Preparation method of drug composition for improving stability of Shengmai drug injection preparation
An injection preparation and stability technology, which is applied in the field of preparation of pharmaceutical compositions to improve the stability of Shenmai drug injection preparations, can solve the problems of easy rancidity, high risk of clinical application of injections, inconvenience in clinical medication and promotion, etc., and achieve pH value Stable, convenient for clinical use and promotion, and the effect of reducing degradation substances of ginseng and wheat
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] A method for preparing a pharmaceutical composition for improving the stability of Shenmai drug injection preparations, comprising the following steps: (1) weighing 0.1 g to 100 g of raw materials based on Shen Mai, 9.0 g of sodium chloride, and 1 mg to 2.0 g of oxalic acid , Sodium oxalate 1mg~2.0g; (2) Oxalic acid and sodium oxalate are prepared into 10%~20% solutions respectively, and set aside. (3) Add to 500ml of water for injection below 40°C, stir until completely dissolved, then add 0.02% (g / ml) activated carbon, stir for 15 minutes, filter and decarburize. (4) Adjust the pH value of the filtrate to 3.0-7.0 with oxalic acid or sodium oxalate solution, add water for injection below 40°C to 1000ml; (5) filter the medicinal solution until it is clear, fill it, and sterilize it.
[0026] The specific components and contents thereof of the present embodiment are as follows:
[0027]
[0028] Oxalic acid and sodium oxalate were prepared into 10% to 20% solutions r...
Embodiment 2
[0030] Alternatively, the above pharmaceutical composition for injection that improves the stability of the Shenmai drug injection preparation is prepared according to the following steps:
[0031] (1) Weigh 0.1g-100g of the raw material medicine, 1mg-2.0g of oxalic acid, and 1mg-2.0g of sodium oxalate; (2) Prepare 10%-20% solutions of oxalic acid and sodium oxalate respectively for later use. (3) Add to 500ml of water for injection below 40°C, stir until completely dissolved, then add 0.02% (g / ml) activated carbon, stir for 15 minutes, filter and decarburize. (4) Adjust the pH value of the filtrate to 3.0-7.0 with oxalic acid or sodium oxalate solution, add water for injection below 40°C to 1000ml; (5) filter the medicinal solution until it is clear, fill it, and sterilize it.
[0032] The specific components and contents thereof of the present embodiment are as follows:
[0033] ginseng hydrochloride 20g
[0034] Oxalic acid 1.0g
[0035] Sodium oxalate 2.0g
[0036] Oxa...
Embodiment 3
[0038] Comparative Test of Stability of Shenmai Sodium Chloride Injection
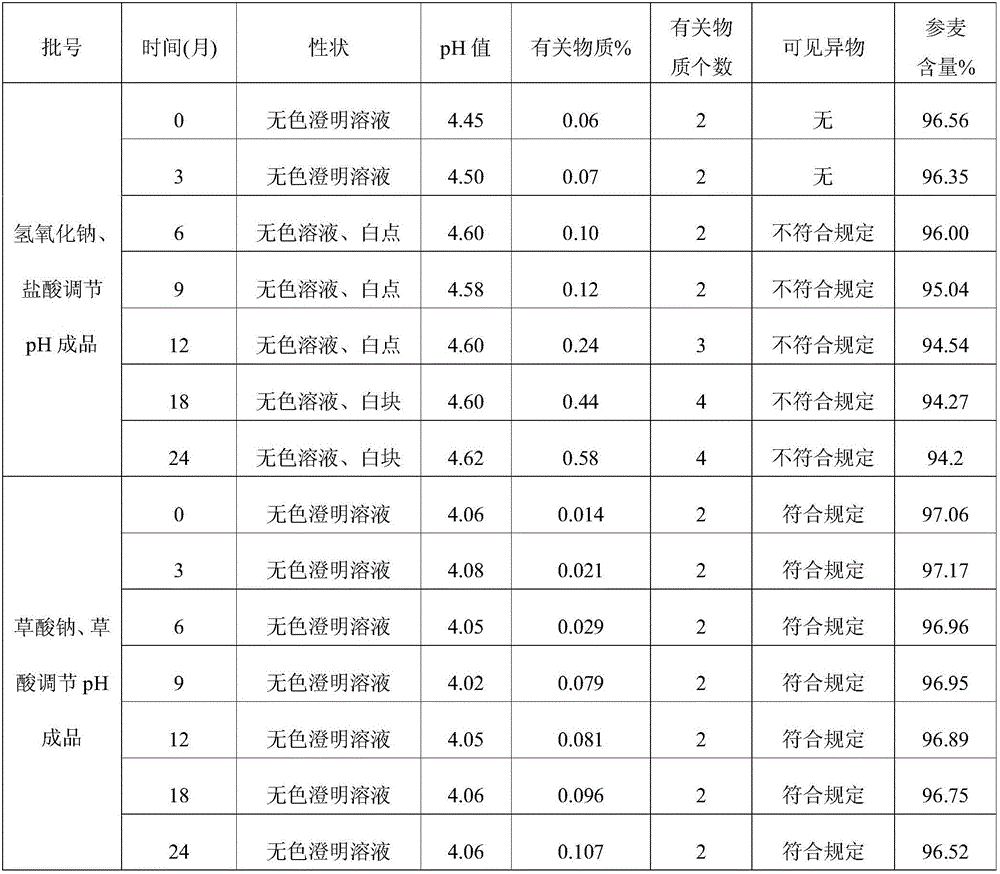
[0039] The detection of visible foreign matter in the Shenmai sodium chloride injection prepared by the present invention complies with the provisions of the drug quality standard, and the stability of the solution is very good. In the case of avoiding the use of other co-solvents that increase the risk of clinical application, the problem of Shenmai sodium chloride injection is solved. Sodium chloride injection is prone to problems such as small white spots, white lumps, and cloudy solution during storage. The Shenmai Sodium Chloride Injection prepared by utilizing the present invention was investigated respectively in 24 months at 25°C and 6 at 40°C according to the relevant requirements of the Chinese Pharmacopoeia 2005 edition two appendix XIXC pharmaceutical preparation stability test guidelines. The stability of the drug can be obtained by placing it at 60°C for 10 days, and at 0-5°C for 20 days....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com