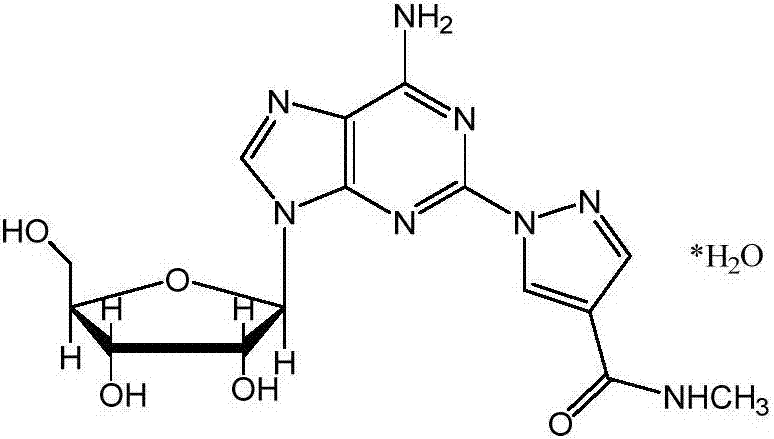
Amplified production method of Regadenoson injection
A technology of regadeson and its production method, which is applied in the direction of pharmaceutical formula, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., and can solve problems such as incomplete application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Add 122.5L of 60°C water for injection into a 200L pre-dissolution tank, use a heating jacket outside the pre-dissolution tank to maintain the temperature in the tank at 60°C, add 52.5kg of 1,2-propylene glycol and stir to dissolve, then add 28.01g of anhydrous Regadeson Stir to dissolve; weigh 3.045kg anhydrous disodium hydrogen phosphate, 1.645kg anhydrous sodium dihydrogen phosphate and 350.02g disodium edetate and stir until completely dissolved. Transfer the liquid in the pre-dissolving tank to the liquid mixing tank, use a heating jacket outside the liquid mixing tank, and also maintain the temperature in the tank at 60°C; add 60°C water for injection to make the volume to 350L, stir for 0.2h, and the liquid mixing takes 0.42h, Take the medicinal liquid to test the content is 99.98%, related substances: total impurities 0.87%, maximum simple impurities 0.39%. After the medicine solution is cooled to room temperature, it is filtered in the workshop, and then filled...
Embodiment 2
[0044] Add 122.5L of 65°C water for injection into a 200L pre-dissolved barrel, use a heating jacket outside the pre-dissolved barrel to maintain the temperature inside the barrel at 65°C, add 52.5kg of 1,2-propanediol and stir to dissolve, then add 29.43g of Regadeson monohydrate Stir to dissolve; weigh 3.818kg disodium hydrogen phosphate dihydrate, 1.892g sodium dihydrogen phosphate monohydrate and 350.25 g disodium edetate and stir until completely dissolved. Transfer the liquid in the pre-dissolving tank to the liquid mixing tank, use a heating jacket outside the liquid mixing tank, and also maintain the temperature in the tank at 65°C; add 65°C water for injection to make the volume to 350L, stir for 0.2h, and the liquid mixing takes 0.38h, The liquid medicine was taken for detection and the content was 100.06%, related substances: total impurities 0.92%, maximum simple impurities 0.43%. After the medicine solution is cooled to room temperature, it is filtered in the work...
Embodiment 3
[0046] Add 122.5L of 75°C water for injection into a 200L pre-dissolved barrel, use a heating jacket outside the pre-dissolved barrel to maintain the temperature in the barrel at 75°C, add 52.5kg of 1,2-propanediol and stir to dissolve, then add 30.73g of Regadeson dihydrate Stir to dissolve; weigh 3.812kg disodium hydrogen phosphate dihydrate, 1.895g sodium dihydrogen phosphate monohydrate and 350.05g disodium edetate and stir until completely dissolved. Transfer the liquid in the pre-dissolving tank to the liquid mixing tank, use a heating jacket outside the liquid mixing tank, and also maintain the temperature in the tank at 75°C; add 75°C water for injection to make the volume to 350L, stir for 0.2h, and the liquid mixing takes 0.45h, The liquid medicine was taken for detection and the content was 100.01%, related substances: total impurities 0.88%, maximum simple impurities 0.37%. After the medicine solution is cooled to room temperature, it is filtered in the workshop, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com