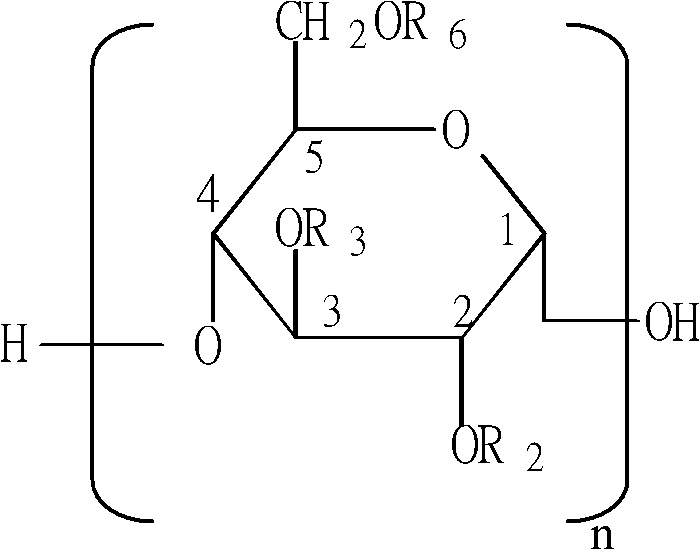
A clean production method of medium molecular weight hydroxyethyl starch
A technology of hydroxyethyl starch and production method, which is applied in the field of medicine and chemical industry, can solve the problems of by-product generation and unsuitable hydroxyethyl starch raw material medicine, etc., and achieve the effects of reducing the probability of pollution, facilitating the production of preparations, and shortening the process flow
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] 100Kg of 15% crude product solution containing hydroxyethyl starch 130 / 0.4 is processed by a hollow fiber ultra-micro-membrane composed of 304-type pipeline valves. The time is 5.5h, and the net wind is spray-dried, and the final product is 70Kg.
[0058] The resulting product is lower than the bacterial endotoxin limit value 1.25EU / g, and the product solution (5%) is transparent and colorless, reaching the Chinese Pharmacopoeia 2010 edition No. 1 turbidity liquid standard, and the absorbance at 400nm is 0.012, which is better than the national drug quality standard (bacterial endotoxin Toxin limit 5EU / g, No. 1 turbidity solution, absorbance at 400nm 0.05). Other indicators are in line with pharmaceutical standards.
Embodiment 2
[0060] The 20% crude product solution containing 100Kg hydroxyethyl starch 200 / 0.5 is decolorized by medicinal charcoal, kept at 80°C, then filtered through a 0.8 micron microporous filter element, and then passed through a ceramic membrane ultrafiltration membrane composed of 304 stainless steel pipe valves Treatment, the solvent added during the treatment is purified water (endotoxin limit 2.5EU / ml), so far, the treatment time is 4.5h, spray-dried with clean air, and the final product is 73Kg. The above process was repeated twice, and the equipment was cleaned and sterilized on-line before each implementation, and 85Kg and 66Kg of final products were obtained respectively.
[0061] The obtained three batches of final products are lower than the bacterial endotoxin limit of 2.5EU / g, and the product solution (5%) is transparent and light in color, reaching the standard of No. 2 turbidity liquid in the 2010 edition of the Chinese Pharmacopoeia, and the absorbance at 400nm is 0.0...
Embodiment 3
[0063] The 20% crude product solution containing 100Kg hydroxyethyl starch 200 / 0.5 is decolorized by medicinal charcoal, kept at 80°C, filtered through a 0.8 micron microporous filter element, and then treated by a ceramic ultrafiltration membrane composed of 304 stainless steel pipe valves At the same time, a 0.45-micron microporous filter element filtration system is connected in parallel. The solvent added in the process is water for injection (endotoxin limit 1.25EU / ml). So far, the treatment time is 4.5h, and the net air spray is dried, and the final product is 73Kg.
[0064] The final product is lower than the bacterial endotoxin limit of 1.25EU / g, and the product solution (5%) is transparent and light in color, reaching the standard of No. 1 turbidity liquid in the Chinese Pharmacopoeia 2010 edition, and the absorbance at 400nm is 0.010, reaching the national drug quality standard (bacterial endotoxin The limit value is 8EU / g, No. 2 turbidity solution, the absorbance at ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com