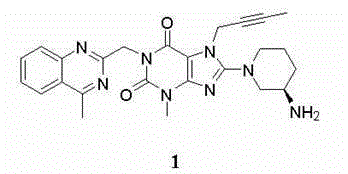
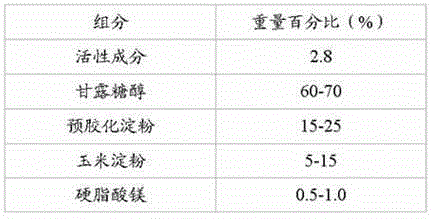
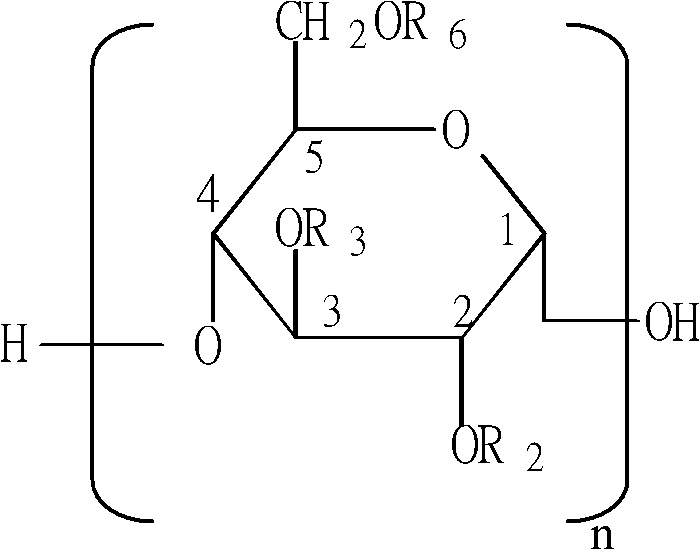
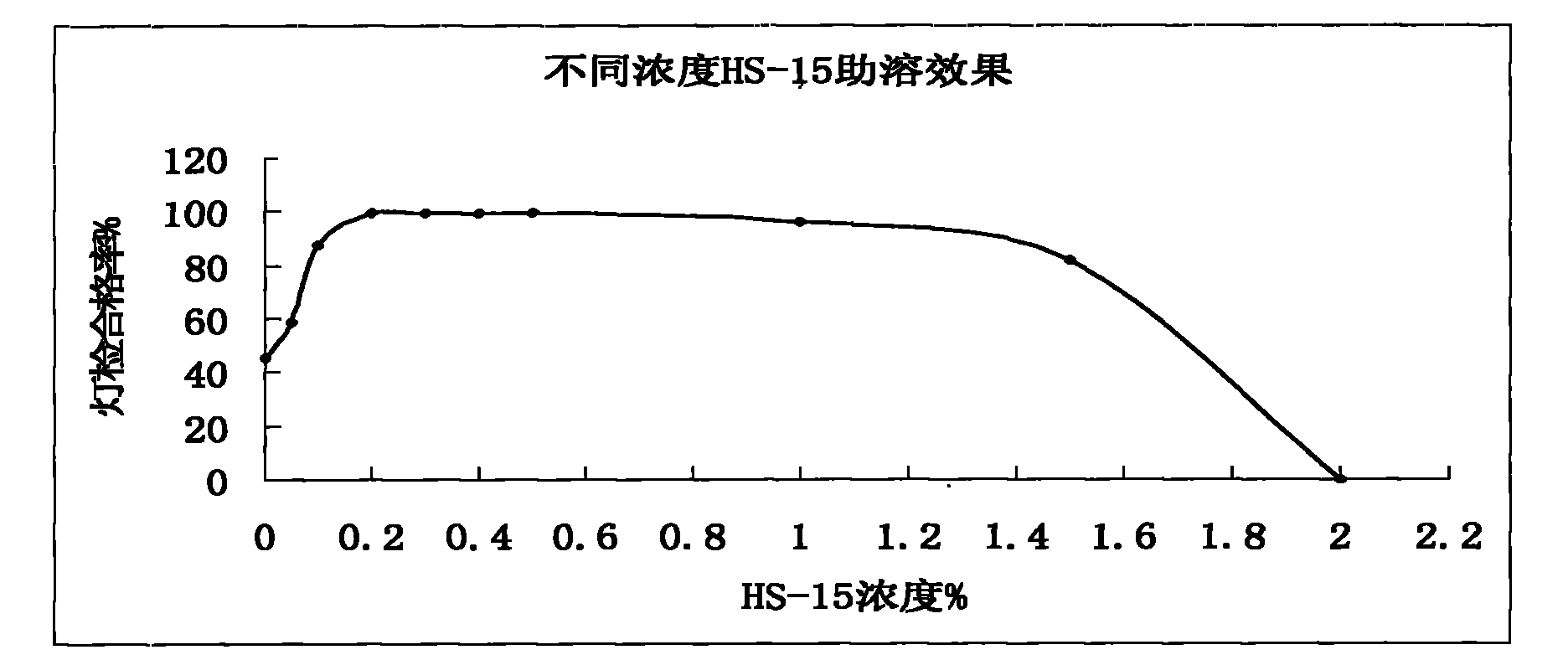
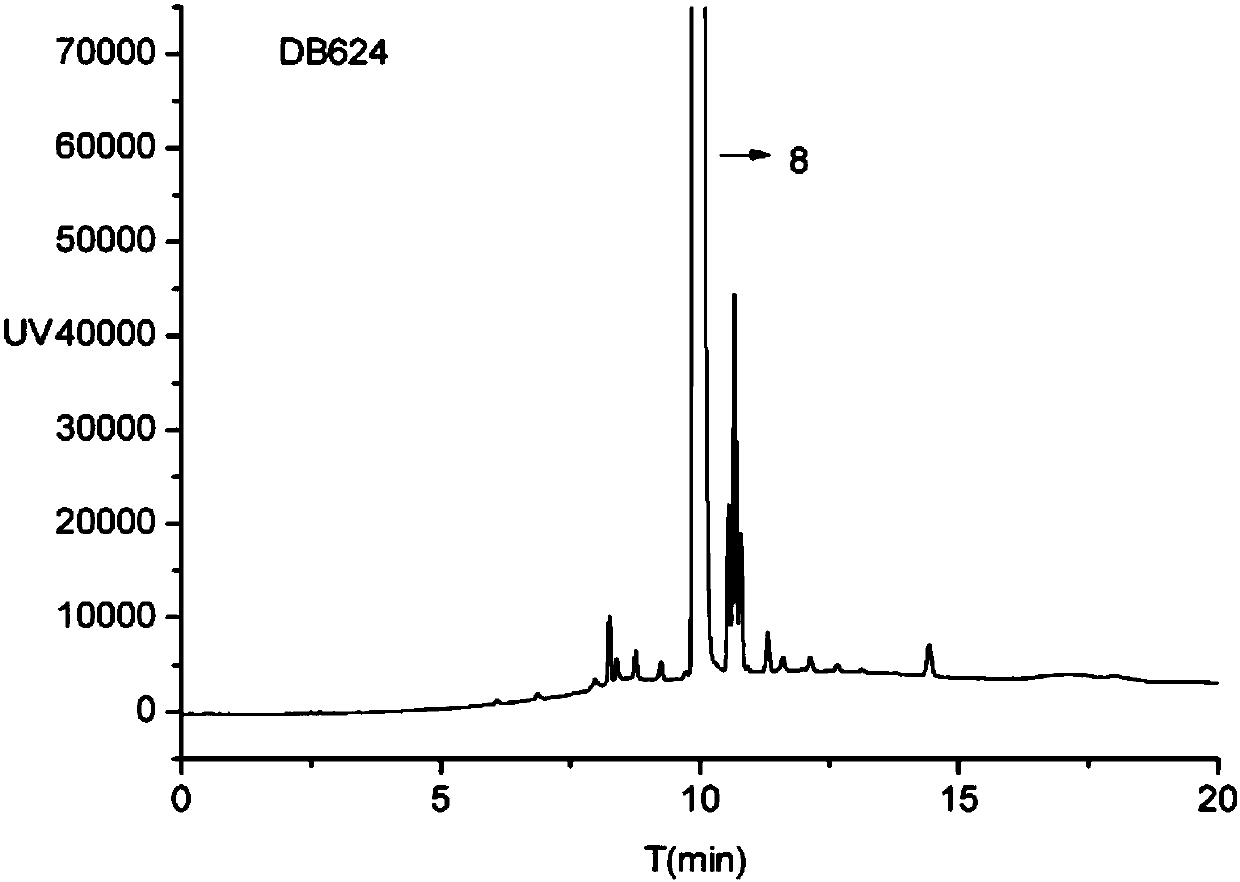
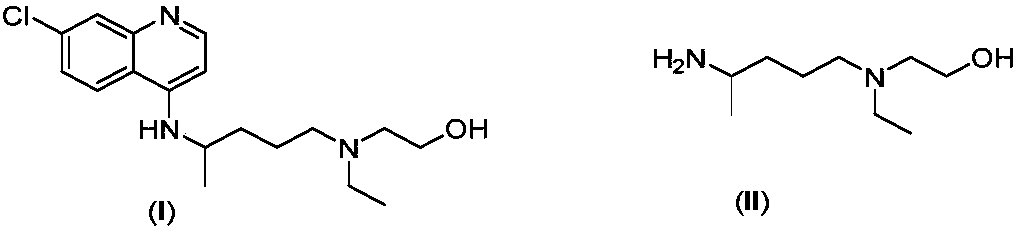
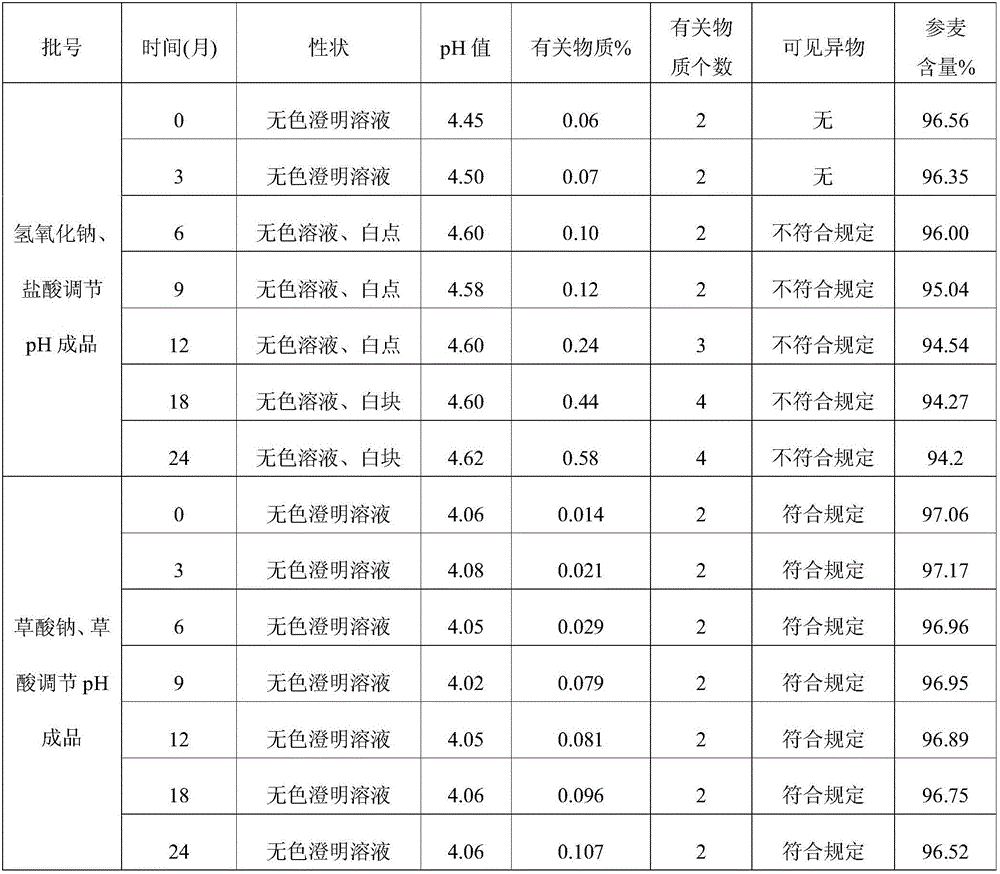
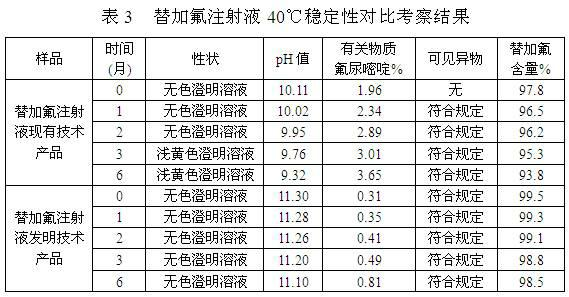
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
118 results about "Drug quality/standard" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Levels of excellence which characterize drugs based on accepted standards of quality; standards of adequacy, acceptable performance, identification, quality and potency.
Method for extracting coarse oil from coix seed with hypercritical carbon dioxide
InactiveCN1485418AAcidity meetsAcidity meets requirementsFatty-oils/fats productionWater contentSupercritical carbon dioxide
A method or extracting crude oil of coix seed by supercritical carbon dioxide extraction. It comprise:1) drying, the water content of coix seed is below 10úÑú”2) pulverizing, naturally lowering temperature, after testing, pulverizing into 10-60 meshes; 3) supercritical carbon dioxide extraction, in the extracting vessel, the temperature is 30í½60degree Cú¼ the pressure is 19í½24MPaú”in the separating column, the temperature is 30í½60degree Cú¼ the pressure is 6í½15Mpaú”in the resolving vessel, the temperature is 30í½60degree Cú¼ the pressure is2í½6Mpaú”the flow rate is 10í½5000L / Hrú”the extraction time is 1í½4h.
Owner:江西康莱特新森医药原料有限公司
Headspace gas chromatography detection method of residual solvents in macroporous resin extract
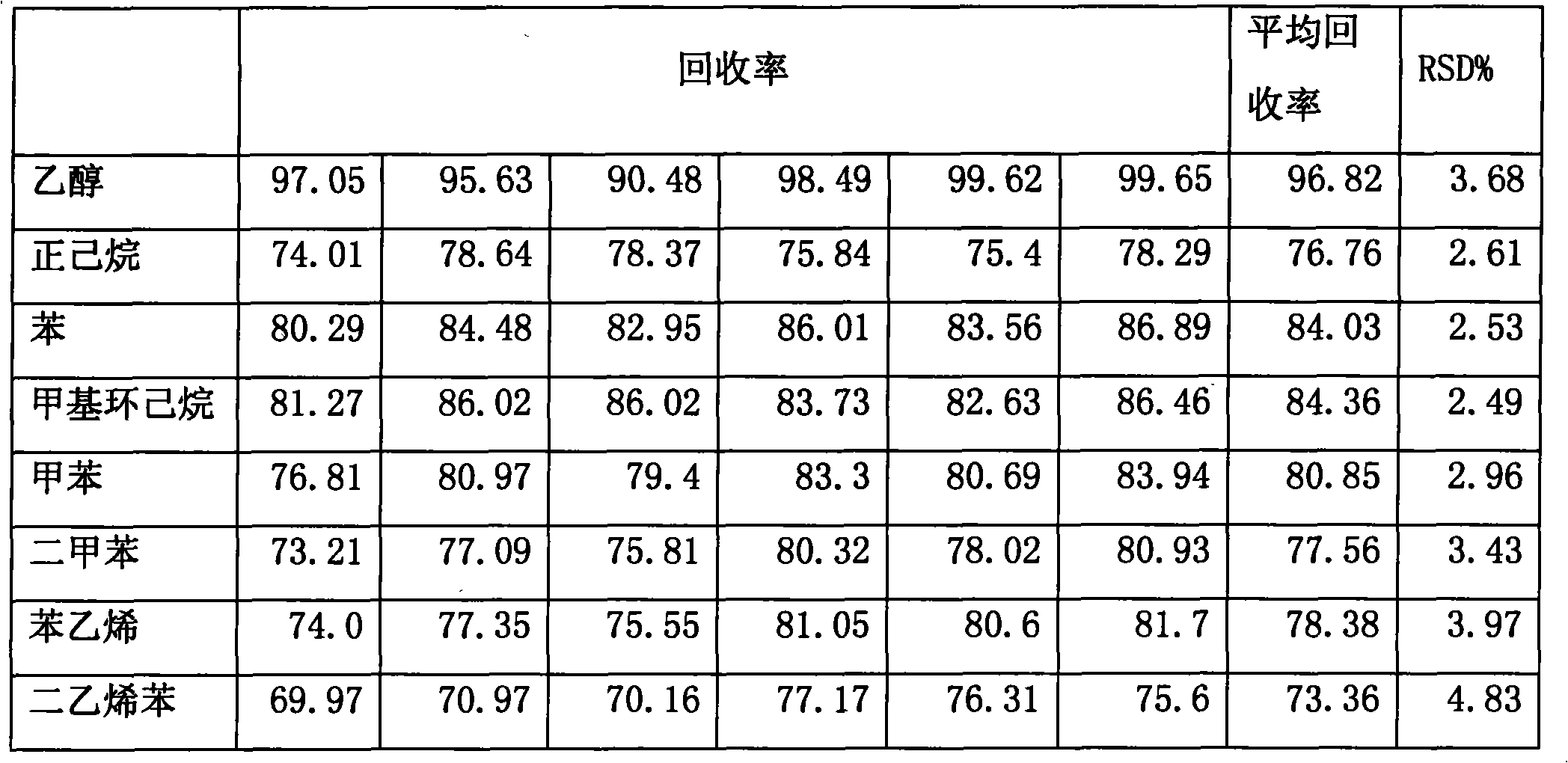
The invention discloses a headspace gas chromatography detection method of residual solvents in a macroporous resin extract, which can be used for simultaneously measuring normal hexane, benzene, methylbenzene, methylcyclohexane, dimethylbenzene, styrene and divinylbenzene in the macroporous resin extract. The method comprises the following steps of: (1) carrying out tests of chromatographic conditions and system suitability; (2) preparing a comparison product solution; (3) preparing a test product solution; and (4) measuring. The specificity, the system suitability, the linearity, the precision, the minimum detection limit, the recovery rate, and the like of the method all meet the requirements of drug quality standard analysis method verification and guiding principles in the first appendix of the Chinese pharmacopoeia (Version 2005).
Owner:BEIJING UNION PHARMA FACTORY
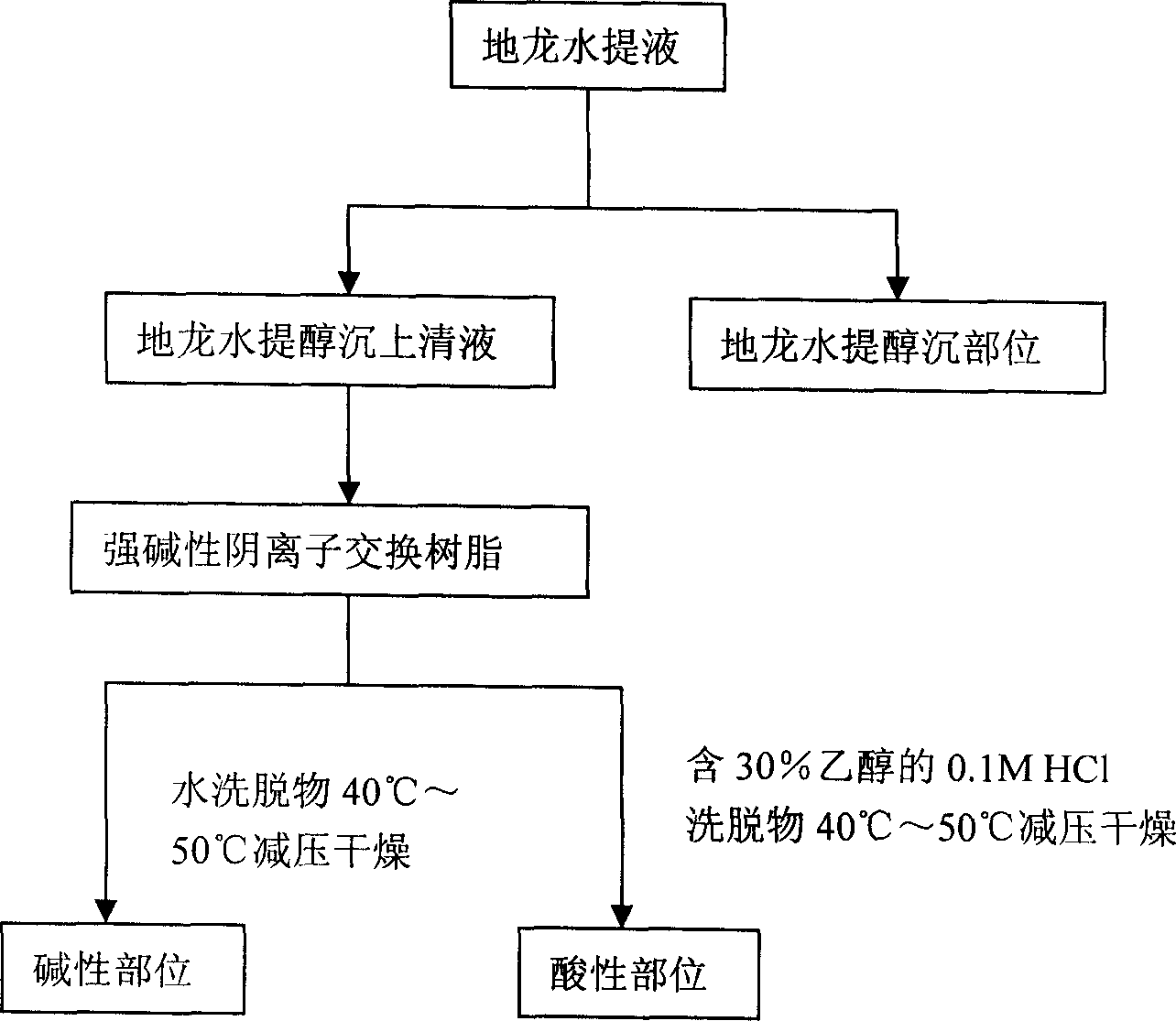
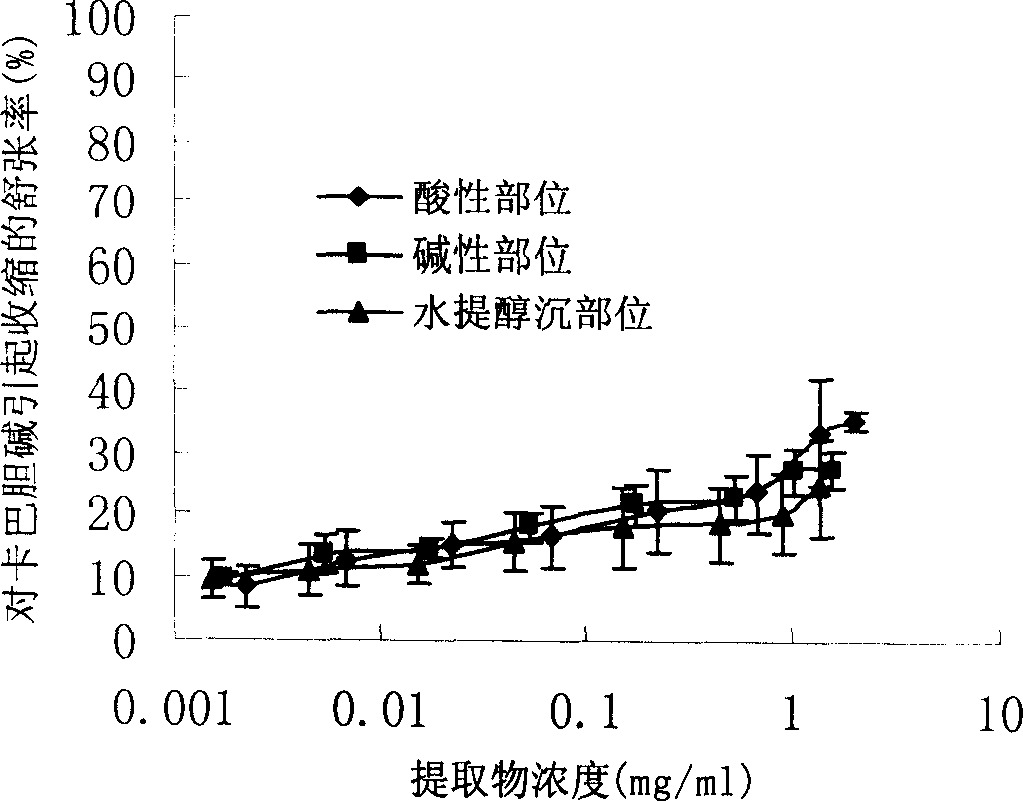
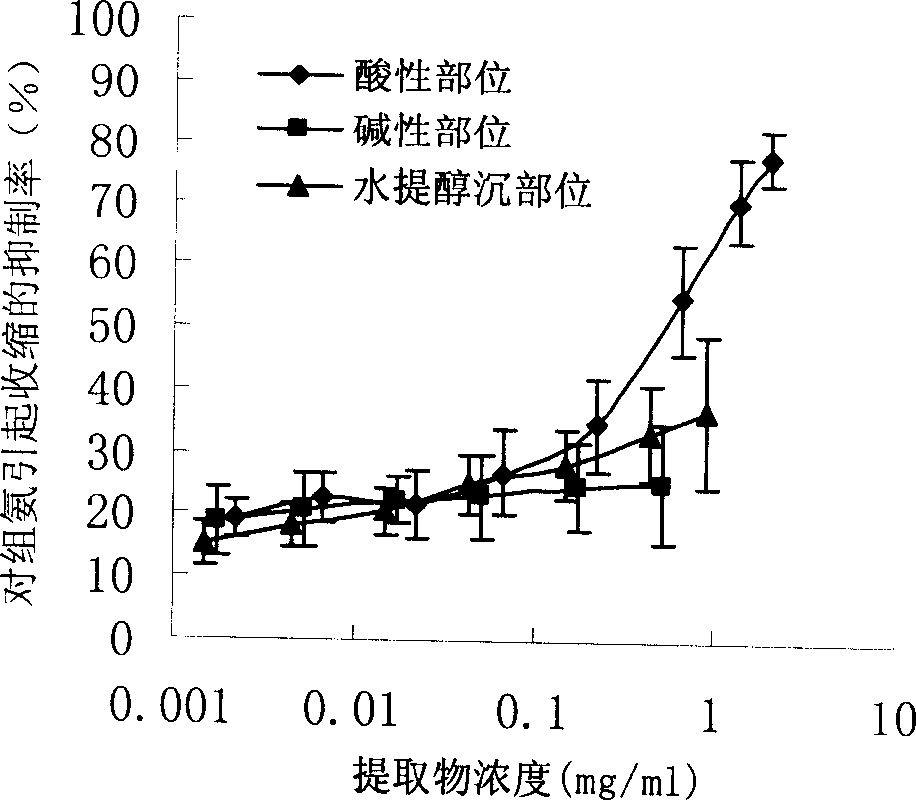
Earthworm acidic-part medicine for treating cough asthma disease and preparing method
InactiveCN1850118AEasy to acceptIncreased content of anti-asthma active ingredientsRespiratory disorderLeech/worm material medical ingredientsDiseasePurine
The present invention relates to an earthworm acid region medicine for curing cough and asthma diseases and its preparation method. Said earthworm acid region medicine contains fatty acid, amino acid and purine compound, and can be used for curing the diseases of asthma and chronic bronchitis, etc. Its preparation method includes the following steps: using strong base anion-exchange resin to adsorb aqueous extract of earthworm, then using dilute hydrochloric acid or alcohol-containing dilute hydrochloric acid to make elution.
Owner:SHANGHAI JIAO TONG UNIV
Synthesis method of middle-molecular-weight hydroxyethyl starch
The invention provides a synthesis method of middle-molecular-weight hydroxyethyl starch. The synthesis method comprises the following steps: carrying out hydroxyethyl substitution reaction on waxy corn starch hydrolyzate and a hydroxyethyl substituting agent under the conditions that water is used as a solvent and sodium hydroxide is taken as a catalyst; and carrying out ultrafiltration interception membrane separation and activated carbon decoloration, filtering, and carrying out spray drying on filtrate so as to obtain the white powdery middle-molecular-weight hydroxyethyl starch product. According to the invention, an adopted aqueous phase synthesis method has the advantages that an ideal molar degree of substitution (MS) of hydroxyethyl and an ideal substitution position ratio (C2:C6) are conveniently controlled in the reaction, and the byproducts of the reaction are easy to separate; and under the condition of the aqueous phase synthesis method, the reaction is a room temperature reaction, reactants and the catalyst are completely dissolved in the reaction system, the substitution positions are uniformly distributed in the product, the reaction byproducts such as sodium chloride, chlorohydrin, cyclochloroethane, glycol and the like can be easily removed once through a membrane separation technology, the obtained product has proper MS and a ratio of C2:C6, and the obtained product quality can reach or be superior to the existing national drug standard.
Owner:WUHAN HUST LIFE SCI & TECH
Process for Producing Aliprazo
InactiveCN1576273AStrong response specificityHigh yieldOrganic chemistryPurification methodsAripiprazole
The present invention relates to the preparation process of Aripiprazole. Aripiprazole is prepared with two kinds of compound and through condensation. The preparation process of the present invention has mild reaction condition, less side products, simple operation and controllable quality of the intermediate, and the Aripiprazole product of the present invention may reach relevant medicine standard without needing several times of re-crystallization.
Owner:重庆凯林制药有限公司 +1
Dipeptidyl peptidase IV inhibitor pharmaceutical composition, use and preparation method thereof
ActiveCN105456270AReduce typesLow costMetabolism disorderPharmaceutical non-active ingredientsStarch cornMagnesium stearate
The invention discloses a pharmaceutical composition containing a dipeptidyl peptidase IV inhibitor linagliptin, use and preparation method thereof. The linagliptin containing pharmaceutical composition provided by the invention consists of linagliptin or a salt thereof serving as the active ingredient, and pharmaceutical excipients mannitol, pregelatinized starch, corn starch and magnesium stearate. The linagliptin containing pharmaceutical composition provided by the invention reduces the types of excipients, increases the stability of the preparation, reduces the cost of raw materials, and solves the hardness and friability problems of linagliptin tablets by controlling the particle size of the key excipient mannitol. The obtained table has all indicators especially the dissolution rate in line with the drug quality standards, and the process is simple, thus being more suitable for large scale production.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD
A clean production method of medium molecular weight hydroxyethyl starch
The invention provides a clean production method of medium molecular weight hydroxyethyl starch, in particular to a clean production method in the refining process of chemical raw materials hydroxyethyl starch 200 / 0.5 and hydroxyethyl starch 130 / 04 used for plasma expanders. Bacterial endotoxin is the key toxic substance leading to clinical infusion pyrogenic reaction. The bacterial endotoxin in the raw material drug of hydroxyethyl starch exceeds the limit, which can easily lead to the limit of bacterial endotoxin in the final product of the preparation, hydroxyethyl starch injection, and the unqualified rate increases. The method of the invention is controlled by necessary process conditions such as clean air spray drying under clean conditions, microbial control under high temperature conditions, and on-line cleaning and disinfection of equipment, so that the quality of the bacterial endotoxin index of the obtained final product is obviously better than the current national drug quality standards. At the same time, it provides the necessary conditions for the direct preparation method of raw materials without microfiltration, which is helpful for the qualification of the insoluble particle index of the preparation, shortens the process flow of the final preparation, and reduces the probability of pollution.
Owner:WUHAN HUST LIFE SCI & TECH
Compound angelica medicament injection preparation containing polyethylene glycol 12-hydroxystearate and preparation method thereof
InactiveCN101884658AHigh clarityGood for clinical usePowder deliveryAntipyreticPolyethylene glycolHydroxystearic Acid
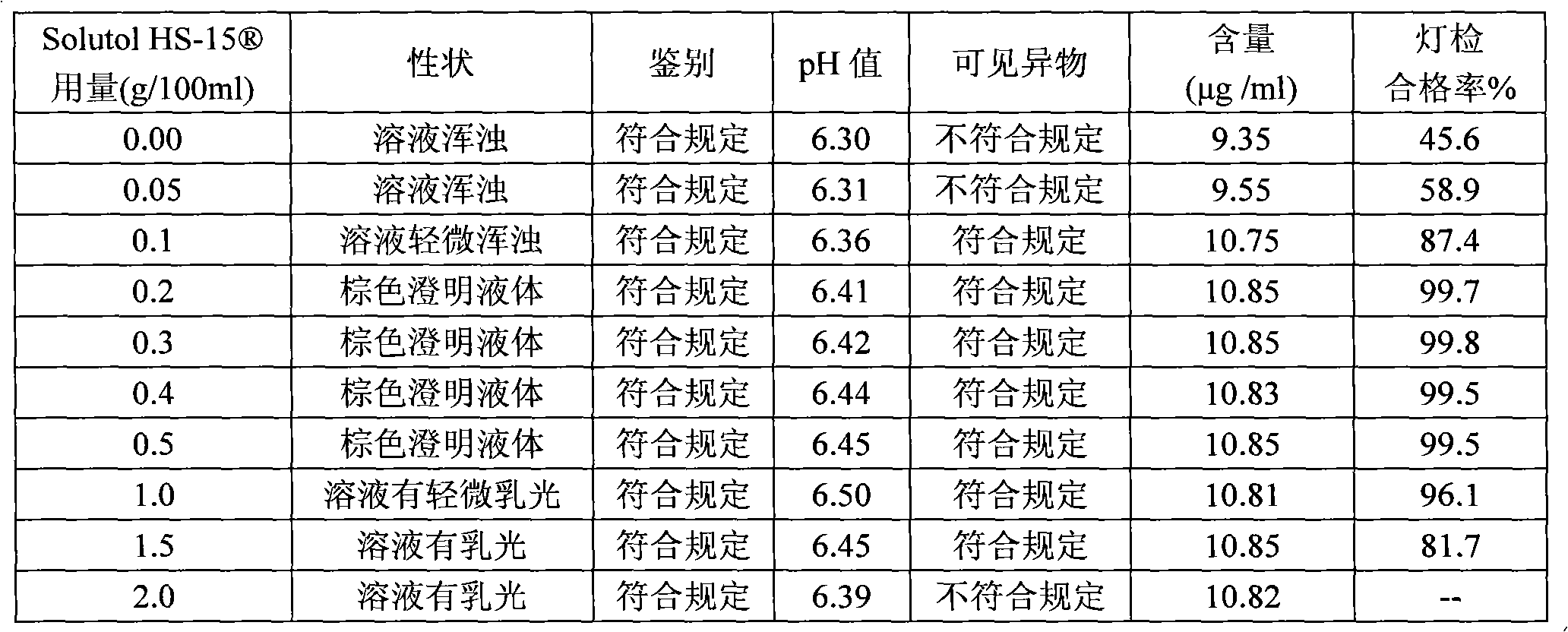
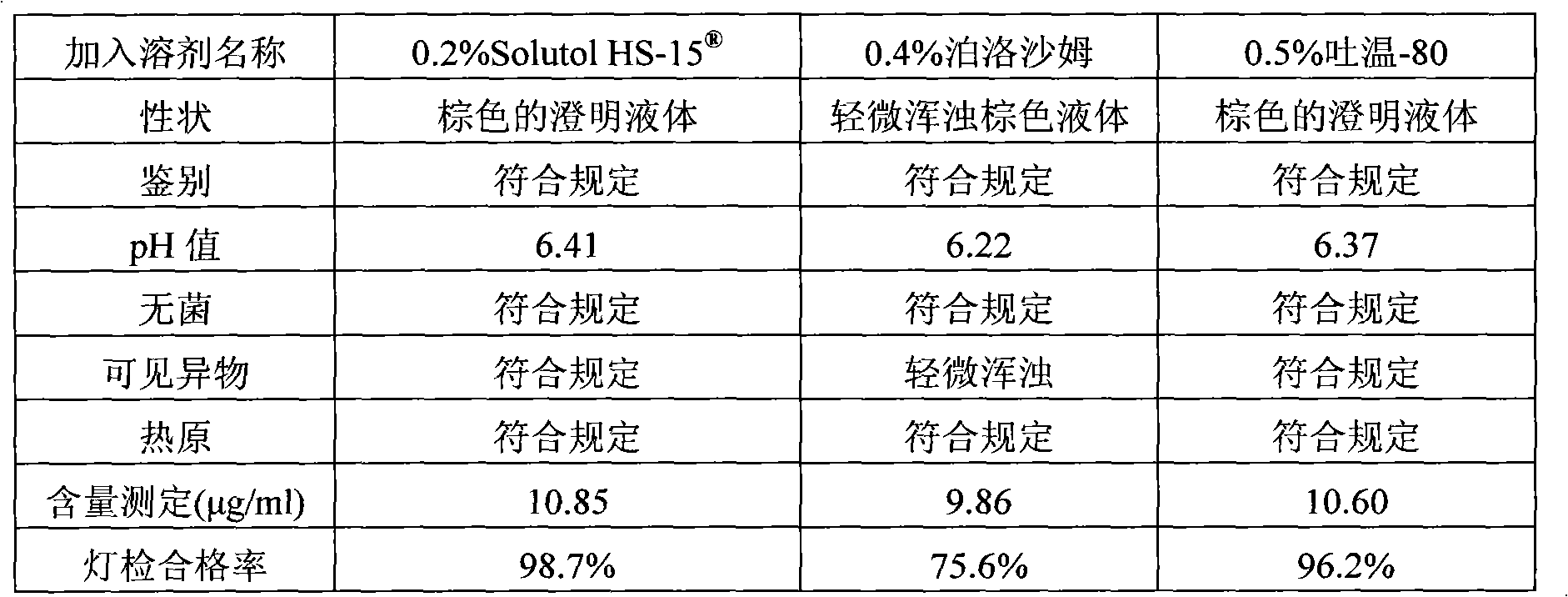
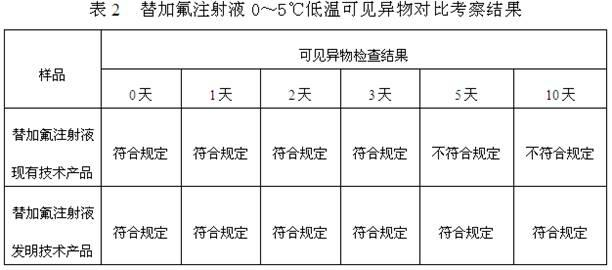
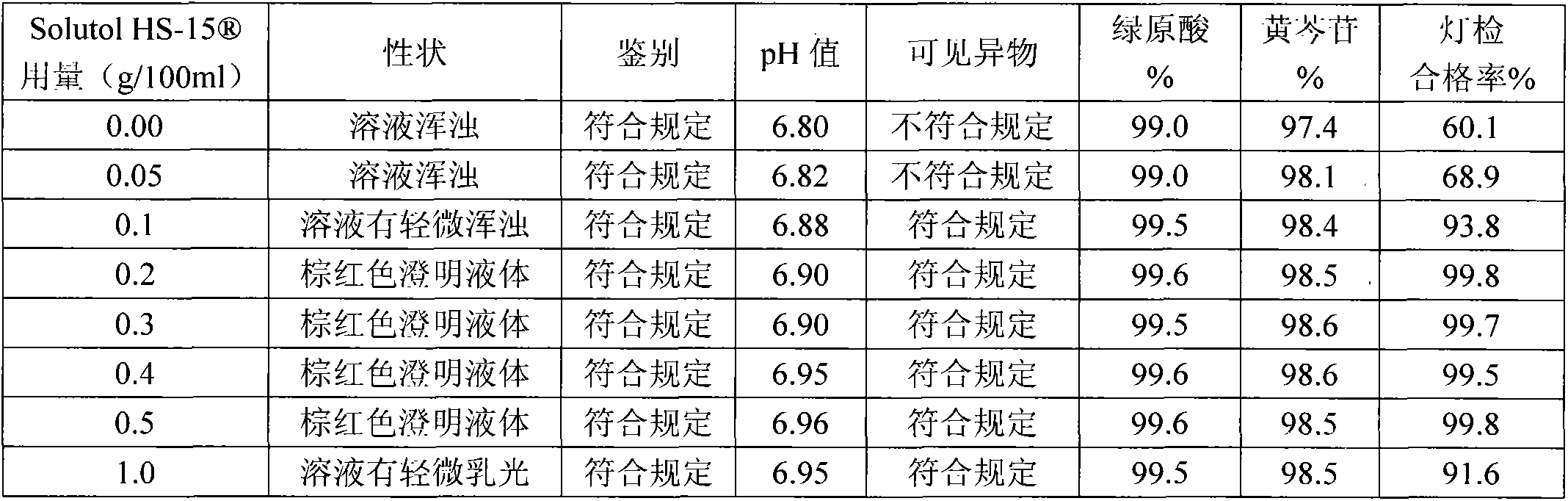
The invention discloses a compound angelica medicament injection preparation containing polyethylene glycol 12-hydroxystearate and a preparation method thereof. The compound angelica medicament injection preparation is the injection medicament mainly prepared by dissolving an angelica extract, a rhizoma chuanxiong extract, a safflower extract and polyethylene glycol 12-hydroxystearate for improving the clarity of the injection in injection water, wherein the using amount of the polyethylene glycol 12-hydroxystearate is 0.1g-1.0g / 100ml. The compound angelica medicament injection preparation of the invention can improve the clarity of compound angelica injection, stably ensure that the detection for visible foreign matters of the injection complies with the medicament quality standard especially under the condition that the compound angelica injection is preserved for a longer time (over 24 months), solve the problems of small white spots, white blocks and turbidity of the compound angelica medicament injection which adopts the conventional cosolvent (Tween-80) and is preserved for a longer time, and ensure that the detection of the visible foreign matters of the injection complies with the medicament quality standard so as to facilitate clinic administration and popularization.
Owner:SICHUAN SUNNYHOPE PHARM CO LTD
Heart-soothing and lipid-lowering tablet medicine quality standard detecting method
ActiveCN101953978ASimple and fast operationEasy to separateMetabolism disorderComponent separationLipid formationMedicine
The invention provides a heart-soothing and lipid-lowering tablet medicine quality standard detecting method. The method comprises a process for identifying giant knotweed in the heart-soothing lipid-lowering tablet medicine, a process for identifying red peony in the heart-soothing lipid-lowering tablet medicine, a process for detecting content of the giant knotweed in the heart-soothing lipid-lowering tablet medicine and the like. In the method, emodin in the giant knotweed and paeoniflorin in the red peony in the heart-soothing lipid-lowering tablet are identified and inspected by thin layer chromatography, so the method has the technical characteristics of simple operation, good separation effect, strong specificity and the like; and content of polydatin in the main medicine giant knotweed in the formula is detected by adopting high performance liquid chromatography (HPLC). The method has the characteristics of good separation effect, accurate, sensitive and quick detection, strong specificity and the like.
Owner:YUNNAN PHYTOPHARML
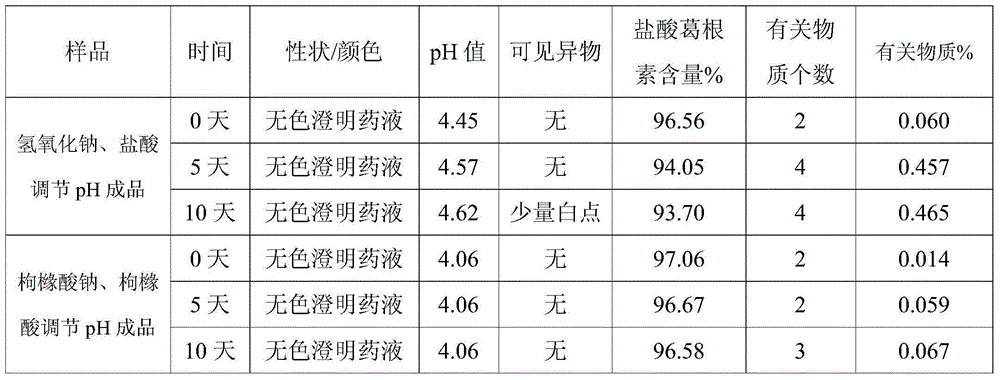
Preparation method of injecting drug improving stability of puerarin drug injection preparation
InactiveCN105125485ADecreased substancesStable pHOrganic active ingredientsNervous disorderDrug injectionMedicine
The invention discloses a preparation method of injecting drug improving the stability of a puerarin drug injection preparation. An injecting drug composition is mainly prepared by dissolving puerarin salt in injecting water, adding citric acid and / or sodium citrate as a pH adjusting agent to adjust the pH value of the medicine liquid, and the dosage of the citric acid and / or sodium citrate is 0.1 to 200 mg / 100ml. By adopting the preparation method, the pH value of the injecting liquid is more stable, puerarin degraded substances are greatly reduced compared with the prior art, under the situation of not utilizing other co-solvent increasing the clinical application risk, the clarity of the puerarin injecting liquid is improved, the problems that the puerarin injecting liquid adopting a product of the prior art has small white points, white blocks and turbidity under the situation that the storage time is relatively long can be solved, the inspection on visible foreign matters of the product is enabled to meet the stipulation of the drug quality standard, and the clinical drug application and popularization are facilitated.
Owner:CHENGDU AIBIKE BIOTECH
Soft capsule used for clearing away heat and toxic materials and preparation method thereof
ActiveCN101711808AEvenly dispersedHigh availability of bioactive ingredientsDigestive systemCapsule deliveryVegetable oilAdditive ingredient
The invention discloses a soft capsule used for clearing away heat and toxic materials and a preparation method thereof. The soft capsule is prepared by adopting a traditional preparation process after mixing and preparing clear extract powder, vegetable oil, soybean phosphatide and beeswax into suspension. The preparation method of the soft capsule comprises the following steps: decocting coptis root, rhubarb and baikal skullcap root, preparing a clear paste, then spraying, drying, preparing coptis root powder, rhubarb powder and baikal skullcap root powder and mixing evenly to obtain clear extract powder; mixing the clear extract powder, vegetable oil, soybean phosphatide and beeswax evenly according to the mixture ratio to prepare suspension; and taking gelatin, glycerol and water to prepare gum and then filling and pressing the suspension into the soft capsule. The invention adopts the natural type vegetable oil and the beeswax as a dispersion medium and a suspending agent respectively, enables the medicines to be evenly dispersed, has high utilization degree of the biologic active components of the medicines, enhances the stability of the medicines and the application safety and effectiveness effectively, enshrouds the discomfortable taste of the medicines, is easy to swallow, shortens the disintegration time limit of the soft capsule and enhances the quality standard of the medicines.
Owner:HAINAN HAISHEN TONGZHOU PHARM CO LTD
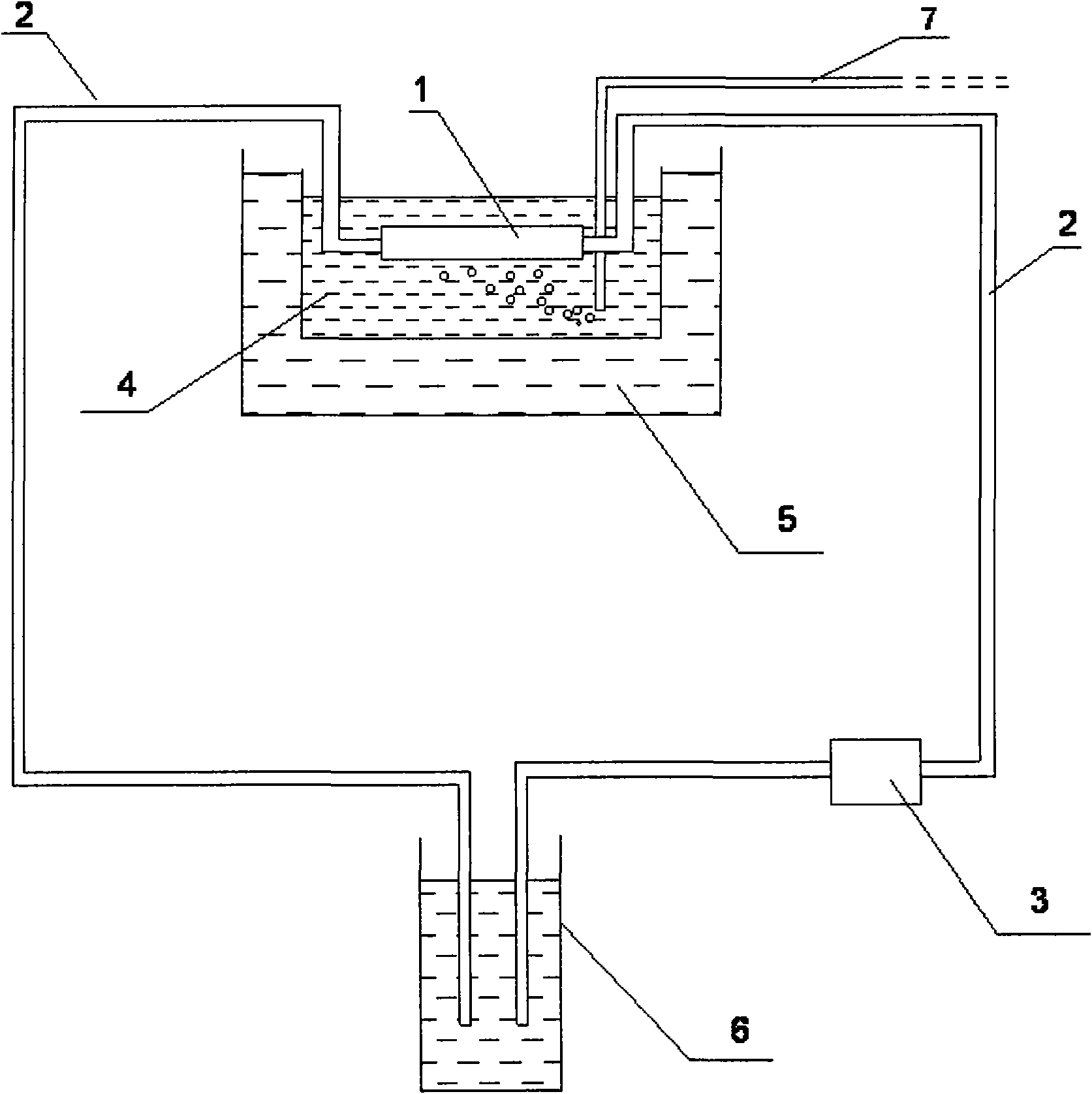
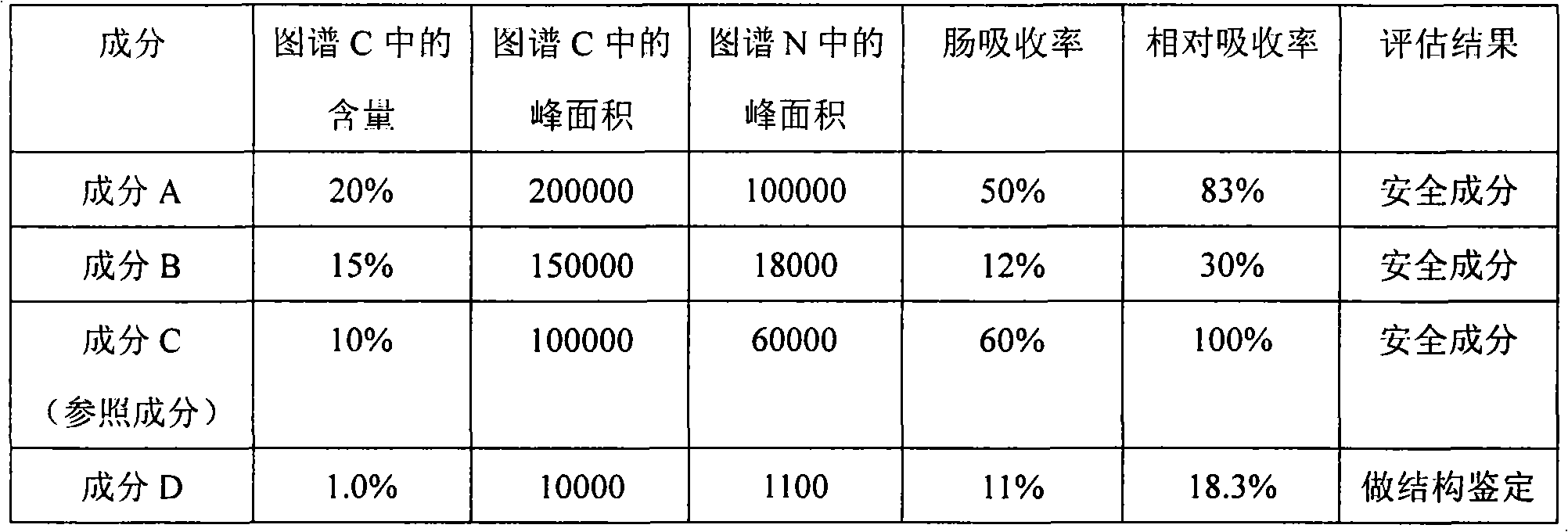
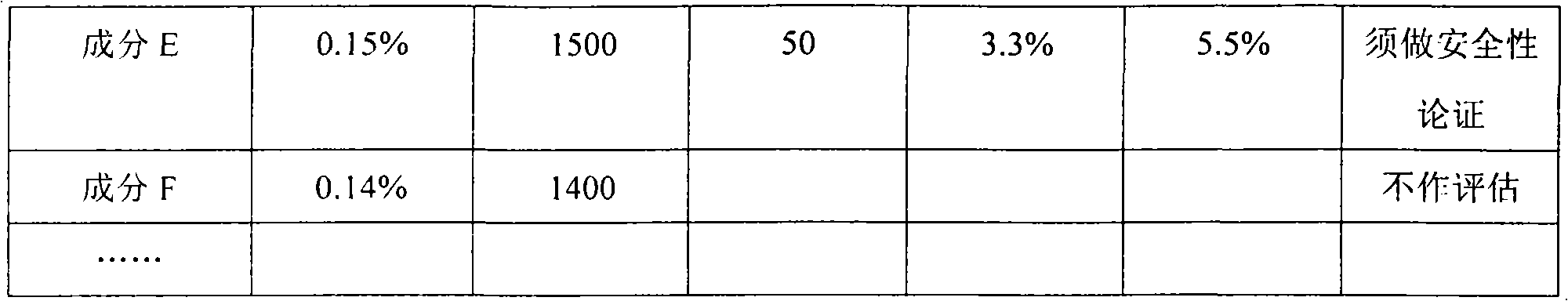
Application of intestinal absorption test in identifying potential unsafe components in Chinese medicine injection
InactiveCN101839902ASimplify workloadSafety and Quality Control StandardsComponent separationTesting medicinal preparationsHplc fingerprintMedicine
The invention discloses application of an intestinal absorption test in identifying potential unsafe components in a Chinese medicine injection. An intestinal absorption test method and a liquid chromatography HPLC (High Performance Liquid Chromatography) fingerprint, and a pharmacokinetic method is firstly applied to a drug-quality standard research. By applying the intestinal absorption test to the security evaluation of the Chinese medicine injection, the application of the intestinal absorption test is helpful to establish a scientific quality control standard which can ensure the safety of the Chinese medicine injection and solve the problems of quality stability and safety of Chinese medicine injection products.
Owner:孙悦平
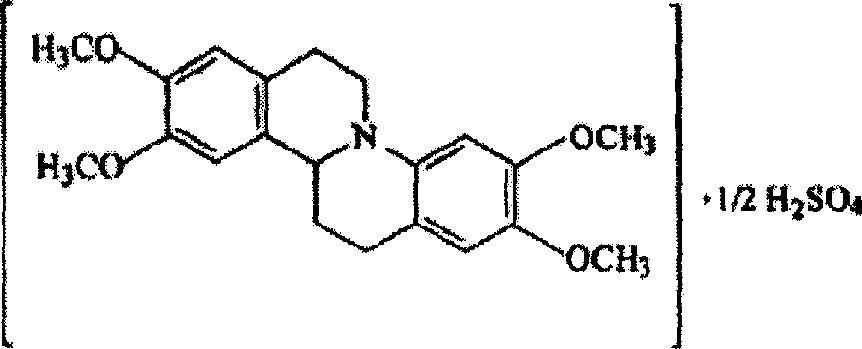
Dripping pills of tetrahydropalmatini sulfas and its preparation process
InactiveCN1729980AHigh rounding rateLittle difference in pellet weightOrganic active ingredientsNervous disorderPolyethylene glycolStearate
Owner:COSCI MED TECH CO LTD
Preparation method of compound amino acid (15) dipeptide (2) injecta
The invention relates to a preparation method of compound amino acid (15) dipeptide (2) injecta, specifically comprising the following steps of: under the whole-course protection of nitrogen, taking water for injection with a certain quantity, sequentially adding glycyl-L-glutamine, glycyl-L-tyrosine and arginine with prescription quantity, stirring and dissolving, clearly dissolving, adding aspartate, glutamic acid, leucine, isoleucine and phenylalanine with prescription quantity which is not less than 60 screen meshes, dissolving and clearly dissolving, adding alanine, histidine, L-lysine monoacetate, methionine, proline, serine, threonine, tryptophan and valine with prescription quantity, stirring and clearly dissolving, adjusting the pH at 5.4-5.8 by citric acid, adding full dose of 0.10% (w / v) of activated carbon, stirring for 30min under the temperature of 60DEG C, fixing the volume to total volume by the water for injection after filtering in a decarbonizing way, sieving by a filter membrane with 0.22 micrometers, filling, charging nitrogen, adding a plug, pricking an aluminum cap, and sterilizing for 8-12min in a hot-press way under the temperature of 121DEG C (F0 value is larger than 8), wherein the quality of the injecta can achieve the quality standard of an imported drug of the finished product of the German Fresenius Corporation.
Owner:北京紫萌医药科技有限公司
Preparation method of stable tegafur injection liquid
ActiveCN103989629AReduce contentGood for clinical useOrganic active ingredientsPharmaceutical delivery mechanismAntioxidantColor changes
The invention discloses a stable tegafur Injection liquid and a preparation method thereof. The stable tegafur Injection liquid is mainly prepared from tegafur and injection water by using a sodium hydroxide solution and / or an acid solution to adjust the injection liquid pH value to be 11.1-12.0, adding an antioxidant and a pH value buffer, charging nitrogen for protection, and then sterilizing at high temperature. The preparation method can make the tegafur Injection liquid more stable, high temperature sterilization can be performed, compared with the prior art, related substances are greatly reduced, especially the problems that pH value is decreasing, the solution color changes into yellow, the visible foreign matter testing is unqualified and the related substance testing is unqualified in high temperature sterilization and storage processes of a tegafur Injection liquid prepared from products in the prior art can be solved, and the method can ensure the product meets the requirement of drug quality standards and facilitates clinical medicine use and promotion.
Owner:SICHUAN SUNNYHOPE PHARM CO LTD
Flavone hippophaes drop pills and method for preparing the same
InactiveCN1729969AImprove accuracyHigh rounding rateOrganic active ingredientsMetabolism disorderDrug contentQuantitative determination
The invention relates to a Culiuhuangtong drip pill composition for treating heart diseases and hyperlipemia, which has the advantages of high biological availability, quick-speed medicine release, quick-speed effect, higher medicinal content, accurate quantitative determination, easy administration, low price, and causing no pollution during production. The drop pill is prepared from polyethylene glycol, polyoxyl (40) stearate as the base material, and Culiuhuangtong as the raw material.
Owner:COSCI MED TECH CO LTD
Preparation method of injection medicine for improving stability of quercetin medicine injection preparation
InactiveCN105963247ADecreased substancesStable pHOrganic active ingredientsMetabolism disorderMedicineTurbidity
The invention discloses an injection medicine composition for improving the stability of a quercetin medicine injection preparation, and a preparation method thereof. The injection medicine composition is an injection medicine composition mainly prepared by dissolving salt of quercetin into injection water and adding citric acid and / or sodium citrate as pH regulators to regulate the pH value of medicine liquid. The consumption of the citric acid and / or sodium citrate is 0.1mg to 200.0mg / 100ml. The injection medicine composition has the advantages that the pH value of the injection liquid is more stable; the quercetin degradation substances are greatly reduced through being compared with that in the prior art; under the condition of not using other solutizers increasing the clinic application risks, the clarity of the quercetin injection liquid is improved; the problems of small white points, white blocks and solution turbidity of a product of the quercetin injection liquid in the prior art under the condition of long storage time are particularly solved; the visible foreign matter inspection of the product is enabled to conform to the specification of the medicine quality standard; the clinic medication and popularization are convenient.
Owner:CHENGDU YICHUANGSI BIOLOGICAL SCI & TECH
Alpha isomer impurity of regadenoson and preparation method and use thereof
ActiveCN105968156AProcess conditions are easy to controlEasy to routeSugar derivativesComponent separationRegadenosonPurine
The invention discloses an alpha isomer impurity of regadenoson and a preparation method and a medicinal use thereof. The impurity is obtained through oriented synthesis; 2,6-dichloropurine and beta-D-1,2,3,5-tetra-o-acetyl-d-ribose are taken as starting raw materials; a salt of alpha 2-chloroadenosine is obtained by concentration, ammonolysis, salifying and purification; the regadenoson alpha isomer impurity is obtained directly through hydrazinolysis, concentration and ammonolysis without dissociation. An isomer is taken as a regadenoson impurity reference substance, so that the content of the impurity produced in synthesis can be effectively identifies, thereby raising the medicine quality standard of regadenoson.
Owner:NANJING HERON PHARMA SCI & TECH CO LTD +1
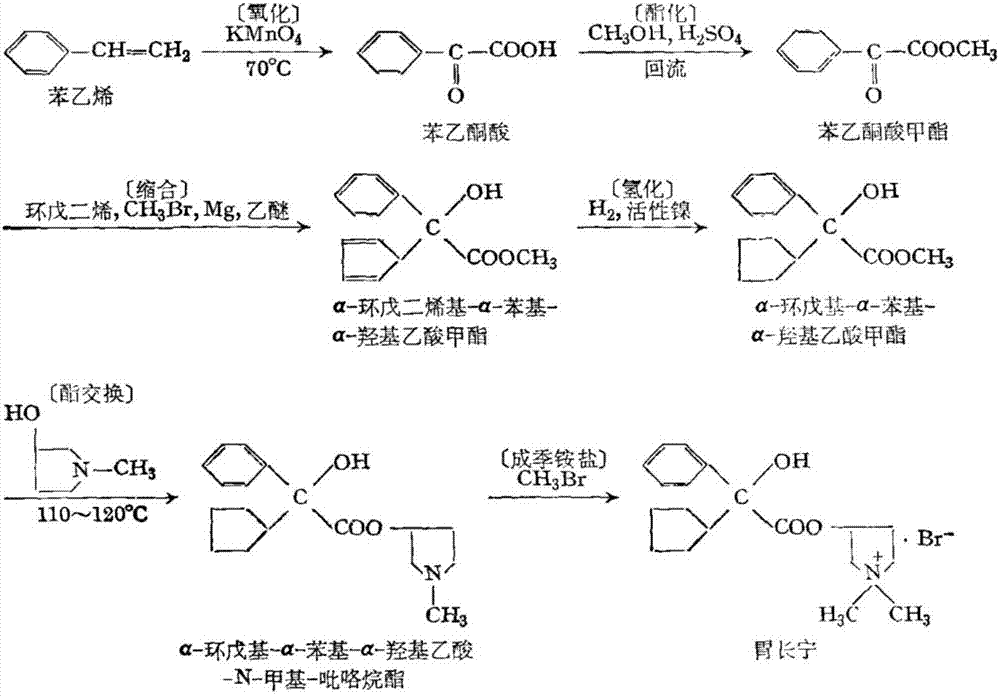
Preparation method of glycopyrronium bromide
The invention discloses a preparation method of glycopyrronium bromide. The preparation method comprises the following steps: firstly, protecting hydroxyl of alpha-cyclopentyl mandelic acid with benzyl; then carrying out esterification with 1-methyl-3-pyrrolidinol by means of a conventional method to obtain a key intermediate ester of pyrrolidinol; carrying out debenzylation on the key intermediate ester under a condition of Pd / C; and finally, carrying out quaterniziation and salification on methyl bromide to separate out a solid, filtering the solid to obtain a coarse product of glycopyrronium bromide, and refining the coarse product to obtain a qualified product of glycopyrronium bromide. In order to prevent side reactions, hydroxyl is introduced with a very low cost to protect benzyl, so that the yield is improved greatly, the post-treatment is reduced, and the wastewater amount is reduced. The method disclosed by the invention has the characteristics of being simple in production operation, low in production cost, easily available in raw material, high in yield, small in pollution and the like. The obtained product meets the drug standard.
Owner:ANHUI DEXINJIA BIOPHARM
Method for carrying out full quality control on Chinese patent drugs by using mixed contrast
InactiveCN101936974ALarge amount of chromatographic informationOvercoming identification bottlenecksComponent separationRetention timeMedicine
The invention relates to a method for identifying on Chinese patent drugs by using mixed contrast, which comprises the steps of: 1, obtaining a supply sample chromatogram map; 2, obtaining a mixed contrast chromatogram map; and 3, comparing whether retention times of chromatographic peaks in the supply sample chromatogram map and the mixed contrast chromatogram map are consistent or not. The invention has the advantages of identifying drug tastes of the Chinese patent drugs by using a supply sample contrast mode under the same chromatogram condition, overcoming the bottleneck problem of identifying the drugs only by the conventional Chinese patent drug quality standard, controlling the integral quality of the drugs, and greatly saving detection time and cost.
Owner:河北省药品检验所
Preparing method and product of mile swertia herb dripping pill
InactiveCN1857391AAccurate dosageNo pollution in the processDigestive systemAntiviralsHepatitisDrug product
The present invention discloses hepatitis treating orally taken medicine preparation, and is especially preparation process of mile swertia herb dripping pill for treating hepatitis. The mile swertia herb dripping pill has fast dissolving and high bioavailability and the production process has no environmental pollution.
Owner:COSCI MED TECH CO LTD
Preparation method for stable composition of breviscapine drug injection preparation
InactiveCN106214629ADecreased substancesStable pHOrganic active ingredientsPharmaceutical delivery mechanismActivated carbonDrug injection
The invention discloses a preparation method for a stable composition of a breviscapine drug injection preparation. The preparation method comprises the following steps that 1, breviscapine, malic acid and / or sodium malate are weighed; 2, a malic acid solution and a sodium malate solution are prepared separately; 3, breviscapine is added into 500 ml of water for injection at the temperature of 30 DEG C to 40 DEG C, after stirring is conducted till the breviscapine is completely dissolved, activated carbon is added, stirring is conducted, and filtering is conducted for decarburization; 4, the pH value of the obtained filtrate is regulated to be 4.0 to 6.0 with the prepared malic acid solution and / or sodium malate solution, and the water for injection is added till the volume is 1,000 ml; 5, filtering is conducted till the filtrate is clarified, filling and sterilizing are conducted, and then the stable composition is obtained. According to the preparation method, the pH value of the injection can be stabler, breviscapine degrading substances are greatly decreased compared with the prior art, the clarification degree of the breviscapine injection is improved on the condition that usage of other cosolvents increasing clinical application risks is avoided, it can be guaranteed that visible foreign matter inspection accords with provisions of drug quality standards, and clinical medication and popularization are convenient.
Owner:成都佳迪璐莎生物科技有限公司
Method for simultaneous detection of hydroxychloroquine side chains, raw materials and intermediates by gas chromatography
ActiveCN107894474AMake sure it's scientificDetermine accuracyComponent separationSide chainLinearity
The invention belongs to the technical field of drug analysis and particularly relates to a method for simultaneous detection of hydroxychloroquine side chains, raw materials and intermediates by gaschromatography. The method can simultaneously determine the hydroxyl side chains, ethylamine, xylene, ethylamine ethanol, chloropentanone, ethylamine diethanolamine, amino pentanone and ethanol, and the specificity, linearity, range, precision, detection line, and accuracy are in line with the requirements of the verification guiding principles for quality standard analysis methods of traditionalChinese medicines in Chinese Pharmacopoeia 2015 Edition Volume IV. The method aims to provide a technical basis for the detection and monitoring of the synthesis process of the hydroxychloroquine sidechains.
Owner:SHANGHAI INST OF TECH
Immune balancing energy restoring pill and its manufacturing method
InactiveCN1559545ASolve medical expensesReasonable compositionUnknown materialsPill deliveryCordycepsMedicine
Owner:张土荣
Preparation method of drug composition for improving stability of Shengmai drug injection preparation
InactiveCN106361969AStable pHDecreased ginseng degraded substancesPharmaceutical delivery mechanismPharmaceutical non-active ingredientsDrug injectionCLARITY
The invention discloses a preparation method of a drug composition for improving the stability of a Shengmai drug injection preparation. The drug composition for injection is mainly prepared by dissolving Shengmai salt in water for injection and adding oxalic acid and / or sodium oxalate to serve as a pH regulator for regulating a pH value of medicinal liquid. The usage amount of the oxalic acid and / or sodium oxalate is 1-100.0 mg / 100ml. The pH value of the injection can be stable, substance degradability of the Shengmai is greatly reduced compared with the prior art, the clarity of the Shengmai injection is improved under the situation that other cosolvents increasing clinical application risks are not used, especially the problem that the Shengmai injection adopting a product in the prior art produces small white points, white blocks and is turbid under the condition of long storage time is solved, and it can be ensured that visible foreign matter inspection of the product meets the stipulations of the drug quality standard and the drug composition is convenient to clinically apply and popularize.
Owner:CHENGDU XIANXIANXIAN BIOTECH CO LTD
Injecting medicine composition for improving stability of ginsenoside medicine injection
InactiveCN105999278ADecreased substancesStable pHPharmaceutical delivery mechanismPharmaceutical non-active ingredientsMedicineCLARITY
The invention discloses an injecting medicine composition for improving the stability of a ginsenoside medicine injection and a preparation method thereof. The preparation method mainly comprises the following steps: dissolving salt of ginsenoside in injecting water; adding citric acid and / or sodium citrate to serve as a pH regulator, and regulating the pH value of the medicinal liquid, wherein the dosage of the citric acid and / or sodium citrate is 0.1-200.0mg / 100ml. By adopting the preparation method, the pH value of the injection can be stable, the ginsenoside degraded substances are greatly reduced in comparison with that in the prior art, the clarity of ginsenoside injection is improved under a condition that other cosolvent capable of increasing clinical application risks is prevented from being used, and the problems that the ginsenoside injection has small spots, white blocks and turbid solution by adopting the prior art under a condition of long storage period can be especially solved, obviously foreign matter inspection of the injection accords with the specification of medicine quality standard, and clinical medication and popularization can be benefited.
Owner:成都市斯贝佳科技有限公司
Stable Tegafur injection and preparation method thereof
ActiveCN102178646AReduce contentGood for clinical useOrganic active ingredientsInorganic non-active ingredientsAntioxidantNitrogen
The invention discloses stable Tegafur injection and a preparation method thereof. The stable Tegafur injection is prepared mainly by the following steps of: mixing Tegafur and water for injection; adjusting the pH value to be 10.5 to 12.0 by using sodium hydroxide solution and / or acidic solution; adding an antioxidant and a pH value buffering agent and introducing nitrogen to perform protection; and performing high-temperature sterilization to prepare the injection. By the method, the Tegafur injection can be more stable; high-temperature sterilization can be performed; relative substances are greatly reduced compared with those in the prior art; particularly, the problems that the pH value is reduced, the color of the solution is changed to yellow, visible foreign matters are detected to be unqualified and relative substances are detected to be unqualified in the high-temperature sterilization and storage process of the Tegafur injection adopting the products in the prior art are solved; and the stable Tegafur injection can guarantee that the products meet the rule of the medicament quality standard and contributes to clinic administration and popularization.
Owner:SICHUAN SUNNYHOPE PHARM CO LTD
A kind of Shuxin Jiangzhi Tablet and its preparation method
The invention provides a heart nourishing and lipid lowering tablet and a preparation method thereof. Through changing accessory formula in an original production process, stability of the heart nourishing and lipid lowering tablet is effectively improved, and quality problems of the heart nourishing and lipid lowering tablet which is easy to absorb moisture and easy to get cracked are solved. Raw material and accessory formula of the production process consists of 20-75% of a dry paste of heart nourishing and lipid lowering tablet, 7-15% of pollen, 10-30% of microcrystalline cellulose, 2-7% of calcium sulfate, 1-5% of dalbergia wood volatile oil, 4.5-20% of carboxymethyl starch sodium and the balance of a lubricating agent. The heart nourishing and lipid lowering tablet prepared by the invention is smooth in surface, stable in performance and suitable for mass production; product quality meets national drug standard requirements.
Owner:YUNNAN PHYTOPHARML
Preparation method of houttuyfonate injection preparation pharmaceutical composition
InactiveCN107753423ADecreased substancesStable pHAntibacterial agentsAntipyreticCLARITYInjection product
The invention discloses a preparation method of a houttuyfonate injection preparation pharmaceutical composition. The pharmaceutical composition for injection is a pharmaceutical composition for injection, which is prepared by dissolving salt of houttuyfonate into water for injection, adding tartaric acid and / or sodium tartrate as a pH regulator and regulating the pH value of medicine liquid, wherein the dosage of the tartaric acid and / or the sodium tartrate is 0.1mg / 100ml to 200.0mg / 100ml. By adopting the preparation method of the houttuyfonate injection preparation pharmaceutical composition, the pH value of injection liquid is more stable; the content of houttuyfonate degraded substances is greatly reduced as compared with that in the prior art,; under the condition that other co-solvents which increase the clinical application risk are not used, the clarity of houttuyfonate injection is improved; and the problems that a houttuyfonate injection product in the prior art has small white spots and white blocks and a solution is turbid under the condition that the product is stored for relatively long time are especially solved, the checking of visible foreign matters of the productcan meet the regulations of drug quality standards and the houttuyfonate injection preparation pharmaceutical composition is convenient for clinical medication and popularization.
Owner:CHENGDU XIANXIANXIAN BIOTECH CO LTD
Honeysuckle and baical skullcap root pharmaceutical preparation containing polyethylene glycol 12-hydroxy stearate and preparation method thereof
InactiveCN103432201AHigh clarityGood for clinical usePowder deliveryAntipyreticMedicineBaical Skullcap Root
The invention discloses a honeysuckle and baical skullcap root pharmaceutical preparation containing polyethylene glycol 12-hydroxy stearate and a preparation method thereof. The honeysuckle and baical skullcap root pharmaceutical preparation is a drug for injection prepared by dissolving a honeysuckle extract, a baical skullcap root extract and polyethylene glycol 12-hydroxy stearate which is used for improving the limpid degree of an injection liquid into water for injection, wherein the amount of polyethylene glycol 12-hydroxy stearate is 0.1 g-1.0 g / 100ml. Under the condition that the honeysuckle and baical skullcap root pharmaceutical preparation is stored for a long time (more than 24 months), the limpid degree of the honeysuckle and baical skullcap root pharmaceutical preparation can be improved, the detection of visible foreign matters of the injection liquid can be stably kept to meet stipulation of drug quality standards, the problems that the injection liquid has a small number of small white dots, white blocks and solution turbidness which are occurring under the condition that a honeysuckle and baical skullcap root pharmaceutical preparation adopts a conventional cosolvent (Twain-80) and is stored for a long time are solved, the detection of the visible foreign matters of the product can be ensured to meet the stipulation of the drug quality standards, and convenience is brought to clinical administration and popularization.
Owner:SICHUAN SUNNYHOPE PHARM CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com