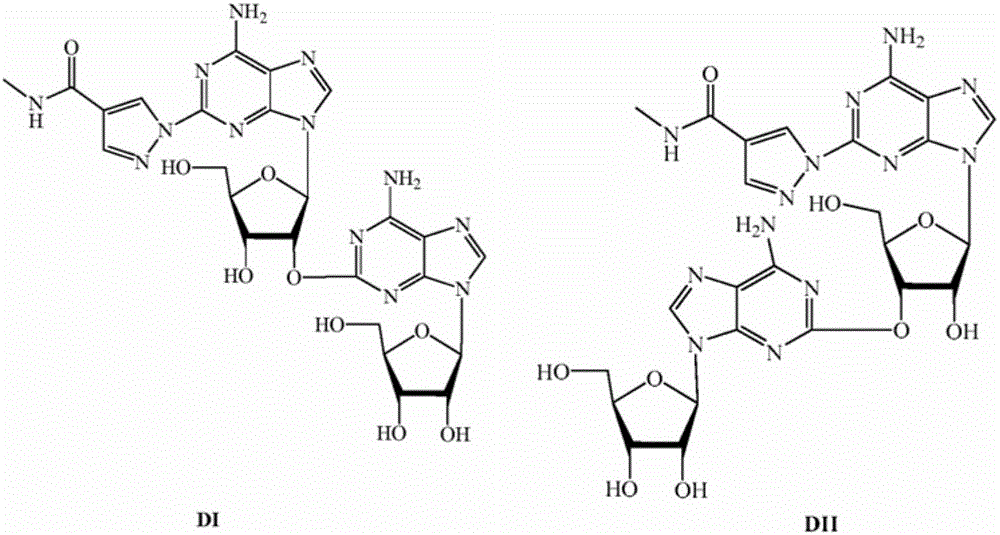
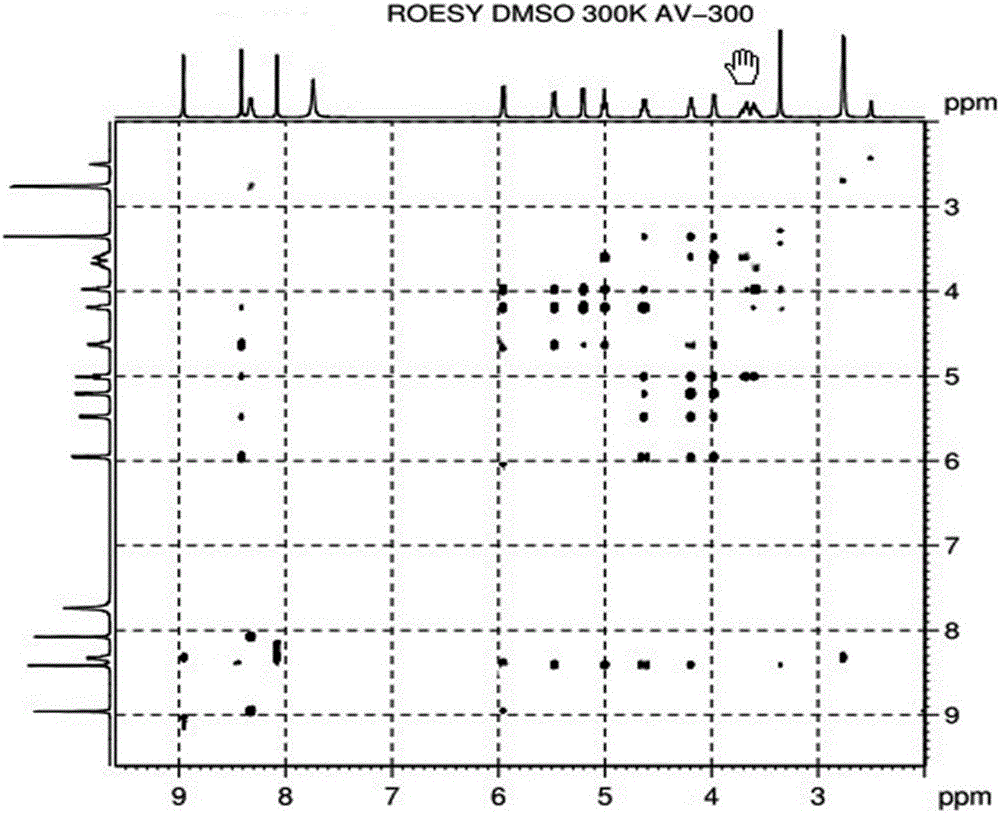
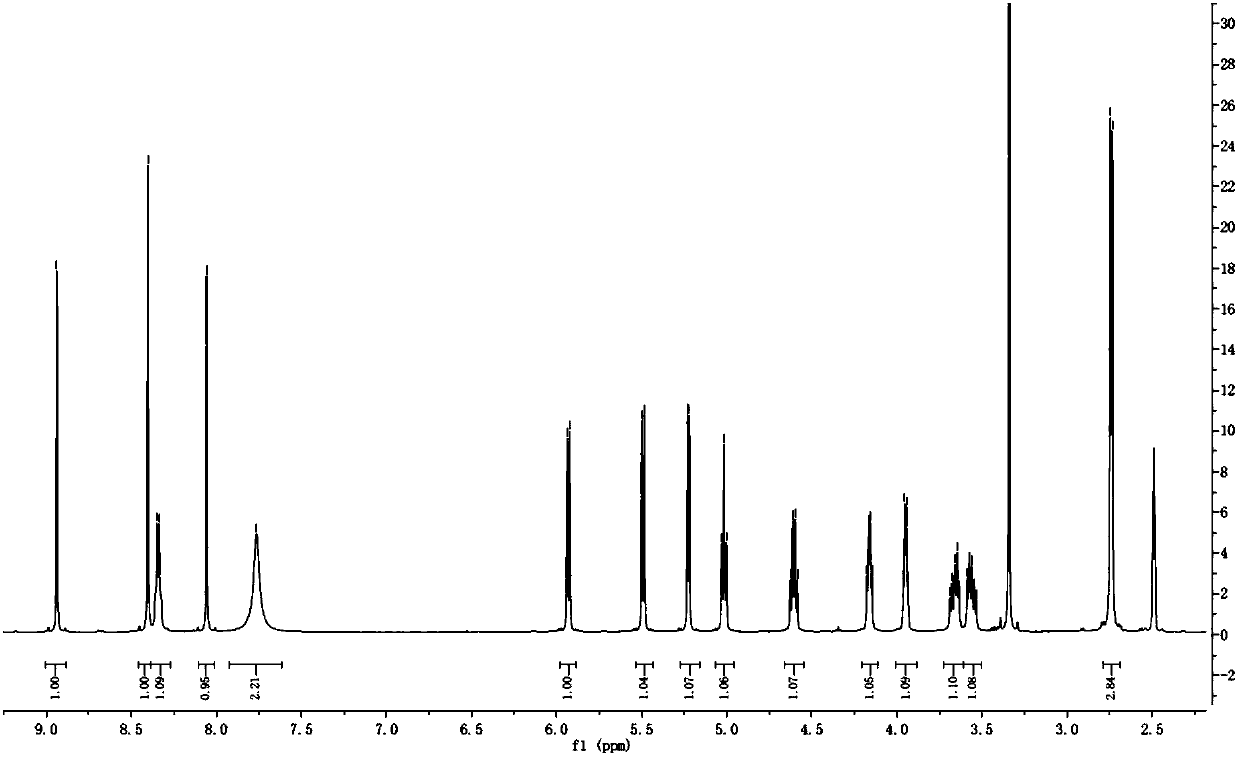
Alpha isomer impurity of regadenoson and preparation method and use thereof
ActiveCN105968156AProcess conditions are easy to controlEasy to routeSugar derivativesComponent separationRegadenosonPurine
The invention discloses an alpha isomer impurity of regadenoson and a preparation method and a medicinal use thereof. The impurity is obtained through oriented synthesis; 2,6-dichloropurine and beta-D-1,2,3,5-tetra-o-acetyl-d-ribose are taken as starting raw materials; a salt of alpha 2-chloroadenosine is obtained by concentration, ammonolysis, salifying and purification; the regadenoson alpha isomer impurity is obtained directly through hydrazinolysis, concentration and ammonolysis without dissociation. An isomer is taken as a regadenoson impurity reference substance, so that the content of the impurity produced in synthesis can be effectively identifies, thereby raising the medicine quality standard of regadenoson.
Owner:NANJING HERON PHARMA SCI & TECH CO LTD +1
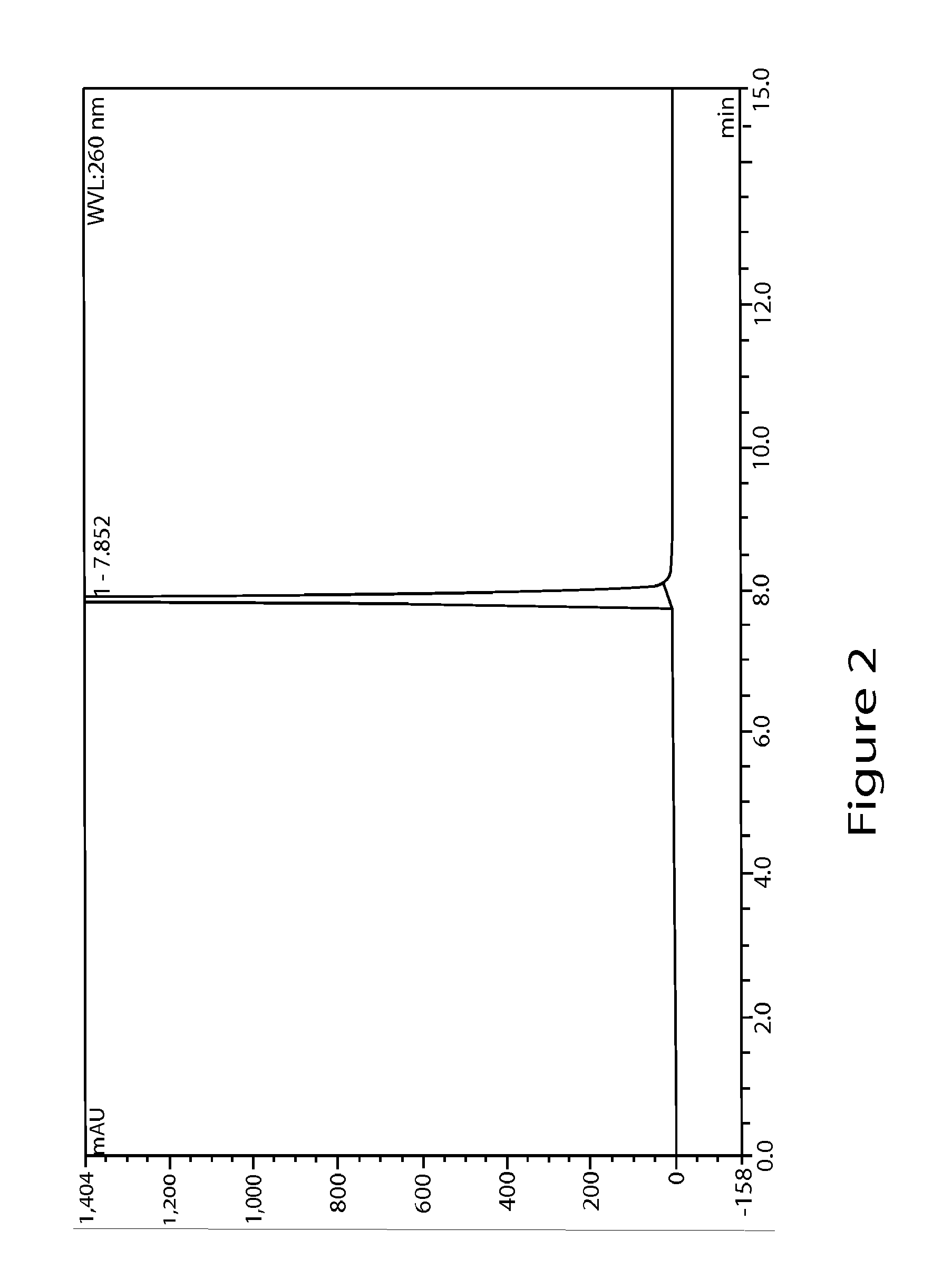
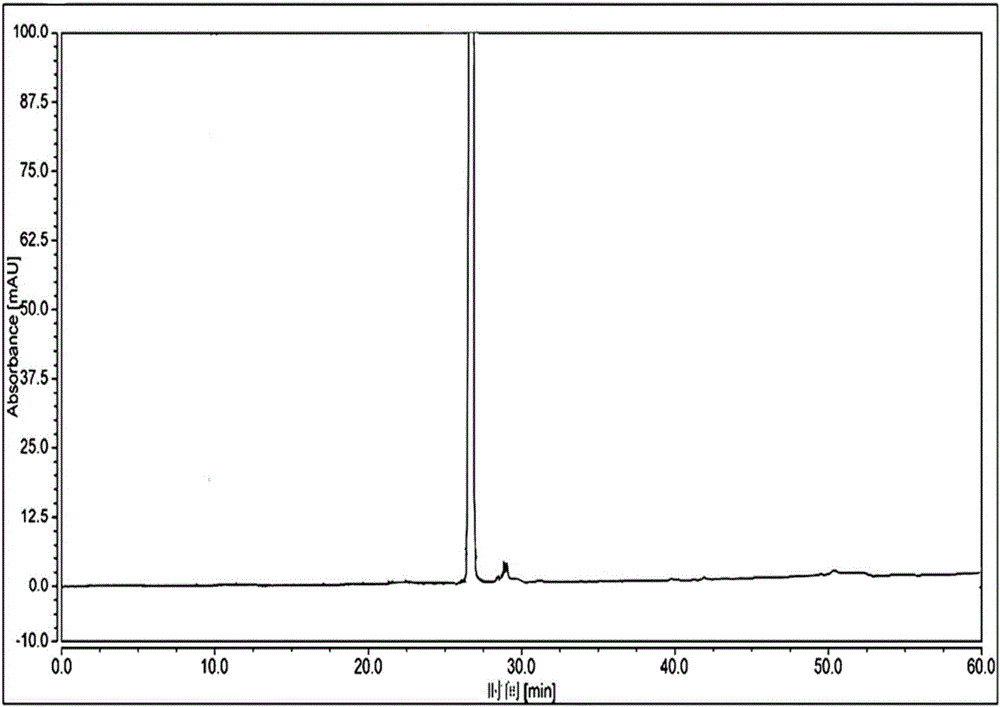
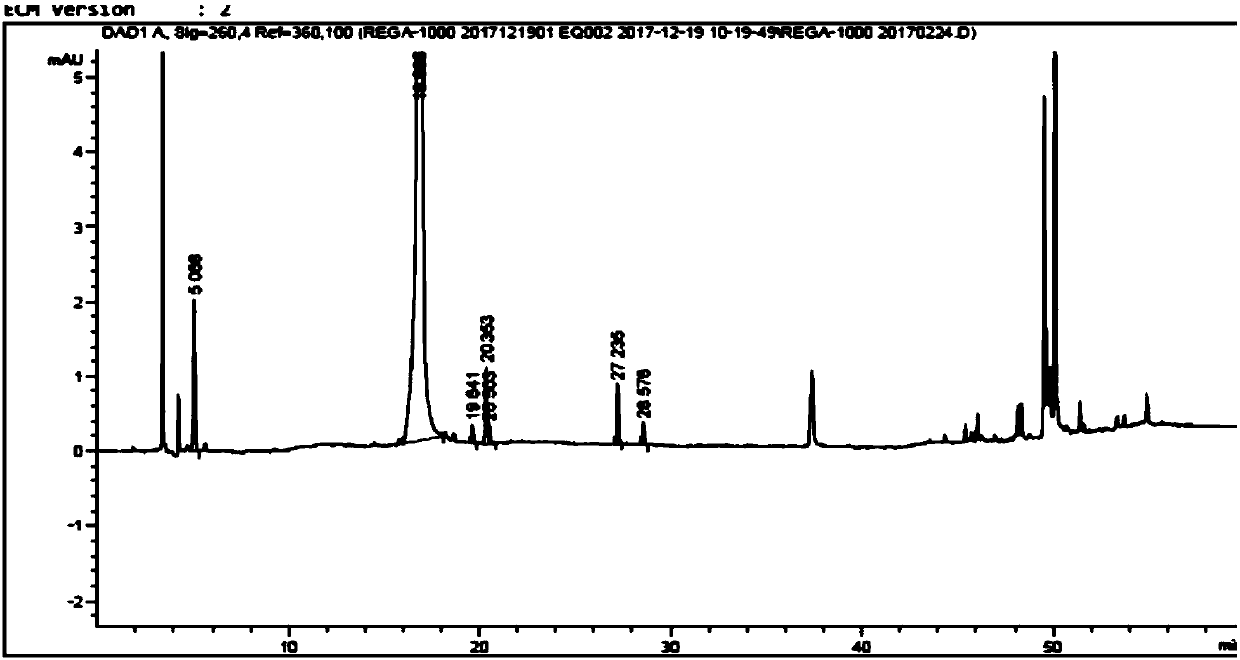
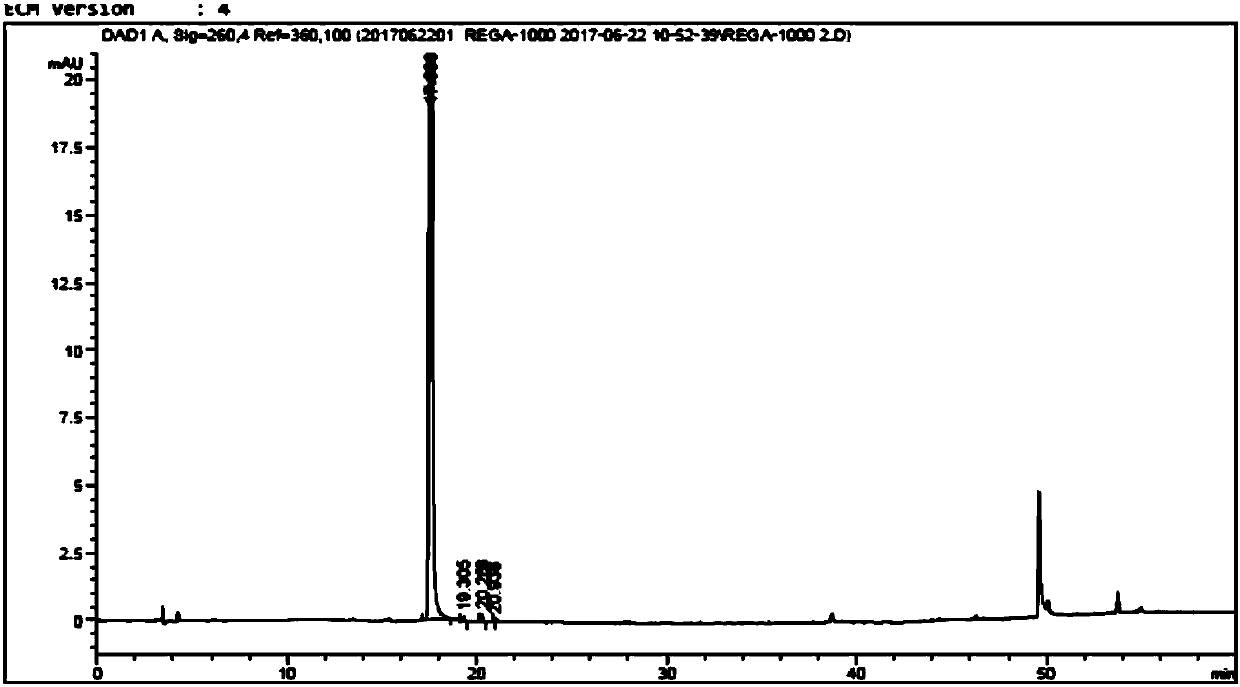
High performance liquid chromatographic analysis method for related substances of regadenoson
ActiveCN105866290AHigh purityReliable impurity profile referenceComponent separationGradient elutionImpurity
The invention discloses a high performance liquid chromatographic analysis method for related substances of regadenoson. According to the method, a reversed phase column and an ultraviolet detector are adopted, and a 1-alkyl sodium sulfonate solution-methanol is adopted as a flowing phase for gradient elution. By the method, all known impurities in regadenoson raw materials and a preparation can be analyzed at the same time, the content of each known impurity can be effectively controlled by a principal component self-contrast method adopting a correction factor, degrees of separation between each impurity peak as well as between a major peak and an adjacent impurity peak are all more than 1.5, and the peak impurity of the major peak and each impurity peak is higher than 1.0. A simple and reliable analysis method is provided for quality control over the regadenoson raw materials and the preparation.
Owner:NANJING HERON PHARMA SCI & TECH CO LTD +1
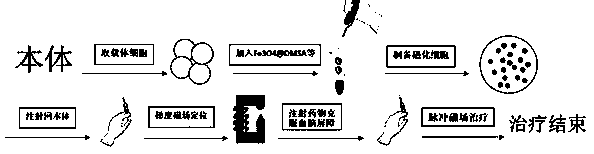
Method for controlling non-invasive brain entry of magnetic nano particles on basis of cell drug loading technology
InactiveCN109125728AEasy to operateWeak antigenicityNervous disorderEnergy modified materialsAgonist drugsMagnetite Nanoparticles
The invention discloses a method for controlling non-invasive brain entry of magnetic nano particles on the basis of a cell drug loading technology. The method comprises a preparation technology of cell (such as red blood cells and neutrophile granulocyte) wrapped magnetic nano particles, a blood brain barrier overcoming technology and a gradient magnetic field positioning and treatment technology. The preparation technology of the cell wrapped magnetic nano particles comprises the steps of hypotonic dilution and isotonic sealing; the blood brain barrier overcoming technology relies mainly onA2a adenosine receptor agonist drugs such as regadenoson; and a gradient magnetic field uses a principle of an electromagnetic effect and is generated by supplying current to a plurality of turns of coils located on an iron core. Magnetic cells of the technology have good magnetism and biocompatibility; accurate targeting and long-time retention of the magnetic cells in a body can be achieved through focusability of the gradient magnetic field; the magnetic nano particles can be positioned in a specific brain area by opening a blood brain barrier; a reaction can be generated in the brain through a pulse magnetic field; and accurate loading of the magnetic nano particles is achieved.
Owner:SOUTHEAST UNIV
Polymorph of regadenoson
The invention provides a novel polymorph of Regadenoson. More particularly, the invention provides propylene glycol solvate of Regadenoson. The invention also provides a process for the preparation of propylene glycol solvate of Regadenoson.
Owner:BIOPHORE INDIA PHARMA PVT LTD
Regadenoson purification method
ActiveCN106397442AEfficient removalHPLC high purityOrganic chemistryPurification methodsOrganic solvent
The present invention discloses a regadenoson purification method, which comprises: under the effect of an alkali water solution, mixing an impurity A-containing regadenoson crude product and a polar organic solvent, and crystallizing, wherein the impurity A content in the regadenoson crude product is 0.1-1.0%. According to the present invention, with the purification method, the impurity A in the regadenoson crude product can be effectively removed, the impurity A content in the obtained regadenoson product is less than 0.10%, and the lowest content is less than 0.04%, such that the HPLC purity of the regadenoson product is further improved, the HPLC purity of the finally-obtained regadenoson product is more than 99.80%, the product quality is good, and impurity limit standard of ICH Q3a is completely met. The formula of the impurity A is represented in the specification.
Owner:CHINA NAT MEDICINES GUORUI PHARMA +1
Process for the preparation of regadenoson
ActiveUS9771390B2Efficient and economic processToxic reductionSugar derivativesRegadenosonMedicinal chemistry
Owner:SCINOPHARM TAIWAN LTD
A kind of Rui Jianuosheng injection and preparation method thereof
ActiveCN105997852BImprove solubilityIncrease dosageComponent separationInorganic non-active ingredientsAntioxidantPhosphate
Owner:NANJING HERON PHARMA SCI & TECH CO LTD +1
Pharmaceutical compositions of regadenoson
The present invention relates to novel pharmaceutical compositions of Regadenoson and its pharmaceutically acceptable salts, solvates or hydrates in the form of solution, wherein the compositions are free from phosphate buffer and EDTA. Further the invention relates to pharmaceutical composition of Regadenoson comprising a tonidty modifier.
Owner:LEIUTIS PHARMA PVT
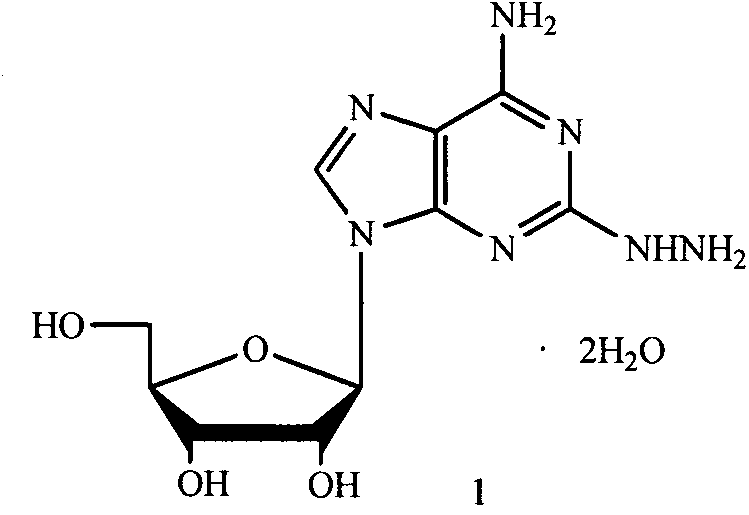
2-hydrazino adenosine and preparation method thereof
The invention belongs to the field of pharmaceutical chemical engineering and relates to 2-hydrazino adenosine and a preparation method thereof. According to the method disclosed by the invention, a chemical compound shown as a formula II reacts with hydrazine hydrate in the presence or the absence of a polar solvent and the reaction formula is shown as follows. The 2-hydrazino adenosine crystal prepared by adopting the method is high in yield, high in purity and low in cost; the method has the advantages of being friendly to the environment, simple and convenient to operate and easy in industrial scale-up and has a very good industrial application prospect; and the 2-hydrazino adenosine can be used for preparing an adenosine receptor agonist, i.e. regadenoson.
Owner:南京海辰药业股份有限公司
A process for the preparation of regadenoson
ActiveUS20160304551A1Toxic reductionEfficient and economic processSugar derivativesBiochemical engineeringRegadenoson
The present invention provides novel processes for the preparation of regadenoson having the formula (I). In some embodiments, the intermediates for the synthesis of regadenoson are also provided.
Owner:SCINOPHARM TAIWAN LTD
Stable solid forms of regadenoson
A process for the preparation of the amorphous form of Regadenoson of formulais disclosed together with new crystalline polymorphic forms E, F and G and methods for their preparation. Regadenoson amorphous form can be prepared in mild reaction conditions with high chemical purity (>99.6%) and high stability to the heating. A particularly thermodynamically stable anhydrous crystalline form of Regadenoson (form G) is also disclosed, provided with high stability not when exposed to 90% RH at 25° C. for 96 hour, but also to the heating up to 200° C.
Owner:AMRI ITAL SRL
A kind of preparation method of Regardson crystal form e
ActiveCN105198950BHigh puritySugar derivativesSugar derivatives preparationOrganic solventRegadenoson
The invention relates to the field of crystal form preparation, in particular to a preparing method for a regadenoson crystal form E. The preparing method includes the steps that regadenoson and first organic solvent are mixed and purified through reversed-phase high-performance liquid chromatography, and the component with the retention time of 30 min is collected to obtain a first intermediate product; the first intermediate product is mixed with second organic solvent to obtain an organic phase, and a second intermediate product is obtained through concentration; the second intermediate product and DMF are mixed and crystallized to obtain the regadenoson crystal form E. The purity of the regadenoson crystal form E can be improved greatly by means of the preparing method.
Owner:SHANGHAI ZIYUAN PHARMA
Pharmaceutical formulations of regadenoson
InactiveUS20190240247A1Organic active ingredientsPharmaceutical delivery mechanismRegadenosonCyclodextrin
The present invention relates to parenteral pharmaceutical formulation of Regadenoson comprising of Regadenoson, one or more cyclodextrins and pharmaceutically acceptable excipients.
Owner:LEIUTIS PHARMA PVT
Regadenoson purifying method and new crystal form of regadenoson
The invention relates to a regadenoson purifying method and a new crystal form of regadenoson. The method comprises the following steps that a regadenoson crude product is heated and dissolved in a mixed solvent, and then stirring cooling, crystallization, filtering and drying are conducted, wherein the mixed solvent is selected from mixed solvents of ethanol, methanol, acetonitrile and water; orthe method comprises the following steps that the regadenoson crude product is heated and dissolved in a good solvent, then a poor solvent is added, and stirring cooling, crystallization, filtering and drying are conducted, wherein the good solvent is selected from DMF, NMP and sulfolane or a mixture of the DMF, NMP and sulfolane, and the poor solvent is selected from ethanol, methanol and water or a mixture of the ethanol, methanol and water.
Owner:SHANGHAI JIANHE PHARM & TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com