Regadenoson purifying method and new crystal form of regadenoson
A technology of regadeson, purification method, applied in the field of preparation of pharmaceutical compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] Example 1 Purification of Regadesone Crude Product and Preparation of Form I
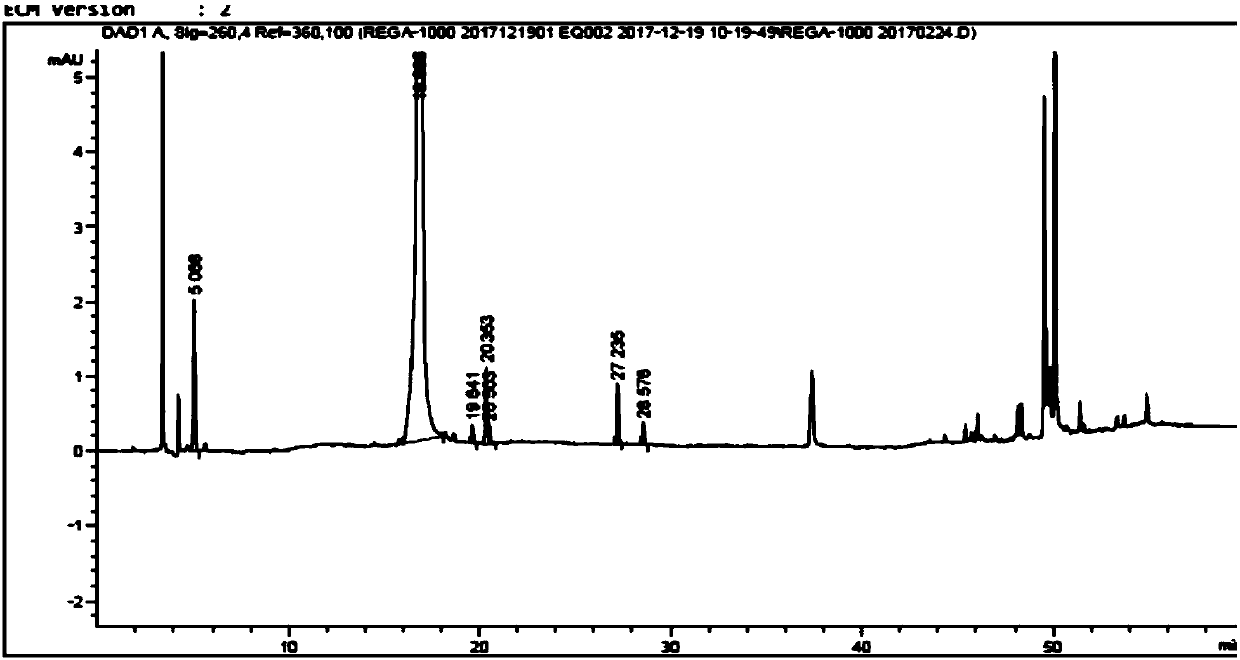
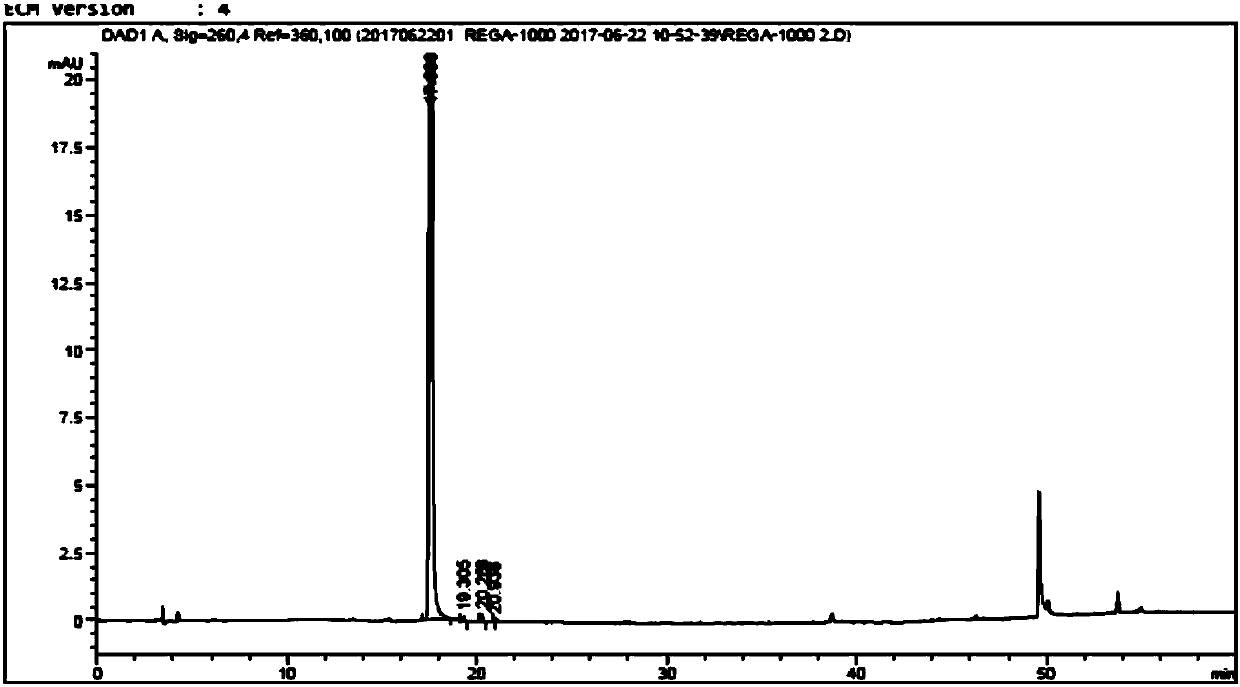
[0070] Add purified water (3450mL) and dehydrated ethanol (3450mL) in the reaction bottle, stir, add the Regardson crude product (115.0g, purity sees attached figure 1 ), heated until the solid dissolved. Then slowly lower the temperature and continue to stir and crystallize for 1-2 hours. After filtering, the filter cake was rinsed with absolute ethanol, and the filter cake was vacuum-dried (50°C, 0.1M Pa) to constant weight. 96.4g of pure product of Deregardson, yield: 83.8%, purity 99.9% (HPLC, impurity of formula 6 is less than 0.1%, see attached figure 2 ).
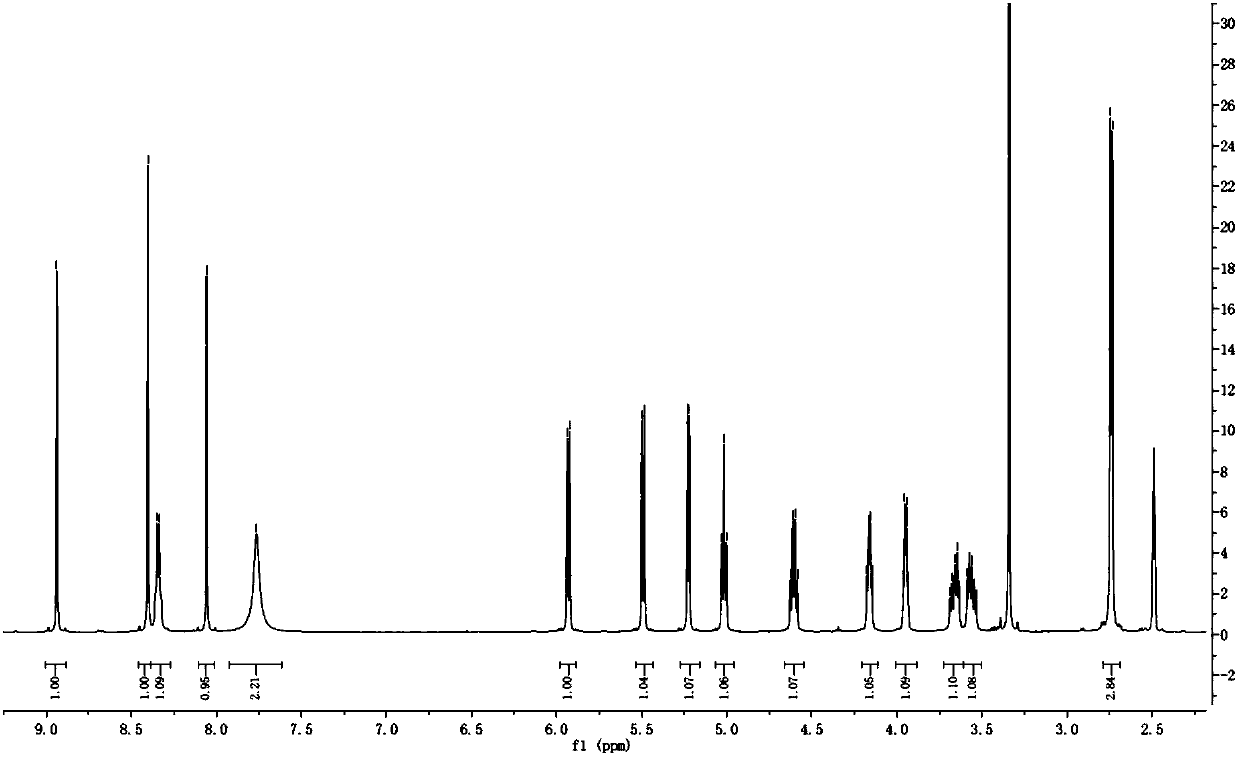
[0071] The structure of the substance is 1 Confirmation by HNMR (see attached image 3 ). Thermal analysis (see attached Figure 4 ) The result proves that the substance contains a part of water, which is a monohydrate.
[0072] X-ray powder diffraction tests were also carried out, see attached Figure 5 The spectrogram da...
Embodiment 2
[0074] Example 2 Purification of Regadesone Crude Product and Preparation of Form I
[0075] Add DMF (75ml) and crude Regadeson (5g) to the reaction flask, stir and heat to 70-80°C to dissolve, add water (150ml) preheated to 80°C, then cool to room temperature, stir and crystallize 1- After 2 hours, filter, rinse the filter cake with absolute ethanol, and vacuum-dry the filter cake (50°C, 0.1M Pa) to constant weight. Obtained 4.3g of pure Regadeson with a yield of 86.0% and a purity of 99.95%.
Embodiment 3
[0076] Example 3 Purification of Regadesone Crude Product and Preparation of Form I
[0077] Add DMF (75ml) and crude Regadeson (5g) to the reaction flask, stir and heat to 70-80°C to dissolve, add water (225ml) preheated to 80°C, then cool to room temperature, stir and crystallize 1- After 2 hours, filter, rinse the filter cake with absolute ethanol, and vacuum-dry the filter cake (50°C, 0.1M Pa) to constant weight. Obtained 4.4g of pure Regadeson with a yield of 88.0% and a purity of 99.98%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com