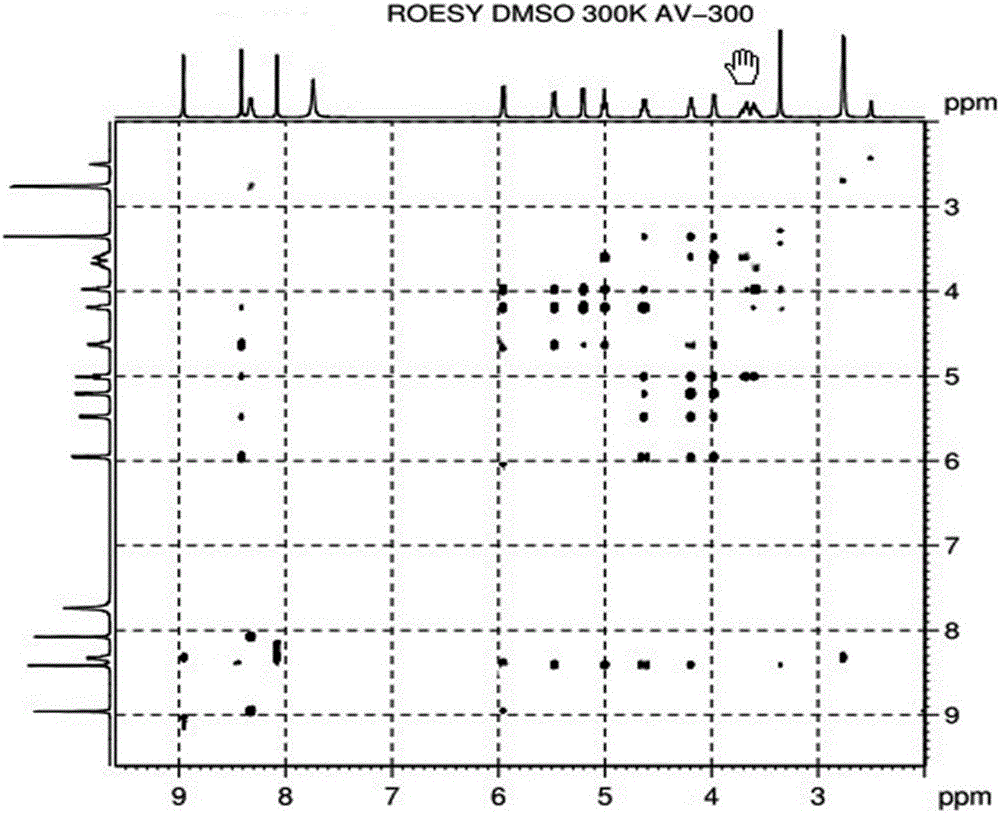
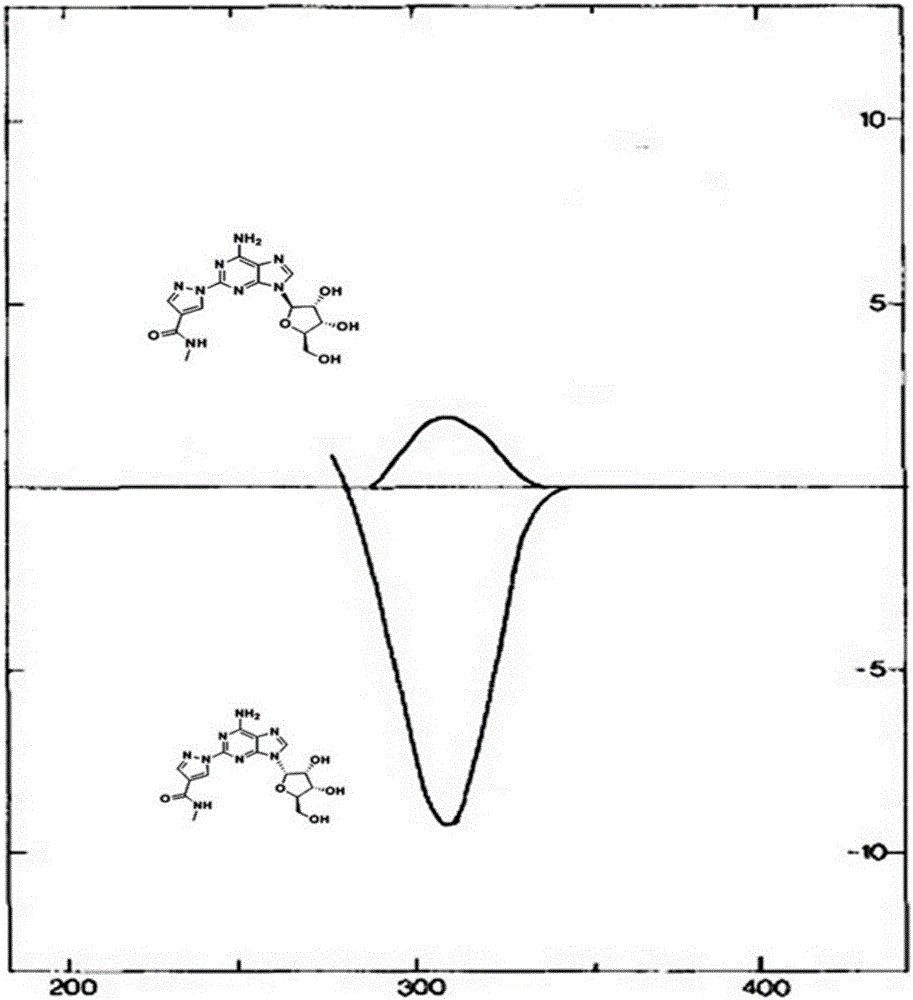
Alpha isomer impurity of regadenoson and preparation method and use thereof
A technology of Reganosine and α isomers, which is applied to the α isomer impurities of Reganosine, and the application field of Reganosine pharmaceutical quality control, which can solve the problem that α isomer impurities cannot be effectively identified and Quality control and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] The preparation of embodiment 1 formula 2 compound
[0075] Under nitrogen protection, dissolve 2,6-dichloroadenine (500g, 2.64mol) and β-D-tetraacetylribose (923g, 2.90mol) in 10L of anhydrous methyltetrahydrofuran, and cool to -5 ℃, add about 250g of acid resin catalyst "Amberlyst15" and about 28.5g of 3A molecular sieve, after stirring evenly, slowly add 800mL of anhydrous methyl tetrahydrofuran solution of about 450g of tin tetrachloride dropwise, and control the reaction temperature at -5 to 0℃ , after dropping, add 5L of isopropyl acetate, slowly rise to 30-35°C and continue to react for 3-4h, TLC monitors that the reaction is complete, filter, and slowly add 10L of saturated sodium bicarbonate solution and dichloromethane to the filtrate under strong stirring Extract (4L*3), combine the organic phases, concentrate to dryness, add about 5L of methyl tert-butyl ether for beating for 1 hour, filter the obtained solid with suction, and dry it to obtain about 1063g of...
Embodiment 2
[0076] The preparation of embodiment 2 formula 3 compound
[0077] The compound of formula 2 (1063 g, 2.37 mol) was dissolved in 25 L of isopropanol, and dry ammonia gas was continuously introduced, stirred and reacted at 30 ° C for 12 h, and concentrated to dryness to obtain about 595 g of compound of formula 3 with a yield of 83%.
Embodiment 3
[0078] The preparation of embodiment 3 formula 4 compound
[0079] Dissolve the compound of formula 3 (595g, 1.97mol) in 4.5L of dioxane, add L-tartaric acid (310g, 2.05mol), stir and react at 60°C for 4h, slowly add 9L of ethyl acetate, drop it, and cool down to 0°C, stirring and crystallizing for 2 hours, suction filtering the resulting solid, and drying to obtain about 740 g of the compound of formula 4, with a yield of 83% and an HPLC purity of 99.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com