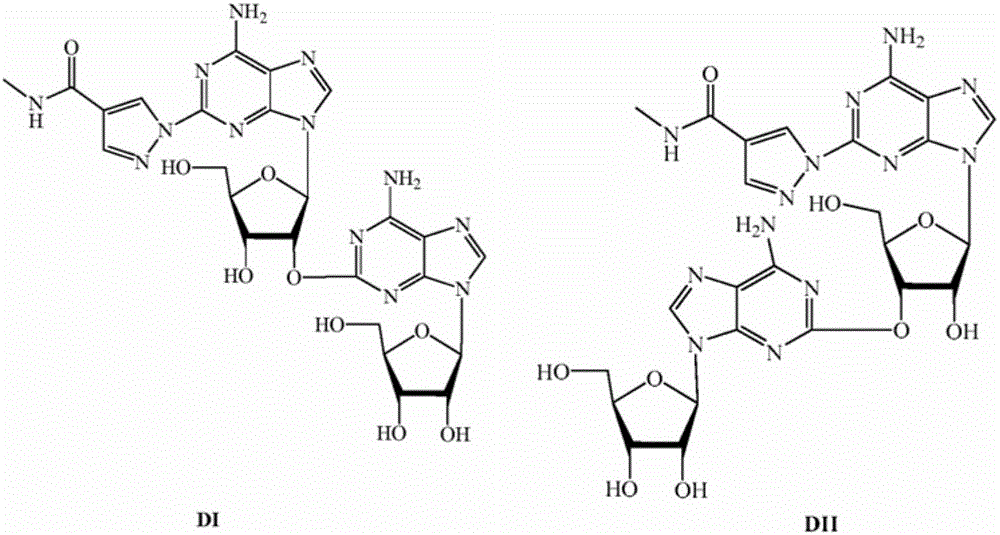
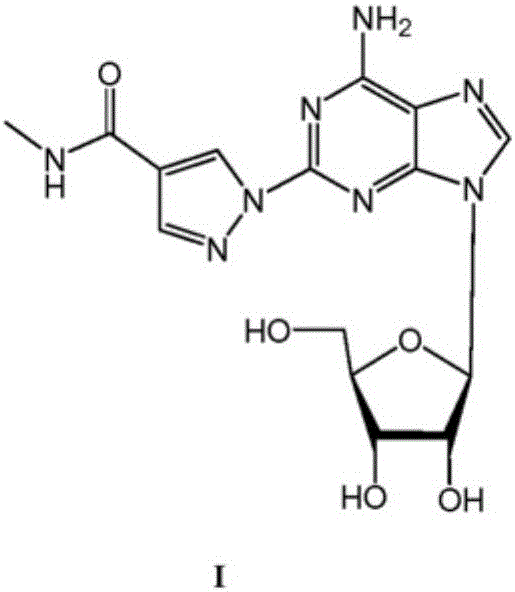
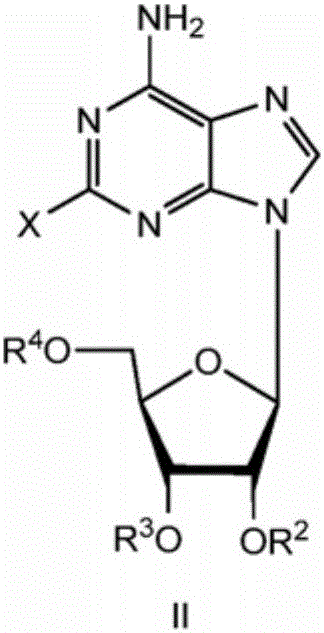
A process for the preparation of regadenoson
A technology of regardson and compound, applied in the field of preparing regardson, can solve the problems of difficult removal, metal pollution of regardson, etc., and achieve the effect of low toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
[0075] Embodiment 2 prepares Regadeson with formula IIb compound
[0076] 2-Chloro-2',3'-O-isopropylidene-adenosine (IIb) was prepared according to Scheme 2.
[0077] Process 2
[0078]
[0079] in N 2(g) 2-chloroadenosine (IIa, 20g, 66.3mmol), 2,2-dimethoxypropane (DMOP, 60mL) and HClO 4 The aqueous solution (70 wt%, 3 mL) was stirred at room temperature for 8 h. with NaHCO 3 Saturated aqueous solution (about 120 mL) slowly adjusted the pH of the reaction mixture to 7-9. After stirring in an ice bath for 2 h, the mixture was filtered, washed with water (50 mL) and then dried under vacuum at 50° C. for 6 h to afford the compound of formula lib with 99% purity in 88% yield.
[0080] 1 H NMR (400MHz, DMSO-d6) δ8.36(s, 1H), δ7.87(s, 2H), δ6.06(d, J=2.4Hz, 1H), δ5.28(dd, J=6Hz ,2.4Hz,1H),δ5.08(t,J=5.6Hz,1H),δ4.94(dd,J=6Hz,2Hz,1H),δ4.21(m,1H),δ3.54(m ,2H), δ1.54(s,3H), δ1.33(s,3H).
[0081] 13 C NMR (100 MHz, DMSO-d6) δ 157.3, 153.6, 150.4, 140.4, 118.6, 113.6, 89.8,...
Embodiment 3
[0092] Embodiment 3 prepares Regadeson with formula IId compound
[0093] 2-Chloro-2',3'-O-cyclohexylidene-adenosine (IId) was prepared according to Scheme 5.
[0094] Process 5
[0095]
[0096] in N 2(g) 2-chloroadenosine (IIa, 5g, 16.58mmol), cyclohexanone (50mL) and HClO 4 (70 wt%, 0.75 mL) was stirred at room temperature for 24 h. The pH of the reaction mixture was slowly adjusted to 7-9 with 6N KOH (3 mL) and diluted with water (100 mL). The aqueous phase was extracted twice with DCM (50 mL x 2). The combined organic phases were washed with water (100 mL) and then washed with anhydrous Na 2 SO 4dry. The separated dry organic phase was evaporated to dryness under vacuum at 50°C. The residue was passed through a silica gel column to obtain the compound of formula IId with 99% purity in 51% yield.
[0097] 1 H NMR (400MHz, CDCl 3 )δ7.82(s,1H),5.97(s,2H),5.81(d,J=5.0Hz,1H),5.40(dd,J=11.6,2.0Hz,1H),5.20(t,J=5.4 Hz,1H),5.10(dd,J=5.9,1.1Hz,1H),4.53(s,1H),4.00(d...
Embodiment 4
[0109] Embodiment 4 prepares Regadeson with the compound of formula IIe
[0110] 2-Chloro-2',3'-O-isopropylidene-5'-O-(2-methoxy-propan-2-yl)-adenosine (IIe) was prepared according to Scheme 8.
[0111] Process 8
[0112]
[0113] in N 2(g) , the compound of formula IIb (5 g, 16.58 mmol), 2,2-dimethoxypropane (DMOP, 25 mL) and TfOH (0.28 g) were stirred at reflux temperature for 3 h. with Et 3 The reaction was quenched with N (1 mL) and evaporated to dryness under vacuum at 50 °C. The residue was suspended in EtOAc / toluene (20 mL) in a ratio of 1:1 (v / v). The isolated precipitate was passed through a silica gel column to afford Ile with 98% purity in 35% yield.
[0114] 1 H NMR (400MHz, DMSO-d6) δ8.32(s, 1H), 7.85(s, 2H), 6.12(s, 1H), 5.39(dd, J=6.1, 2.2Hz, 1H), 4.97(dd, J=6.1, 2.9Hz, 1H), 4.31(td, J=5.2, 3.0Hz, 1H), 3.56-3.39(m, 2H), 2.95(s, 3H), 1.54(s, 3H), 1.34(s ,3H), 1.20(s,3H), 1.17(s,3H).
[0115] 13 C NMR (100 MHz, DMSO-d6) δ 157.3, 153.6, 150.3, 140.6,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com