High performance liquid chromatographic analysis method for related substances of regadenoson
A technology of high-performance liquid chromatography and analysis method, which is applied in the field of drug analysis, and can solve problems such as inability to effectively separate and analyze, drug safety risks, and inability to accurately quantify impurities.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Example 1 High-performance liquid chromatography analysis method for related substances in the raw material drug of Reganoson
[0067] The high-performance liquid chromatographic analysis method for detecting related substances in the raw material drug of Reganosen, specifically includes the following steps:
[0068] (a) Chromatographic conditions setting: Agilent C18 (250×4.6mm, 5μm) reversed-phase C18 column is used, column temperature is 35°C; mobile phase A is a phosphate buffer system of sodium 1-pentanesulfonate, and the 1- The concentration of sodium pentanesulfonate is 15mmol / L, the concentration of phosphoric acid in the buffer system is 0.02mol / L, and the pH is adjusted to 4.2 with sodium hydroxide; the mobile phase B is methanol, and gradient elution is carried out; the flow rate is 1.0ml / min ; The detection wavelength is 247nm; The eluent is composed of mobile phase A and mobile phase B, and the elution procedure is as follows,
[0069] ;
[0070] (b) Pr...
Embodiment 2
[0078] Example 2 High performance liquid chromatography analysis method for related substances in Ruiganuosheng injection
[0079] The high-performance liquid chromatography analysis method for detecting related substances in Reganosheng injection specifically includes the following steps:
[0080] (I) Chromatographic condition setting: Agilent C18 (250×4.6mm, 5 μm) is used as a reversed-phase C18 column, and the column temperature is 35°C; mobile phase A is a phosphate buffer system of sodium 1-pentanesulfonate, and the 1- The concentration of sodium pentanesulfonate is 15mmol / L, the concentration of phosphoric acid in the buffer system is 0.02mol / L, and the pH is adjusted to 4.2 with sodium hydroxide; the mobile phase B is methanol, and gradient elution is carried out; the flow rate is 1.0ml / min ; The detection wavelength is 247nm; The eluent is composed of mobile phase A and mobile phase B, and the elution procedure is as follows,
[0081] ;
[0082] (II) Preparation of...
Embodiment 3
[0090] Example 3 Screening and exploration of mobile phase conditions
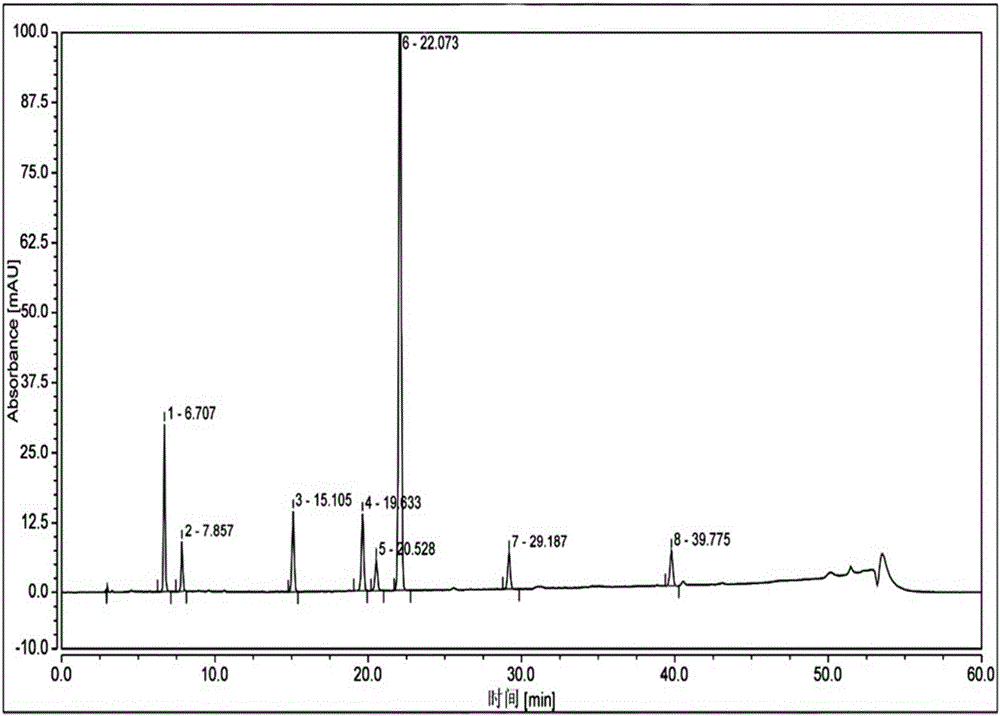
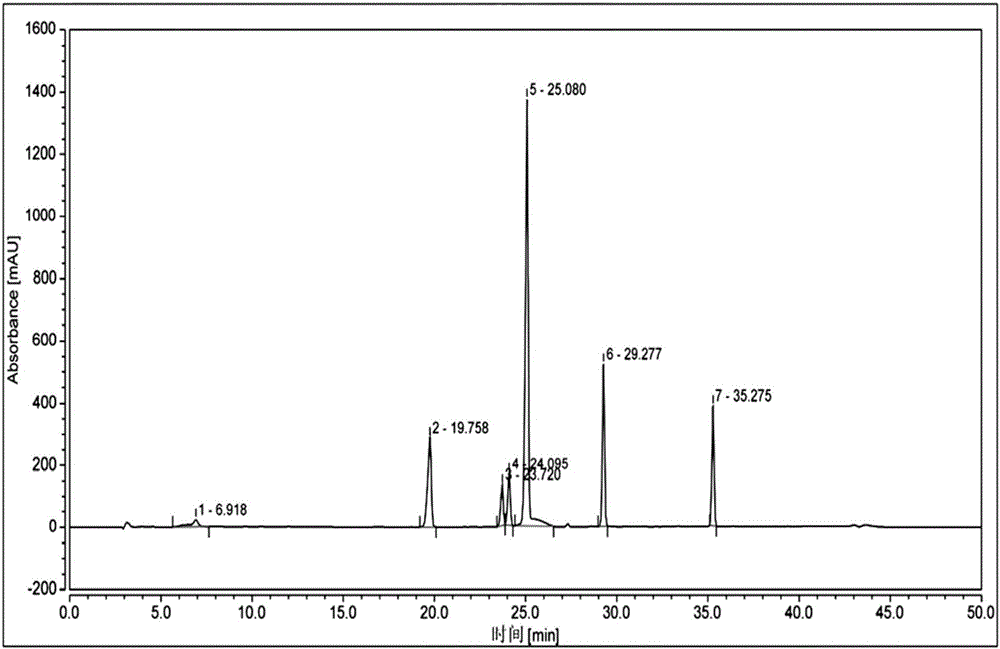
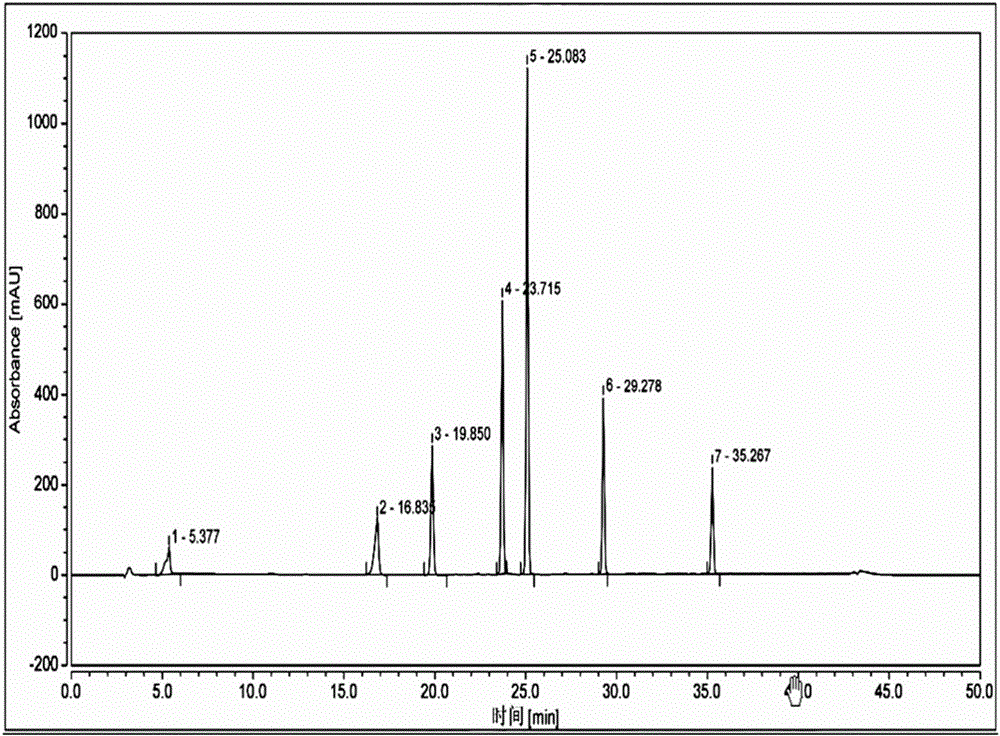
[0091] Take the reference substance of Reganosen, and appropriate amounts of impurities A, D, F, G, H, I, and J, and make a solution with a certain concentration, and use it as a system adaptability solution for investigation. Take an appropriate amount of system adaptability solution and inject it into the high-performance liquid chromatograph. The main test results are summarized in the attached Figure 2-3 And attached figure 1 .
[0092]
[0093] Note 1 The procedure is as follows,
[0094] .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com