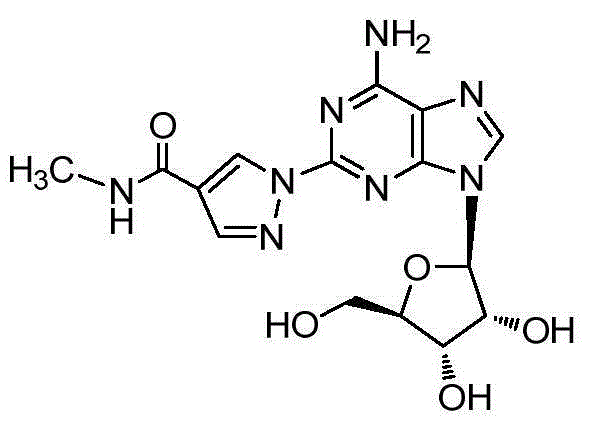
Regadenoson purification method
A technology of regardson and purification method, applied in organic chemistry and other directions, can solve the problems of poor quality, high content of impurity A, inability to obtain, etc., and achieve the effect of good product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
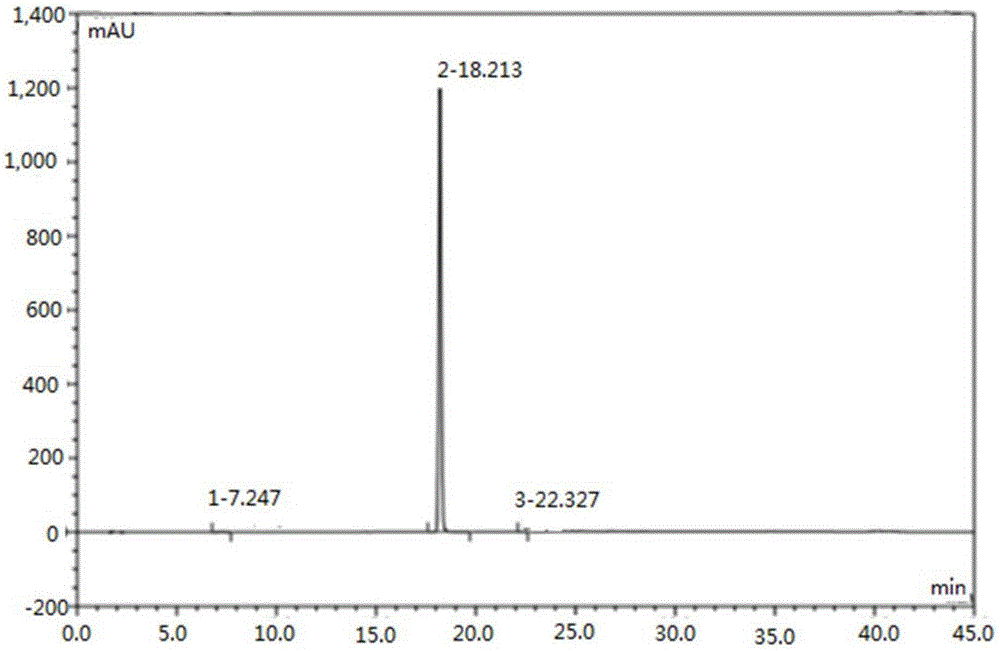
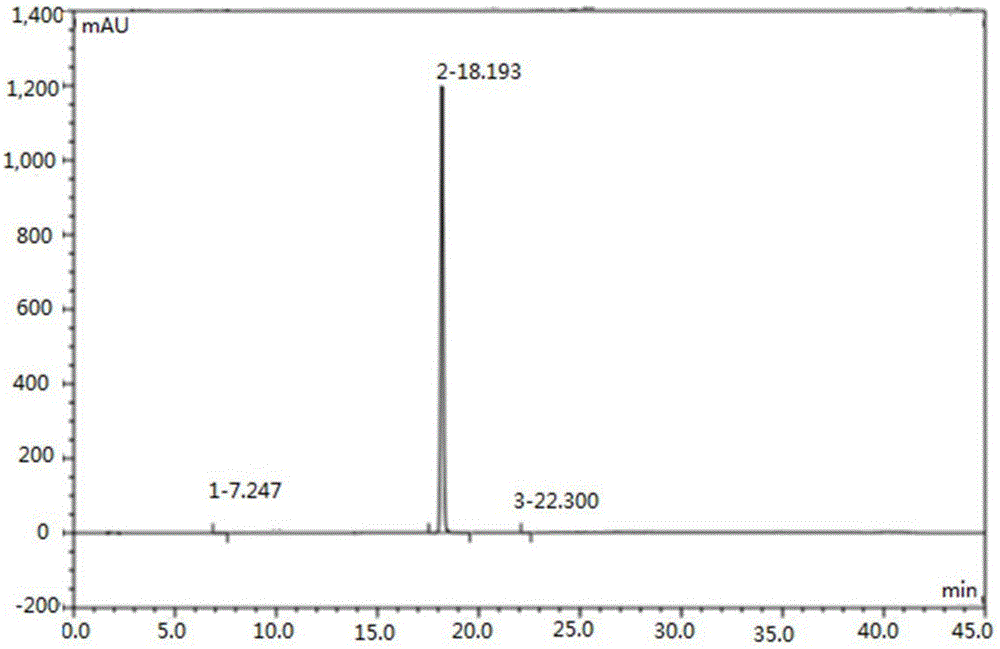
[0038] Take 1 g of Regadeson crude product (HPLC purity 99.58%, impurity A content 0.35%), add 6 mL of DMSO to dissolve, heat to about 80 ° C, dropwise add 9 mL of aqueous sodium hydroxide solution with a concentration of 0.1 mol / L, and gradually precipitate during the dropping process The white solid was naturally cooled to room temperature after the dropwise addition, then stirred for 2 hours, filtered, and the filter cake was washed with 10 mL of pure water and 10 mL of ethanol in turn, and vacuum-dried at 50°C to obtain 0.97 g of Regadeson product. The HPLC results showed that the content of regadeson was 99.89%, and the content of impurity A was 0.05%.
Embodiment 2
[0040] Take 1 g of Regadeson crude product (HPLC purity 98.0%, impurity A content 1.0%), add 20 mL of DMSO to dissolve, heat to about 70 ° C, add dropwise 3 mL of potassium carbonate aqueous solution with a concentration of 0.5 mol / L, and gradually precipitate solid during the dropping process After the dropwise addition, it was naturally cooled to room temperature, stirred for another 3 hours, filtered, and the filter cake was washed with 10 mL of pure water and 10 mL of ethanol in turn, and vacuum-dried at 55°C to obtain 0.85 g of the Regadeson product. HPLC results showed that the content of regadeson was 99.87%, and the content of impurity A was 0.03%.
Embodiment 3
[0042] Take 1 g of Regadeson crude product (HPLC purity 99.50%, impurity A content 0.45%), add 10 mL of DMF to dissolve, heat to about 70 ° C, add dropwise 6 mL of sodium carbonate aqueous solution with a concentration of 0.1 mol / L, and gradually precipitate solid during the dropwise addition After the dropwise addition, it was naturally cooled to room temperature, stirred for another 2 hours, filtered, and the filter cake was washed with 10 mL of pure water and 10 mL of ethanol in turn, and vacuum-dried at 50°C to obtain 0.95 g of Regadeson product. The HPLC results showed that the content of regadeson was 99.89%, and the content of impurity A was 0.04%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com