Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
161results about How to "Guarantee drug safety" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
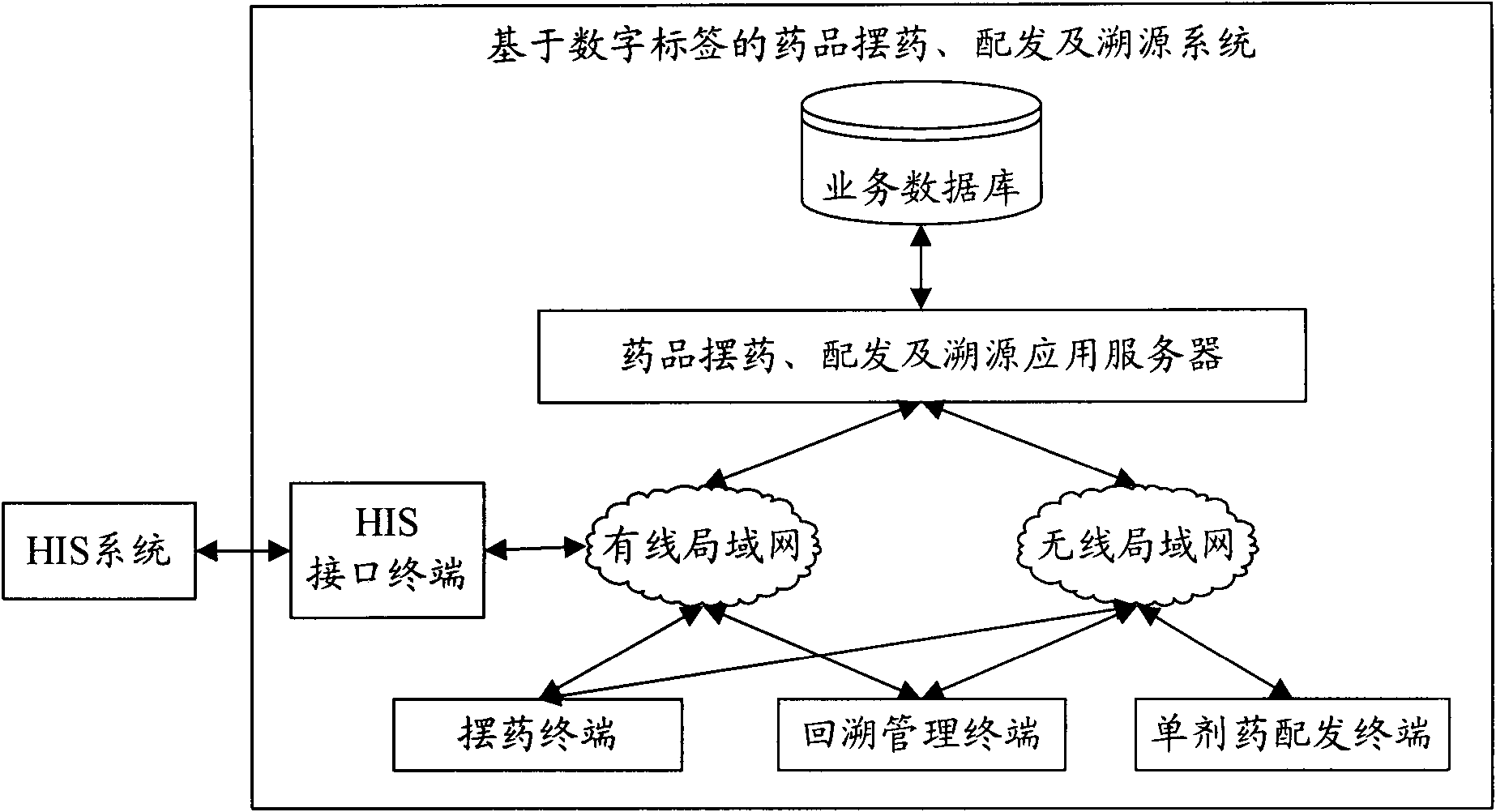
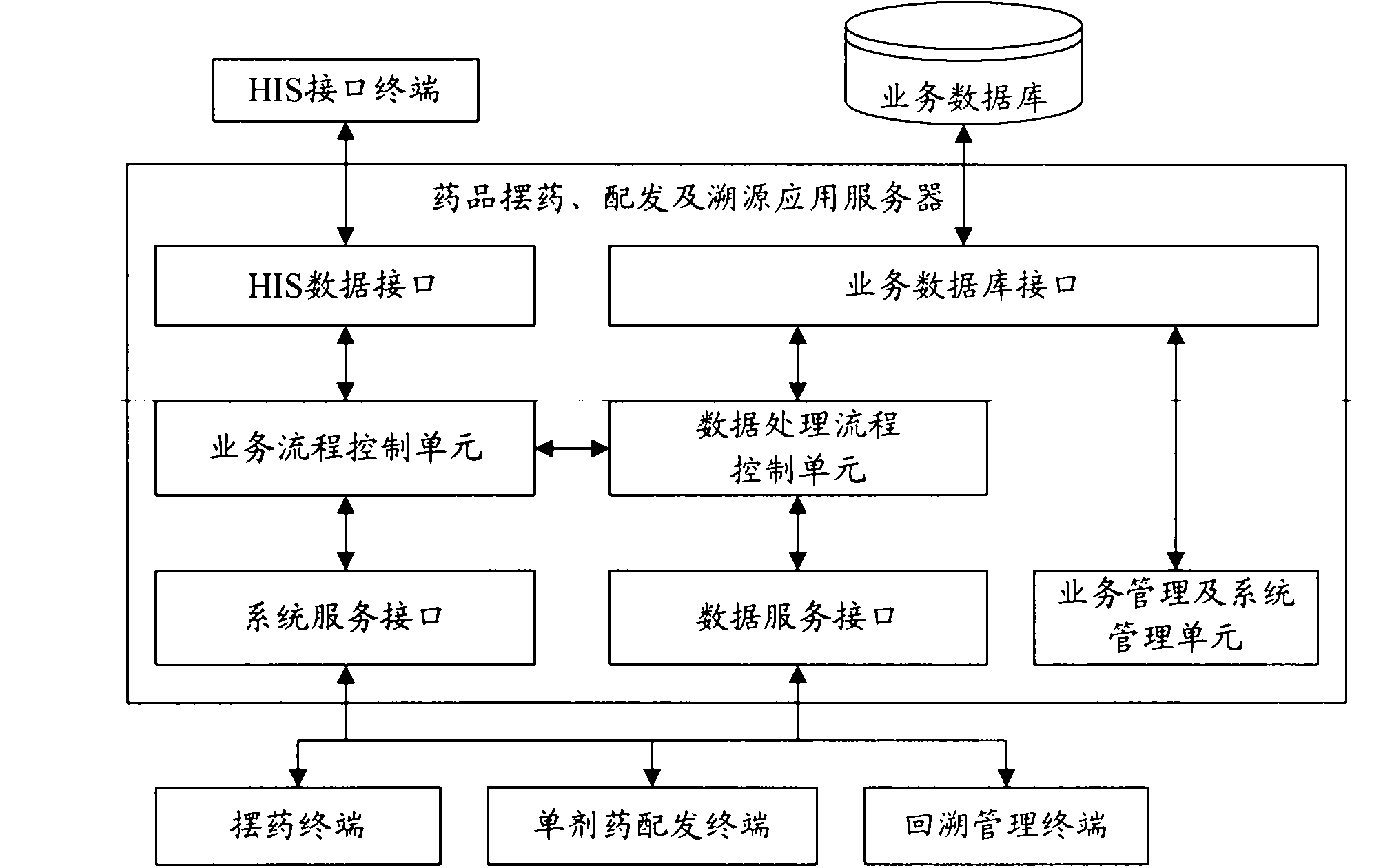
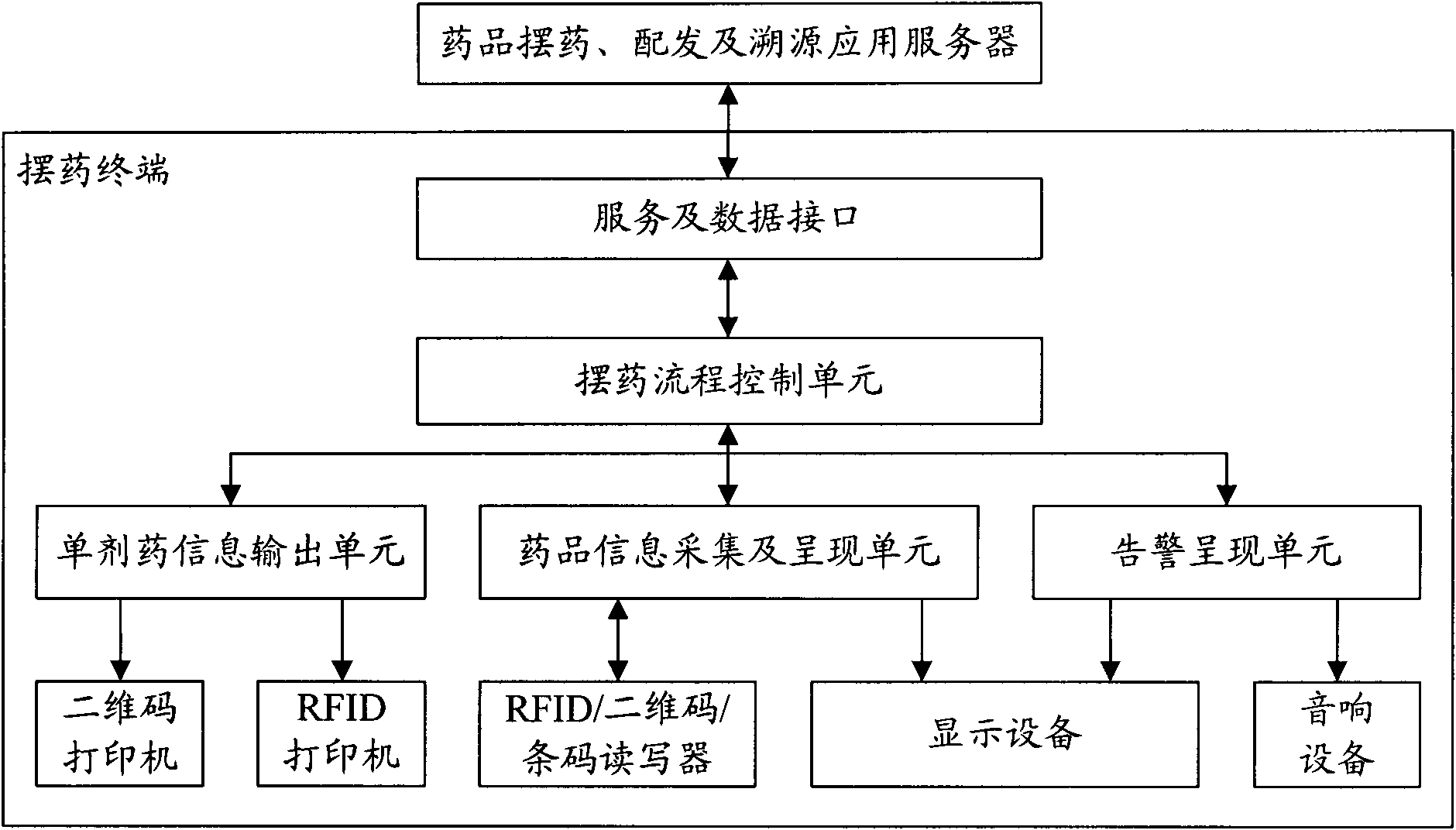
System and method for drug placement, distribution and source tracing based on digital tags
InactiveCN101923606AEnsure medication safetyPrevent medication errorsCo-operative working arrangementsSpecial data processing applicationsPharmacyApplication server
The invention relates to a system and a method for drug placement, distribution and source tracing based on digital tags. The system is formed by connecting the following devices in a wired local network and a wireless local network inside a hospital: a drug placement, distribution and source tracing application server connected with a service database, a plurality of drug placing terminals, a single drug distributing terminal, a source tracing management terminal and an HIS (Hospital Information System) interface terminal interacting information with an HIS system. The system is a set of information system for drug placement, distribution and source tracing constructed by adopting a computer technology, an RFID (Radio Frequency Identification Devices) technology and a two-dimensional code technology. The information system is used for supporting hospital staff to complete two operating steps of drug placement in pharmacies and drug distribution in sick rooms during the drug circulation inside the hospital based on the digital tags, providing scientific and visualized examination means and tools and monitoring and checking each key link during drug placement and distribution. The system can realize the safe control mechanism of drugs for treating patients during the circulation inside the hospital and can reduce the accident potential caused by human mistakes.
Owner:WUXI BUPT SENSING TECH & IND ACADEMY +2
Breviscapine infusion preparation and its preparing method
InactiveCN1425385AInfusion stabilityOvercome the disadvantages of poor clarityOrganic active ingredientsCardiovascular disorderMedicineArginine
The Breviscapine infusion preparation is compounded with Breviscapine as main component, glucose or sodium chloride, propylene glycol, L-arginine, sodium bisulphate, EDTA-2Na and water for injection through certain technological process. The preparation is suitable for great dosage application clinically to avoid cross infection caused by intermediate links. In addition, the present invention is clear and stable.
Owner:上海博泰医药科技有限公司
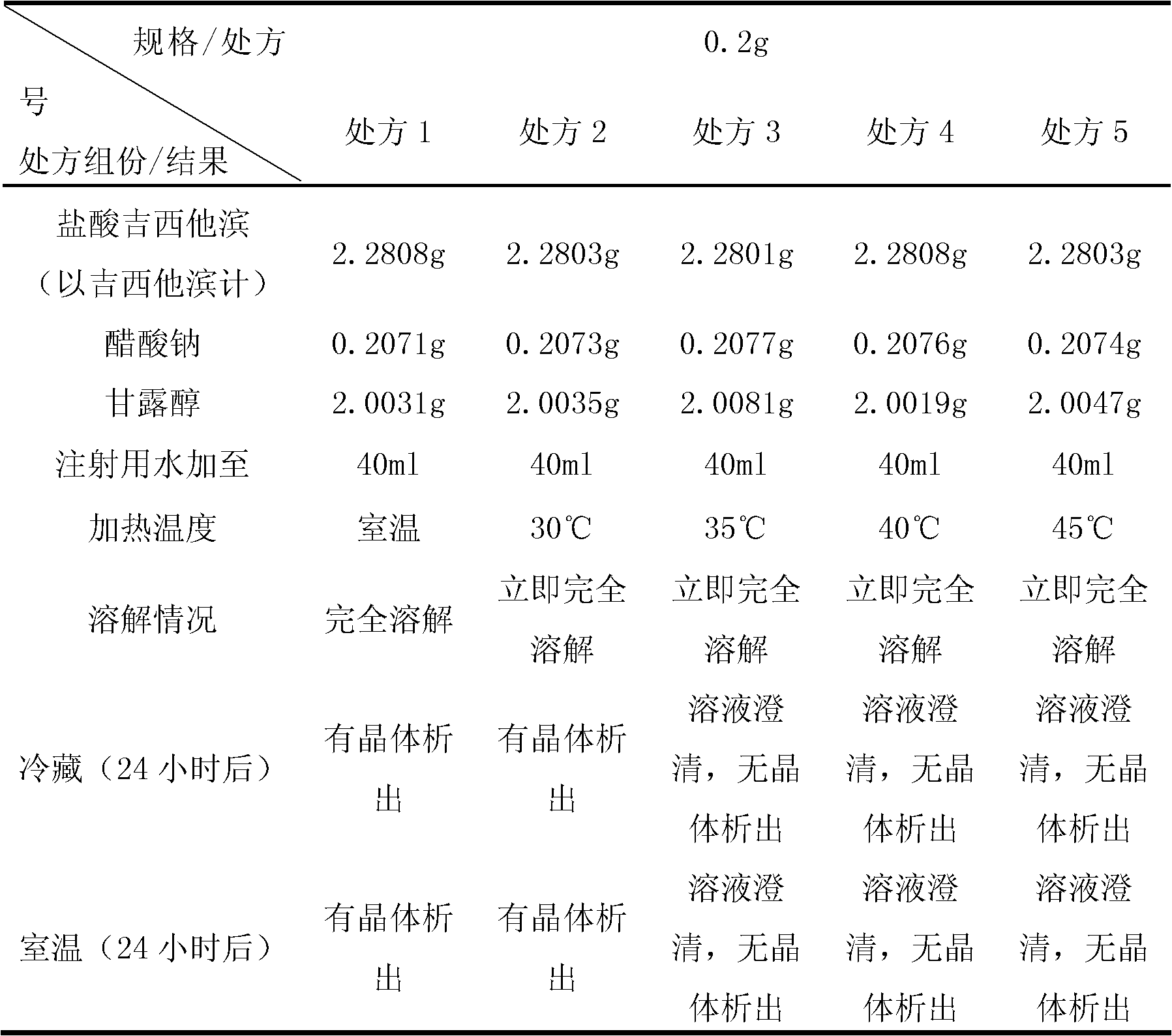
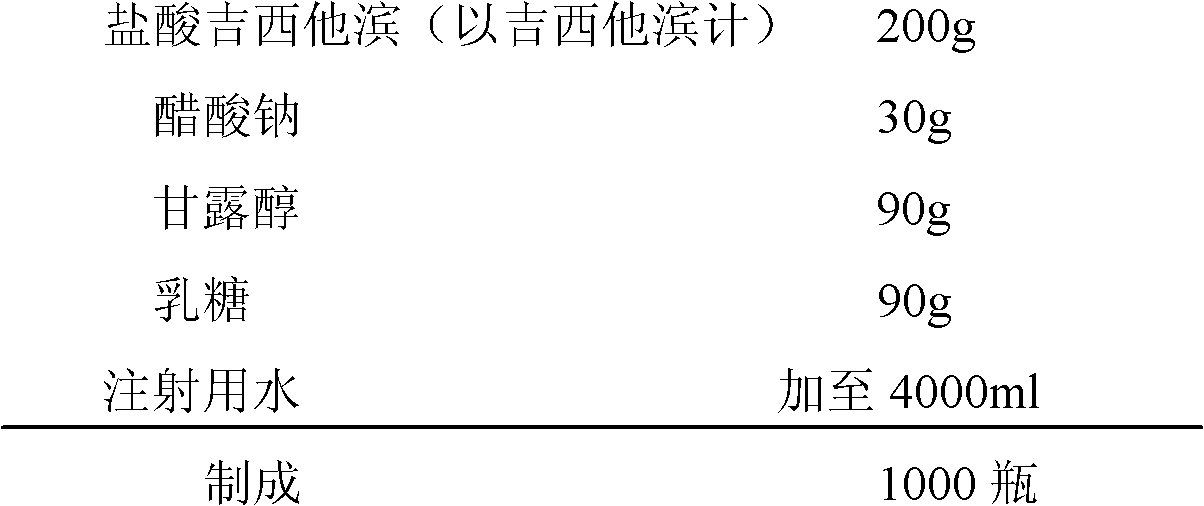
Gemcitabine hydrochloride lyophilized powder injection and preparation method thereof
ActiveCN102144981AReduce dosageImprove stabilityPowder deliveryOrganic active ingredientsSodium acetateAdjuvant
The invention relates to a gemcitabine hydrochloride lyophilized powder injection and a preparation method thereof. The lyophilized powder injection comprises the following components in parts by weight: 20-30 parts of gemcitabine hydrochloride, 5-9 parts of mannitol, and 3-10 parts of sodium acetate. The freeze-drying step includes the following three stages: a pre-freezing stage, a primary drying stage and a secondary drying stage, and the entire freeze-drying time is lower than 20 hours. The gemcitabine hydrochloride lyophilized powder injection provided by the invention has the advantages of less types and amounts of adjuvants, easily-controlled technological parameters, simple process route, short freeze-drying time, convenience in operation, good repeatability, low contents of related substances, and controllable quality; and the redissolved lyophilized powder injection has good clarity and forming performance. The lyophilized powder injection has stable and controllable quality, is easy to realize industrial production, and can generate considerable economic and social benefits.
Owner:HAINAN JINRUI PHARMA CO LTD
Process for extracting ginkgolide, ginkgolide injection and process for preparing same
ActiveCN1594319AEasy to operateReduce pollutionOrganic active ingredientsOrganic chemistrySodium acetateGinkgolide
The invention discloses a process for extracting ginkgolide, ginkgolide injection and process for preparing same, wherein the extracting process consists of disintegrating the ginkgo leaves, leaching by diluted acetone solution, recovering acetone, removing impurities, extracting and purifying with acetic ether, removing impurities with sodium acetate again, reclaiming acetic ether, recrystallizing in ethanol or methanol, filtering, low temperature drying, solubilizing the bilobalide with hydroxypropyl-beta-cyclodextrin to obtain the bilobalide injection.
Owner:HEILONGJIANG ZBD PHARMA
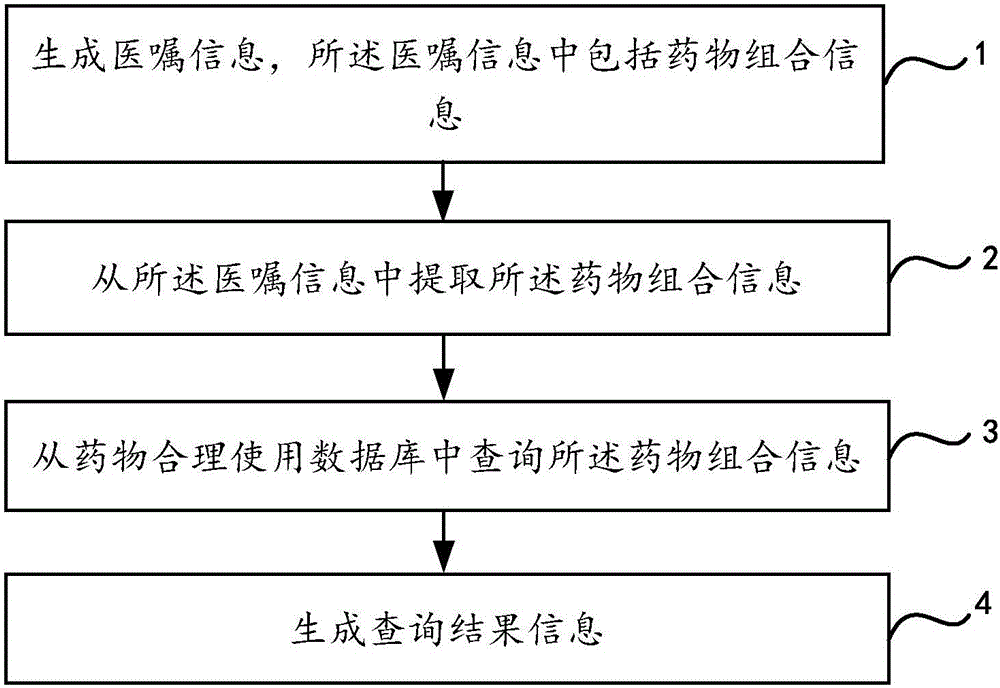
Medicine combination information processing method
InactiveCN106372432AImprove review efficiencyGuarantee drug safetySpecial data processing applicationsInformation processingMedical advice
The invention relates to a medicine combination information processing method, which comprises the following steps of 1, generating medical advice information, wherein the medical advice information comprises medicine combination information; 2, extracting the medicine combination information from the medical advice information; 3, inquiring the medicine combination information from a medicine reasonable use database; 4, generating inquiry result information. The processing method of the medicine combination information provided by the invention has the advantages that the process of performing reasonableness inspection by a prescription-checking pharmacist through brain memory or literature and guide look-up on the complicated medicine use schemes is completed through computer software, so that the manual checking is changed into automatic checking; the checking efficiency on the specific medicine combination medical advice is greatly improved; the medicine use errors due to memory mistakes and familiar condition of the pharmacists to the medication schemes can be avoided; the medicine use safety of patients is ensured to the maximum degree.
Owner:杭州逸曜信息技术有限公司
Intelligent precise traditional Chinese medicine dispensing management system and dispensing method thereof
InactiveCN109775217ARealize automatic medicine grabbingPrecise dispensingApparatus for meter-controlled dispensingStorage devicesMedical prescriptionBiomedical engineering
The invention discloses an intelligent precise traditional Chinese medicine dispensing management system. The intelligent precise traditional Chinese medicine dispensing management system comprises amedicine storage cabinet, a medicine taking device for taking medicine and a control cabinet for managing and controlling the medicine storage cabinet and the medicine taking device. One side of the medicine storage cabinet is provided with five layers, wherein the first layer, the third layer and the fifth layer are running layers, and the second layer and the fourth layer are medicine storage layers; and the other side of the medicine storage cabinet is provided with a lifting mechanism. The medicine taking device is a medicine taking trolley, and the medicine taking trolley comprises a position detection structure, a large wheel driving structure, a small wheel driving structure, a small wheel lifting structure, a medicine bottle lifting structure and a medicine taking structure. The control device is the control cabinet. The control cabinet comprises an input module, a display module, an internal control module, an output module, a communication module and a power supply module. The intelligent precise traditional Chinese medicine dispensing management system can receive prescription information and accurately take medicines according to system control, can take the medicines accurately and precisely according to the instructions and prepare multiple medicines at the same time and can feed back the dosage at any time and feed the dosage back to the staff so as to facilitatethe timely storage of medicines.
Owner:XIAN UNVERSITY OF ARTS & SCI
Method for controlling dichloromethane residue in polymer microsphere preparation
ActiveCN103462901AControl residueKeep intactPharmaceutical non-active ingredientsGranular deliveryPolymer scienceMicrosphere
The present invention relates to a method for controlling dichloromethane residue in a polymer microsphere preparation. According to the present invention, during a polymer microsphere preparation process, an external leakage bridge solvent is added to a dichloromethane oil phase and / or a water phase, dichloromethane is carried to the water phase during a microsphere curing process through the external leakage bridge solvent, and finally drying is performed, such that dichloromethane residue in the polymer microsphere preparation can be controlled to less than 600 ppm; the method has characteristics of high efficiency, controllability, stable process, safety, and reasonability; and with the method, the problem of dichloromethane residue in the polymer microsphere preparation can be effectively controlled, and complete form, high drug loading, high encapsulation efficiency and good release effect of microspheres can be maintained.
Owner:ZHEJIANG HISUN PHARMA CO LTD
Method for detecting contents of dolasetron isomer and salt thereof
ActiveCN102183589ATesting and controlling the quality of pharmaceuticalsControl drug qualityOrganic chemistryComponent separationReversed-Phase Liquid ChromatographyDrug product
The invention relates to a method for detecting the contents of dolasetron or isomers in dolasetron salt or dolasetron salt by adopting high performance liquid chromatography. According to the method, normal phase chromatography hu or reversed phase chromatography is adopted to determine the content of isomers of dolasetron or dolasetron salt raw material or preparations (such as oral preparations and injections). The detection method of the invention can better separate dolasetron from isomers, and excellently detects and controls the content of isomers of dolasetron or dolasetron salt raw material or dolasetron preparations (such as oral preparations and injections) to guarantee safety effectiveness of drugs.
Owner:LIAONING HAISCO PHARMACEUTICAL CO LTD
Preparation method of tegafur gimeracil oteracil potassium capsule
ActiveCN103142607AConducive to labor protectionPromote dissolutionPharmaceutical non-active ingredientsCapsule deliveryPotassium oxonateAqueous solution
The invention provides a preparation method of a tegafur gimeracil oteracil potassium capsule. The preparation method comprises the following steps of: dispersing tegafur, gimeracil and potassium oxonate in a hydrophilic material aqueous solution; grinding by utilizing a ball mill; then spray-drying; evenly mixing the spray-dried material with a lubricant; and filling the mixture into a capsule.
Owner:SHANDONG NEWTIME PHARMA
Establishment method of fingerprint spectrum of traditional Chinese medicine composition
ActiveCN104569252AGuarantee drug safetyGuaranteed therapeutic effectComponent separationRetention timeColtsfoots
The invention provides an establishment method of fingerprint spectrum of a traditional Chinese medicine composition, and a method for detecting the constituents in the traditional Chinese medicine composition. The traditional Chinese medicine composition comprises the following active ingredients: dried tangerine peels, radix peucedant, snakegourd seed, pinellia tuber, Radix Asteris, white mulberry root-bark, perilla fruit, bitter apricot seeds, common coltsfoot flowers, heartleaf houttuynia herb, poria and liquorice roots. The establishment method of the fingerprint spectrum comprises: detecting the retention times of compounds in the traditional Chinese medicine composition by adopting high-performance liquid chromatography.
Owner:GUANGZHOU BAIYUNSHAN CHENLIJI PHARMA FAB CO LTD
Tegafur gimeracil oteracil potassium capsule and preparation method thereof
ActiveCN103816159AConducive to labor protectionPromote dissolutionPharmaceutical non-active ingredientsCapsule deliveryBiomedical engineeringLubricant
The invention discloses a tegafur gimeracil oteracil potassium capsule and a preparation method thereof. The tegafur gimeracil oteracil potassium capsule comprises the following components: tegafur, potassiumoxonate, gimeracil, poloxamer and a lubricating agent. The capsule can be rapidly dissolved, and the preparation method of the capsule is simple to operate and suitable for industrial production.
Owner:SHANDONG NEWTIME PHARMA
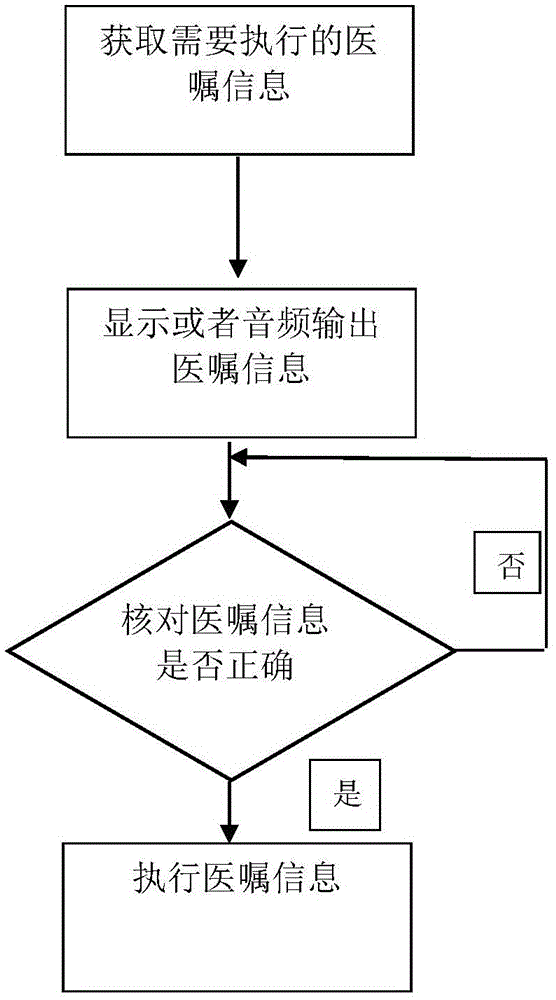
Drug information identification terminal and medical advice information interaction method
InactiveCN105117580ASimple structureEasy to useSpecial data processing applicationsCommunications systemOlder people
A drug information identification terminal comprises a housing, a processor arranged in the housing, as well as a display screen and a switch button which are arranged on the housing, wherein the housing comprises a scanning end and a handheld end; a barcode scanner is arranged on the end surface of the scanning end; the handheld end is provided with a connector and an audio output; the processor comprises an acquisition system, a check system, a storage system and a communication system; the acquisition system is used for acquiring medical advice information; the check system is used for checking whether the acquired medical advice information is correct or not; the storage system is used for storing drug information and executed medical advice information; and the communication system is used for information interaction between the processor and a general medical system. The drug information identification terminal is simple in structure, convenient to use and high in intelligent level, can not only acquire the medical advice information of drug use from a display screen but also play the medical advice information in an audio-play manner, and is convenient for old people to use.
Owner:SUZHOU ART & DESIGN TECH INST
Sulfur-free processing method for codonopsis pilosula
InactiveCN103816201AGuarantee drug safetyEffectively control mildewPlant ingredientsBiotechnologyMedicinal herbs
The invention discloses a sulfur-free processing method for a Chinese herbal medicine, namely codonopsis pilosula. The method comprises the following technological links: washing, stringing, air-drying, rubbing, solar room-temperature drying, hermetically boxing, storing and the like. Codonopsis pilosula deterioration phenomena, such as mildewing and worm eating can be controlled by the method without sulphur fumigating, and the capability of storing and preserving the codonopsis pilosula medicinal material can be greatly improved. The operating method is simple and feasible, the cost is low, ,and the codonopsis pilosula quality preserving effect is great.
Owner:甘肃岷县永康泰中药材有限公司 +1
Rapid nondestructive identification method of anti-AIDS drug
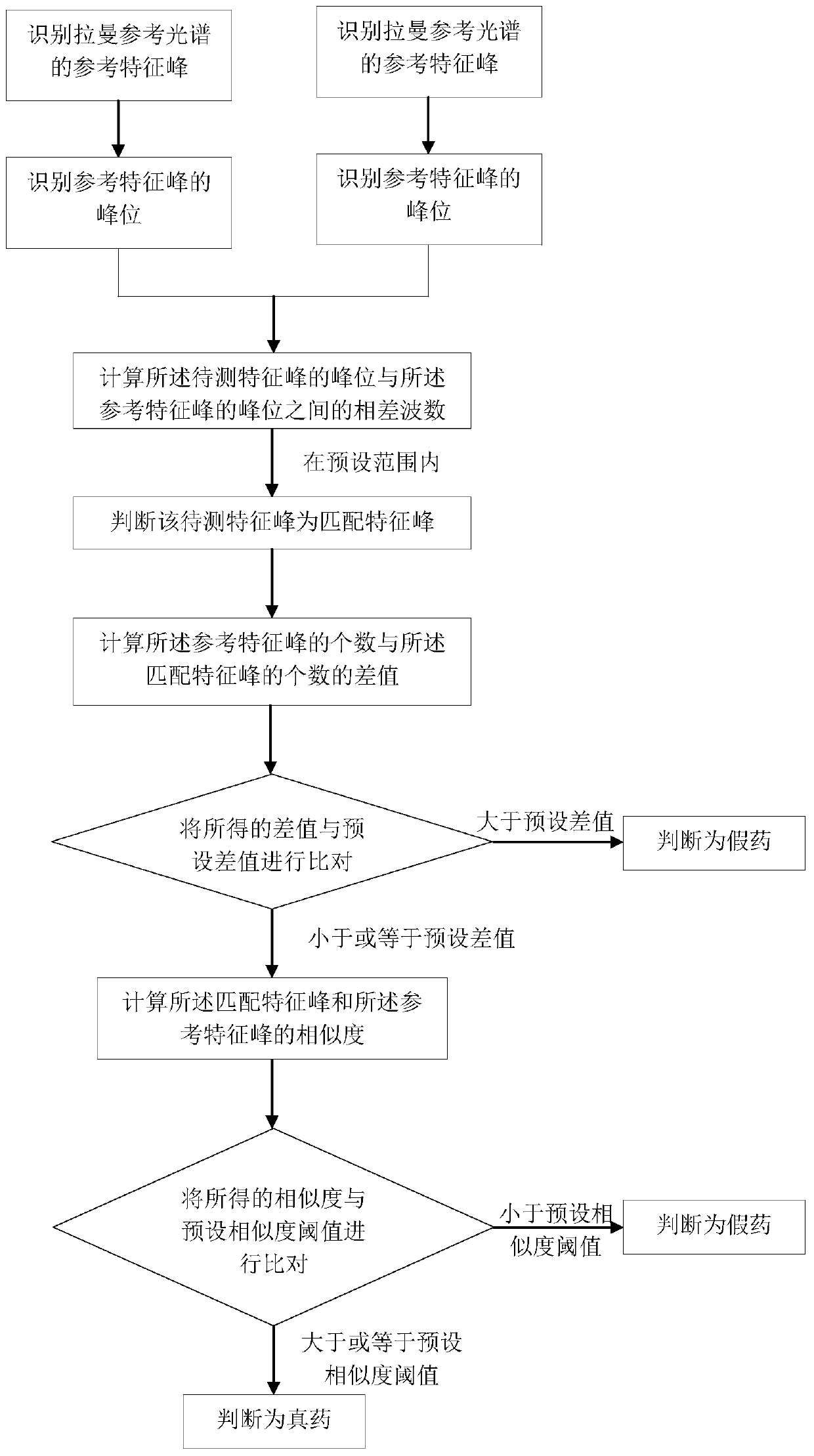
PendingCN111141719AFast non-destructive identificationDifferential scienceRaman scatteringAnti aids drugPharmaceutical drug
The invention discloses a rapid nondestructive identification method of an anti-AIDS drug. According to the method, the Raman reference spectrum database of a standard drug and a drug information database associated with the Raman reference spectrum database are established; a corresponding Raman reference spectrum is obtained according to the source information of a to-be-detected drug, spectrumscanning is performed on the to-be-detected drug by using a Raman spectrometer, so that a Raman to-be-detected spectrum can be obtained; and the Raman to-be-detected spectrum is compared with the corresponding Raman reference spectrum, so that the authenticity of the to-be-detected drug can be determined. The method provided by the invention can scientifically, accurately, simply, conveniently andquickly identify the anti-AIDS drug, will not damage the sample, causes no pollution to the environment, and realizes the rapid nondestructive detection of the drug. The identification method provided by the invention can qualitatively analyze traces and anti-AIDS drugs in order to obtain a stable and reliable analysis result, and is of great significance in the drug research field and the medical health field.
Owner:WUZHOU INST FOR FOOD & DRUG CONTROL
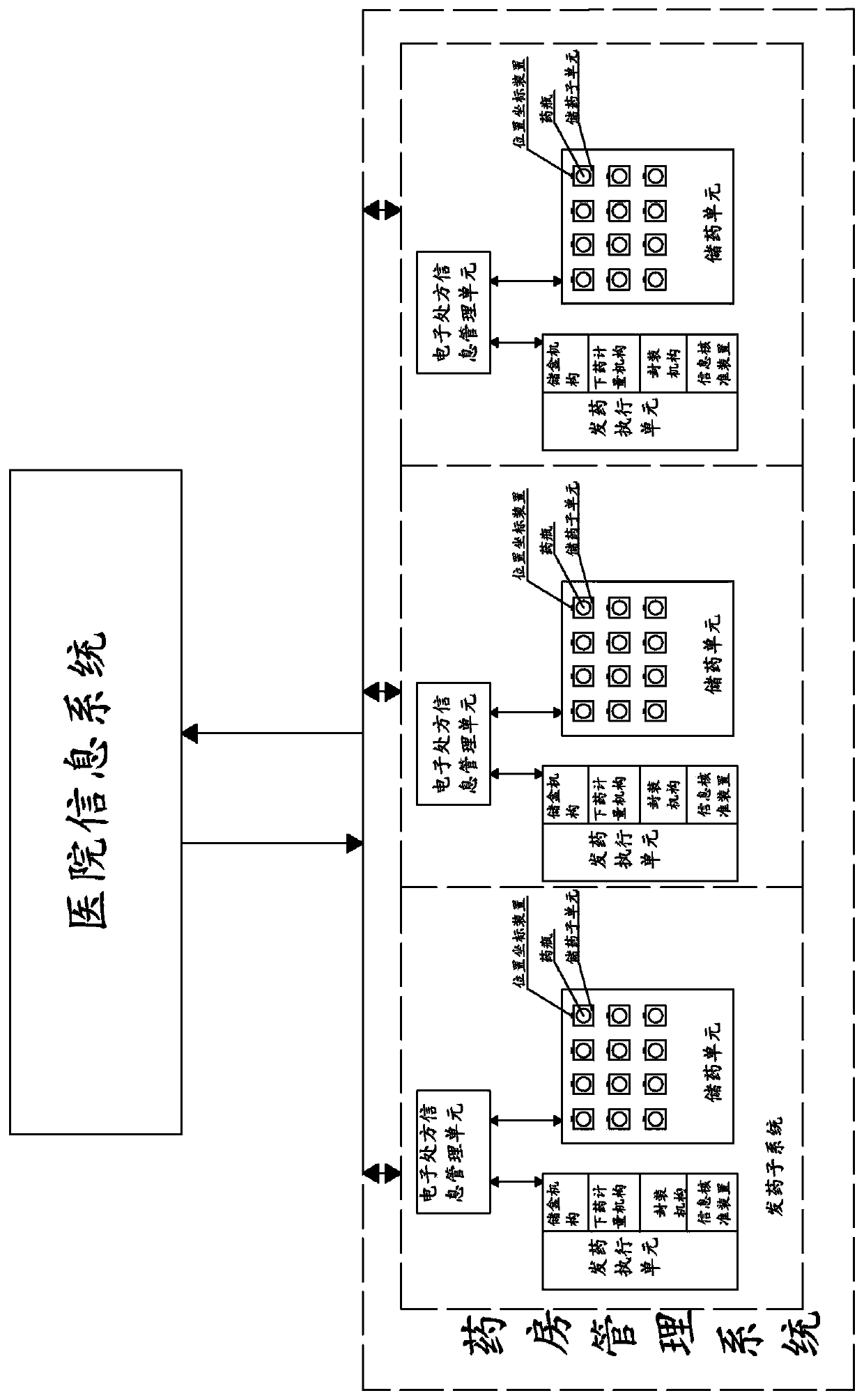
Smart pharmacy system for hospital
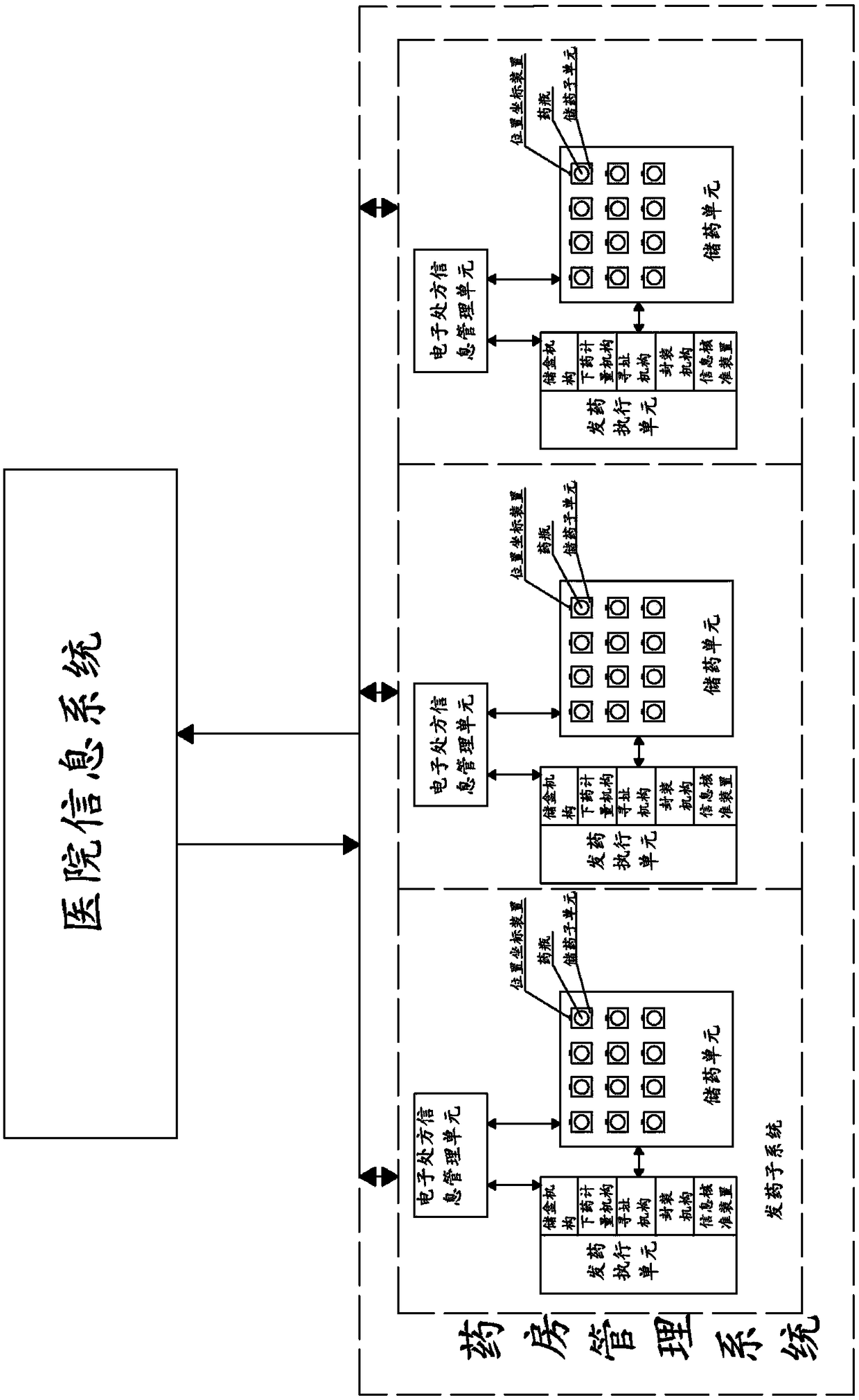
ActiveCN108538353AReal-time understanding of drug distributionAchieve interactionDrug and medicationsHealthcare resources and facilitiesInformation transmissionMedical prescription
The invention, which relates to the technical field of traditional Chinese medicine formula particle regulation, discloses a smart pharmacy system for a hospital. The system is composed of a hospitalinformation system and a pharmacy management system; and communication connection is established between the hospital information system and the pharmacy management system. According to the application, communication connection is established between the hospital information system and the pharmacy management system; and information transmission and interaction between the hospital information system and the pharmacy management system are realized through the communication connection; patient's prescription information can be stored, so that the prescription information is invoked convenientlyand the illness state of the patient is analyzed conveniently when the patient gets medicines next time; the dispensing accuracy and efficiency of the hospital pharmacy are improved effectively; thedrug inventory information is stored and managed conveniently by the hospital; and effective management and statistics are carried out on the dispensing state of the pharmacy.
Owner:CHENGDU YH INTELLIGENT EQUIP TECH CO LTD
Water-soluble vitamin freeze-dried preparation for injection and preparation method thereof
ActiveCN103110656AImprove stabilityGuarantee drug safetyPowder deliveryMetabolism disorderFreeze-dryingSodium ascorbate
The invention discloses a water-soluble vitamin freeze-dried preparation for injection. 1000 bottles of freeze-dried preparations are prepared from the following raw materials: 2.8-3.4g of thiamine mononitrate, 36-44g of nicotinamide, 4.4-5.4g of pyridoxine hydrochloride, 14.8-18.1g of sodium pantothenate compound, 4.4-5.4g of riboflavin sodium phosphate, 102-124g of sodium ascorbate, 54-66mg of biotin, 0.36-0.44g of folic acid, 4.5-6.0mg of vitamin B12, 0.4-0.6g of methyl p-hydroxybenzoate, 280-320g of glycine, 0.4-0.6g of ethylene diamine tetraacetic acid disodium salt and 2000-3000ml of water for injection. The stability of existing sodium pantothenate is obviously improved by the obtained sodium pantothenate compound, thus ensuring that the sodium pantothenate containing water-soluble vitamin freeze-dried preparation for injection has ideal stability and curative effect and further ensuring medication safety of the patients.
Owner:SHANXI PUDE PHARMA CO LTD
Detection method of carbostyril drug residue in veterinary drug
InactiveCN106908560AGuarantee drug safetyImprove securityComponent separationRetention timeProtein mass spectrometry
The invention provides a detection method of a carbostyril drug residue in a veterinary drug. The method comprises the following steps of: determining the carbostyril drug residue in the veterinary drug by means of liquid chromatography-tandem mass spectrometry; firstly, sampling a standard working liquid and a sample solution in a set liquid chromatography-tandem mass spectrometry condition; judging whether the sample contains corresponding detected objects or not, wherein the following conditions need to be met: a mass chromatographic peak retaining time in the sample solution is consistent to a mixed matrix standard working liquid with the allowable deviation of being smaller than + / -2.5%; and if the relative abundance of the set mass spectrum qualitative ions of the drug corresponding to the chromatographic peak is consistent to the relative ion abundance of the matrix standard working liquid equivalent in concentration, and the relative abundance deviation does not exceed the set specification, determining that the drug is contained. During pre-treatment of the sample by the method, the purifying process is cancelled, and the method has the characteristics of being simple in processing program, quick and high in determining accuracy rate and the like. Analyzed from a veterinary drug source, the medicinal safety of breeding industry is effectively ensured, and the animal food safety is improved.
Owner:TIANJIN SHENGJI GRP CO LTD
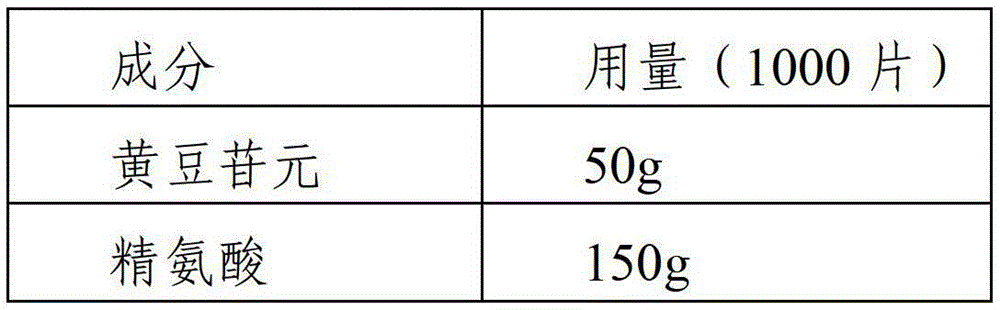
Daidzein-containing tablet composition and preparation method thereof
ActiveCN104095822AHigh dissolution rateImprove stabilityOrganic active ingredientsSenses disorderMedicineArginine
A provided daidzein-containing tablet composition comprises daidzein and arginine with the weight ratio of 1:2.5-4. The tablet composition comprises, in parts by weight, 16 parts of daidzein, 40-64 parts of arginine, 5-7 parts of a disintegrating agent, 14-38 parts of a diluent, 0.2-1.0 parts of a lubricant and 0.9-1.6 parts of an adhesive. By employing the above technical scheme, the water-soluble daidzein-containing tablet composition containing daidzein and arginine with the weight ratio of 1:2.5-4 is provided. The invention further provides a specific prescription and a preparation method. An added weak base enables daidzein to relatively well form a relatively stable easily-soluble salt, and thus improvement of dissolution rate is facilitated, and absorption by human body is facilitated. The technology is simple and the prescription quality is reliable, and batch production of the daidzein tablet can be realized.
Owner:山西国润制药有限公司
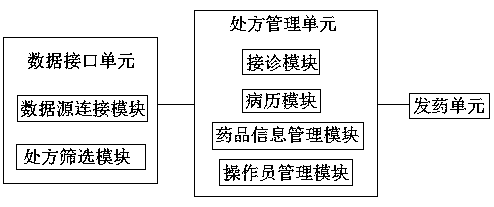
Intelligent traditional Chinese medicine pharmacy system
PendingCN108766523AStructural specificationCompact structureDrug and medicationsHealthcare resources and facilitiesContraindicationData interface
The invention discloses an intelligent traditional Chinese medicine pharmacy system, and belongs to the technical field of medicine. The intelligent traditional Chinese medicine pharmacy system is characterized by comprising a data interface unit, a prescription management unit and a medicine dispensing unit, which are connected electrically; the data interface unit sends a queried and obtained prescription data signal to the prescription management unit; the prescription management unit sends the reviewed prescription data signal to the medicine dispensing unit; and the medicine dispensing unit receives the prescription data signal and then sends medicines through an intelligent medicine dispensing machine. According to the invention, the safety of the medication is ensured by the arrangement of contraindication compatibility; detailed information of a prescription can be accurately checked before the medicines are dispensed, and subsequent inspection is not required; the entire electronic prescription is standardized in procedure and strong in effectiveness from the treatment to the completion of the medicine dispensing, thereby greatly improving the accuracy of medicine dispensing and the efficiency of medicine dispensing, and saving the time for taking medicines for patients; the medicine dispensing machine is compact in structure, small in space occupation and simple to operate; and the system is controlled by a mechanical automatic program, the prescription is simply written and the medicine packing speed is high.
Owner:CHENGDU YH INTELLIGENT EQUIP TECH CO LTD
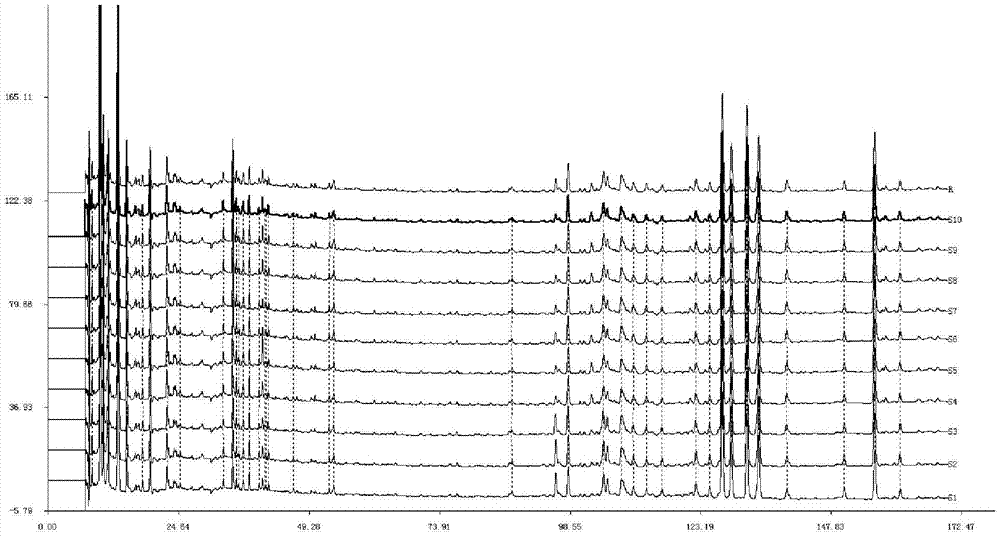
Establishing method of fingerprint of traditional Chinese medicinal composition
ActiveCN106918673AMany characteristic peaksGood peak shapeComponent separationRetention timePhosphoric acid
The invention provides an establishing method of the fingerprint of a traditional Chinese medicinal composition, and a technology for detecting the components of the traditional Chinese medicinal composition by using the fingerprint established through the method. The raw materials of the traditional Chinese medicinal composition comprise Rhizoma Cibotii, Fructus Rosae Laevigatae, Caulis Spatholobi, Philippine flemingia root, Kadsura coccinea, Millettia specisoa Champ, steamed Ligustri Lucidi Fructus, steamed Chinese Taxillus Twig, brine processed semen cuscutae, processed Rhizoma Corydalis, Zanthoxylum nitidum, processed Boswellia carteri and processed myrrh. The establishing method of the fingerprint is characterized in that the retention time of every compound and every medicinal material in the traditional Chinese medicinal composition is detected through high performance liquid chromatography; and the conditions of the high performance liquid chromatography are as follows: octadecylsilane bonded silica is adopted as a stationary phase, a mobile phase comprises acetonitrile and an aqueous phosphoric acid solution with the weight percentage concentration of 0.1%, the volume percentage of the acetonitrile in the mobile phase uniformly increases to 90% from 5% at 0-170 min, the flow velocity is 1 ml / min, the column temperature is 30 DEG C, and the ultraviolet detection wavelength is 254 nm.
Owner:GUANGZHOU CHEN LI JI PHARMA FACTORY
Chinese medicinal granule semi-automatic dispensing method
ActiveCN108766520AAvoiding Misdosed Medication SituationsAvoid situations where the wrong drug is dispensedDrug and medicationsApparatus for meter-controlled dispensingSemi automaticInteraction interface
The invention discloses a Chinese medicinal granule semi-automatic dispensing method, and relates to the technical field of Chinese medicinal granule dispersing. The method comprises the electronic prescription acquisition step, the electronic prescription analysis step, the medicine bottle taking step, the checking step, the medicine dispensing measurement step and the sealing step. The medicinebottle taking step and the checking step require to be manually completed. The medicine dispensing information of the medicine is manually checked for multiple times in the whole dispensing process through the human-computer interaction interface and the information approval device so that the situation of mistaken medicine dispensing can be avoided. Besides, the medicine can be supplemented in time by the semi-automatic dispensing mode when shortage of the medicine stock is discovered, and the medicine dispensing accuracy can be ensured through multi-level approval. After the electronic prescription information is received, the medicine stock information is checked firstly so that the problem of medicine dispensing interruption caused by insufficient medicine stock can be avoided, the medicine dispensing efficiency can be ensured and the probability of medical dispute can be reduced.
Owner:CHENGDU YH INTELLIGENT EQUIP TECH CO LTD
Cefotaxime sodium preparation method
ActiveCN109575048AIncrease contentGood color gradeOrganic chemistryBulk chemical productionSolvent7-aminocephalosporanic acid
The invention provides a cefotaxime sodium preparation method. The method is characterized in that after an active group of 7-aminocephalosporanic acid is protected by a silanization reagent, subjecting to condensation reaction with AE-active ester to generate cefotaxime acid containing a protecting group; after deprotection of the cefotaxime acid containing the protecting group under the action of a deprotection agent, sequentially subjecting to aqueous-phase acidification and crystallization to obtain cefotaxime acid; subjecting the cefotaxime acid to salification and solvent crystallizationto obtain a pure product of cefotaxime sodium. In a whole technical process, a product degradation process is reduced, product quality is evidently improved, product market competitiveness is improved, and medication safety is further guaranteed.
Owner:辽宁美亚制药有限公司 +1
Tanshinone IIA infusion solution and its preparation method
InactiveCN1732915AEasy to useEasy to operateOrganic active ingredientsPharmaceutical delivery mechanismAdjuvantMedicine
The invention discloses a tanshinone IIA infusion solution and its preparation method, wherein each 100ml of the transfusion agent contains water-soluble tanshinone II A 2-50mg, water for injection 100ml and medicinal carrier. The preparing process comprises the following steps, (1) cleaning transfusion bottles, plugs and aluminium caps, sterilizing for later use, (2) dissolving prescription amount of water-soluble tanshinone II A with water for injection, charging adjuvant proportionally, (3) subjectint the solution to active carbon decolouration, adjusting pH, passing through 0.22-0.45 um microporous filtering film, filtering and split charging, filling nitrogen, inserting plugs, sterilizing, inspecting and packaging.
Owner:GUANGZHOU DIHAO PHARMA
Nalmefene hydrochloride compound and preparation method thereof
ActiveCN103204859ANot harmful to healthNo pollution in the processOrganic chemistryChemical structureAlcoholisms
The invention belongs to a technical field of medicines. Specifically, the invention relates to a nalmefene hydrochloride compound and a preparation method thereof. Nalmefene hydrochloride is a derivative of water-soluble naltrexone, and due to the chemical structure of 6-bit methylene of the nalmefene hydrochloride, the nalmefene hydrochloride has the characteristics of long action time, more medication ways, high bioavailability, less side effects, stronger physiological activity, easier biofilm penetration and the like, has different degrees of effects on keeping the normal functions of breathing, circulation, digestion, endocrine and nervous systems, and is applied to the antagonism of respiratory depression of narcotic analgesics, the treatment of heart failure and shock, the treatment of alcoholism and addiction, weight loss and the like. According to the invention, the drug stability is improved, and the medication safety is ensured.
Owner:海思科制药(眉山)有限公司
Method for detecting trace genotoxic impurities in voriconazole
The invention discloses a method for detecting trace genotoxic impurities in voriconazole. The method comprises the following steps of carrying out derivatization treatment on a to-be-detected by using biphenyl-4-thiophenol and detecting a genotoxic impurity by adoption of a high performance liquid chromatography, wherein the to-be-detected sample is a raw medicine of voriconazole and the genotoxic impurity is (R / S)-4-(1-Bromoethyl)-5-fluorin-6-chlorine-pyrimidine. The method is not capable of realizing the effective separation of voriconazole and related genotoxic impurities , but also capable of realizing the quantitative detection of trace contents, so as to effectively control the contents of the genotoxic impurities in the raw medicines and ensure the medication safety. By adoption ofcommon agents, the method is convenient to operate, good in separation degree and high in sensitivity. The method is capable of realizing detection through common high performance liquid chromatography without purchasing and combining other instruments, so that the operation is convenient and the detection cost is low.
Owner:WUHAN LL SCI & TECH DEV +2
Breviscapine injection liquid and its preparing process
InactiveCN1732970ASolve the stability problem of high-dose useImprove stabilityOrganic active ingredientsMetabolism disorderMass compositionActivated carbon
The invention discloses a breviscapine injection liquid and its preparing process, wherein each 1000 weight parts of the Breviscapine injection comprises, Breviscapine 0.5-0.9 part, sodium thiosulphate 0.01-2 parts, glucose 50-100 parts, ethylenediaminetetraacetic acid disodium salt 0.01-1 part, adsorbing with activated charcoal 0.1-0.3 part, and balancing water for injection.
Owner:HANGZHOU MINSHENG PHARM CO LTD
Rapid detection method for drugs on basis of handheld Raman spectrometer and handheld intermediate infrared spectrometer
InactiveCN104990909ASmall amount requiredAccurate counterfeitingRaman scatteringComputer scienceSpectrometer
The invention discloses a rapid detection method for drugs on the basis of a handheld Raman spectrometer and a handheld intermediate infrared spectrometer. The rapid detection method comprises the following steps: step 1, establishing Raman spectral models and intermediate infrared spectral models of a plurality of different drug samples; step 2, respectively detecting the Raman spectrums and the intermediate infrared spectrums of to-be-detected samples by utilizing the handheld Raman spectrometer and the handheld intermediate infrared spectrometer; step 3, comparing the Raman spectrums with the Raman spectral models to determine the drug names of the to-be-detected samples according to characteristic peaks, and then comparing the intermediate infrared spectrums with the intermediate infrared spectral models to determine the drug names of the to-be-detected samples according to characteristic peaks. The rapid detection method for drugs on the basis of the handheld Raman spectrometer and the handheld intermediate infrared spectrometer, provided by the invention, can meet the on-site rapid detecting conditions of high portability, solidity, long continuous working time, automation in analysis and judgment, low operation cost, relatively high accuracy, and environmental friendliness.
Owner:WUZHOU INST FOR FOOD & DRUG CONTROL
Oil-in-water type hydroquinone emulsion, and preparation method and application thereof
PendingCN112043671AImprove transdermal absorption rateImprove bioavailabilityCosmetic preparationsHydroxy compound active ingredientsHydroquinoneOil phase
The invention aims to provide an oil-in-water type hydroquinone emulsion. The emulsion comprises 10-40% (w / w) of an oil phase, 1-5% (w / w) of an oil-in-water type emulsifier, 50-80% (w / w) of a water phase and a pharmaceutically acceptable carrier. The hydroquinone emulsion in the invention has the advantages of high transdermal absorption rate, high bioavailability and the like.
Owner:广东人人康药业有限公司
Smart Pharmacy System for Hospitals
ActiveCN108538353BReal-time understanding of drug distributionAchieve interactionDrug and medicationsProgram controlInformation transmissionPharmacy facility
The invention, which relates to the technical field of traditional Chinese medicine formula particle regulation, discloses a smart pharmacy system for a hospital. The system is composed of a hospitalinformation system and a pharmacy management system; and communication connection is established between the hospital information system and the pharmacy management system. According to the application, communication connection is established between the hospital information system and the pharmacy management system; and information transmission and interaction between the hospital information system and the pharmacy management system are realized through the communication connection; patient's prescription information can be stored, so that the prescription information is invoked convenientlyand the illness state of the patient is analyzed conveniently when the patient gets medicines next time; the dispensing accuracy and efficiency of the hospital pharmacy are improved effectively; thedrug inventory information is stored and managed conveniently by the hospital; and effective management and statistics are carried out on the dispensing state of the pharmacy.
Owner:CHENGDU YH INTELLIGENT EQUIP TECH CO LTD
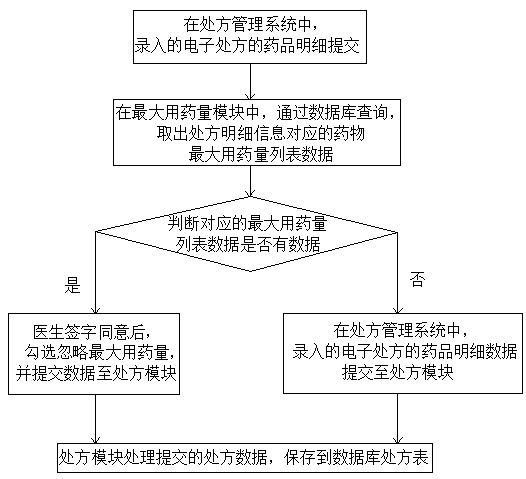
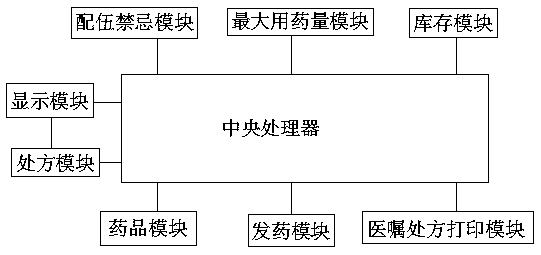
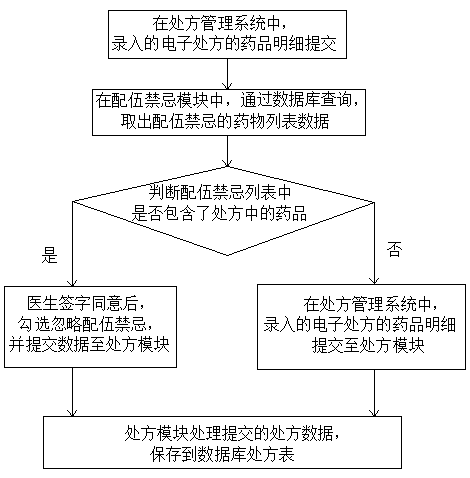
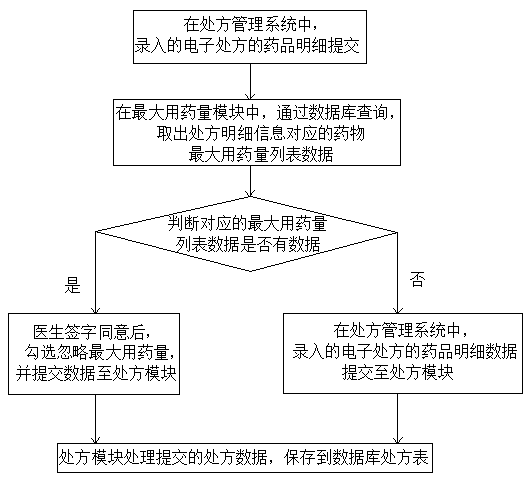
Intelligent medicine delivery machine
PendingCN108777162AEasy to parseShorten the time to collect medicineDrug and medicationsAlternative medicinesElectricityMedicine
The invention discloses an intelligent medicine delivery machine, and belongs to the technical field of medicine equipment. The intelligent medicine delivery machine is characterized by comprising a display module, a recipe module, an incompatibility module, a maximum dosage module, an inventory module, a medicine module and a medicine delivery module which are arranged on the medicine delivery machine and are electrically connected, wherein the display module transmits electronic recipe signals to the recipe module; the recipe module respectively transmits the obtained recipe detail information to the incompatibility module, the maximum dosage module and the inventory module; the incompatibility module, the maximum dosage module and the inventory module transmit the recipe detail signalspassing the detection to the medicine module; the medicine module transmits the medicine delivery signal to the medicine delivery module; the medicine delivery module controls the medicine delivery and charges the medicine into a medicine box. In the whole process of the electronic recipe from processing to medicine delivery completion, the flow process is specified, the effectiveness is very high; the medicine delivery accuracy and the medicine delivery efficiency are greatly improved; the medicine taking time of a patient is reduced; the structure of the medicine delivery structure is compact; the occupied space is small; the operation is simple and convenient.
Owner:CHENGDU YH INTELLIGENT EQUIP TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com