Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
31 results about "Sodium pantothenate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
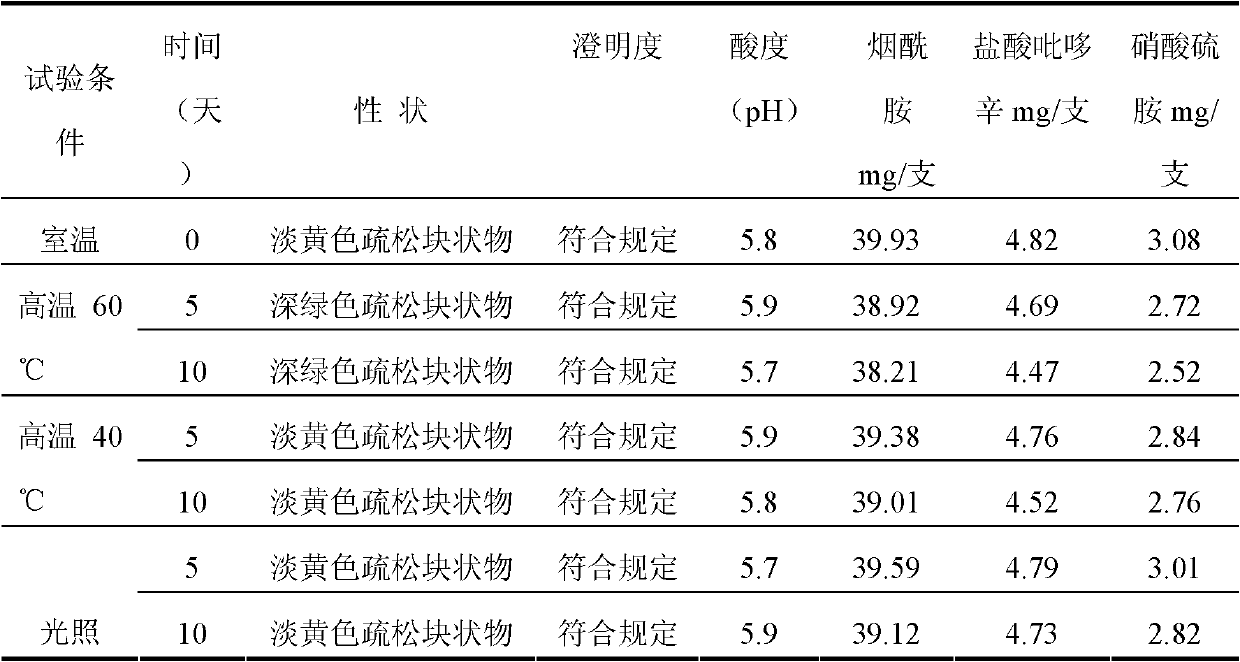
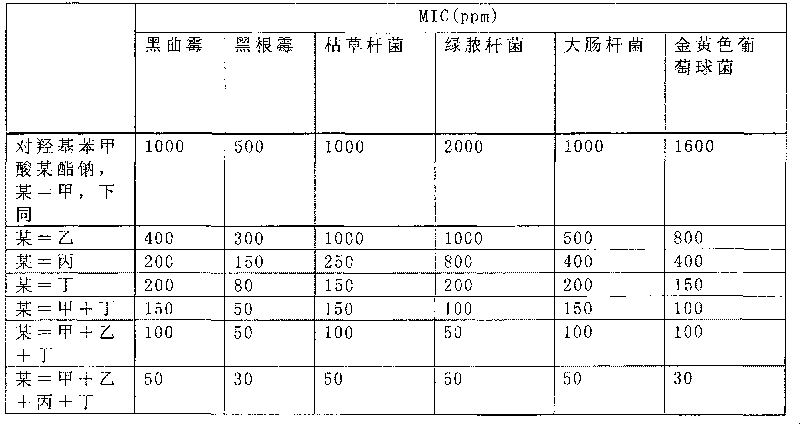
Sodium pantothenate used as a nutrient and/or dietary supplement in animal drugs, feeds, and related products is generally recognized as safe when used in accordance with good manufacturing or feeding practice.
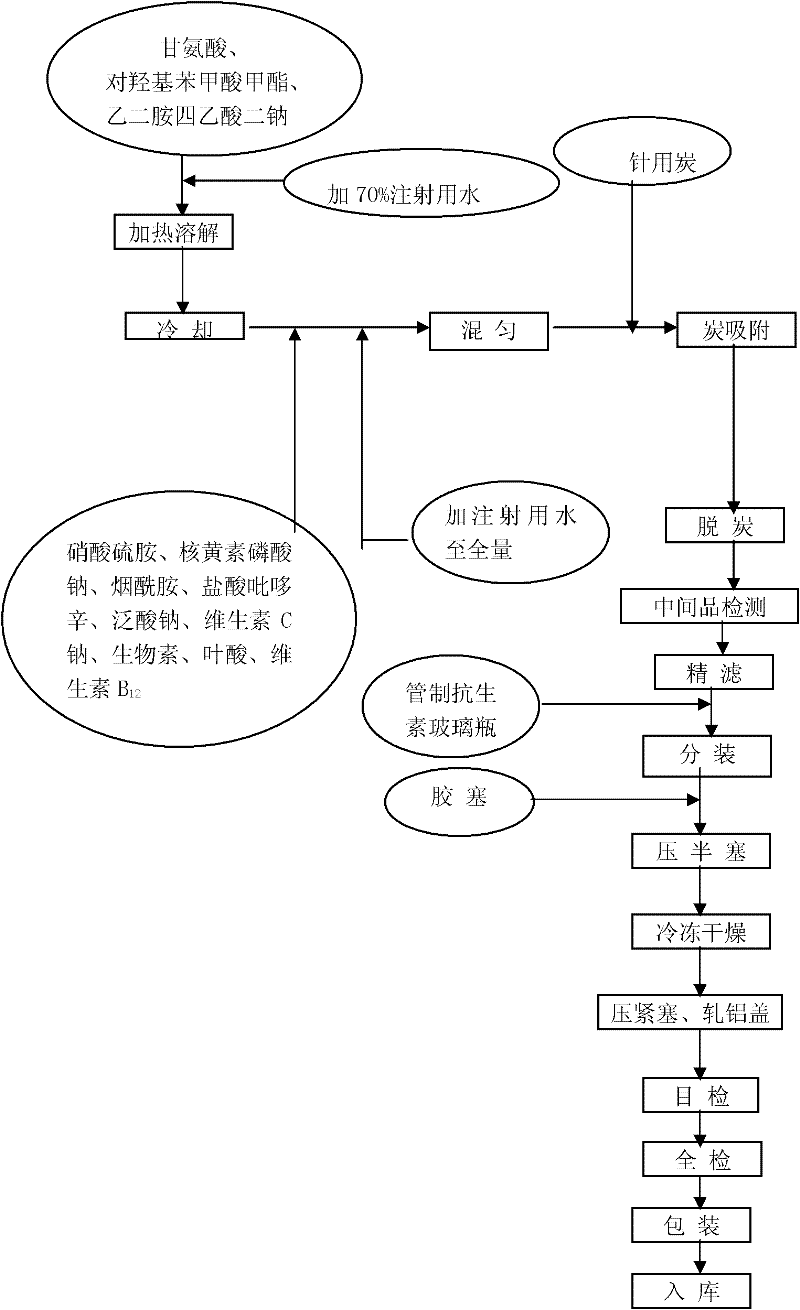
Water-soluble vitamin composition freeze-drying preparation for injection
ActiveCN101904862AGranularity adjustableConcentrated particle size distributionPowder deliveryMetabolism disorderFreeze-dryingSodium pantothenate
The invention discloses a water-soluble vitamin composition freeze-drying preparation for injection. The freeze-drying preparation comprises the following raw materials and is prepared into 1,000 bottles: 2.8 to 3.4 grams of thiamine mononitrate, 36 to 44 grams of nicotinamide, 4.4 to 5.4 grams of pyridoxine hydrochloride, 14.8 to 18.1 grams of sodium pantothenate, 4.4 to 5.4 grams of riboflavin sodium phosphate, 102 to 124 grams of sodium vitamin C, 54 to 66 milligrams of biotin, 0.36 to 0.44 gram of folic acid, 124.5 to 6.0 milligrams of vitamin B, and 0.4 to 0.6 gram of methyl-p-hydroxy benzoate, wherein the nicotinamide is nicotinamide hydrate, and the sodium pantothenate is sodium pantothenate hydrate. The water-soluble vitamin for injection has adjustable grain size, concentrated grain size distribution, glabrous surface, high product fluidity, greatly improved stability and high dissolution rate, and a preparation process of the preparation is simple and is favorable for popularization and use.
Owner:SHANDONG YUXIN PHARMA CO LTD
Serum-free medium used for culture of elderly people cartilage cells
InactiveCN104120105AMaintain biological propertiesMeet the clinical applicationSkeletal/connective tissue cellsCartilage cellsVitamin C
The invention discloses a serum-free medium used for culture of elderly people cartilage cells, the-free medium comprises a basal medium and an additive comprising the components of the following concentration: 233muM-287 muM of vitamin C, 3.7mu M-8.9 muM of linoleic acid, 9muM-17 muM of cholesterol, 6nM-15 nM of dexamethasone, 43 muM-58mu M of acetyl cysteine, 18 mug / mL-32 mu g / mL of transferrin, 22nM-52 nM of sodium selenite, 13 muM-24 muM of sodium pantothenate, 28 muM-43 muM of biotin, 7 mug / mL-18 mu g / mL of insulin, 2 ng / mL-9 ng / mL of epidermal growth factor, 2 ng / mL-9 ng / mL of fiber growth factor, 2 ng / mL-9 ng / mL of platelet derived growth factor, and 0.5%-2.5% of human serum albumin. The cell culture time is short, and the cell biological performance is good.
Owner:HANGZHOU LONGHILL BIO MEDICATION TECH
High safety polyene phosphatidyl choline for injection and preparation method thereof
InactiveCN101940586ASolve the Stability ConundrumReduce dosageOrganic active ingredientsPowder deliveryPolyene phosphatidylcholineMedicine
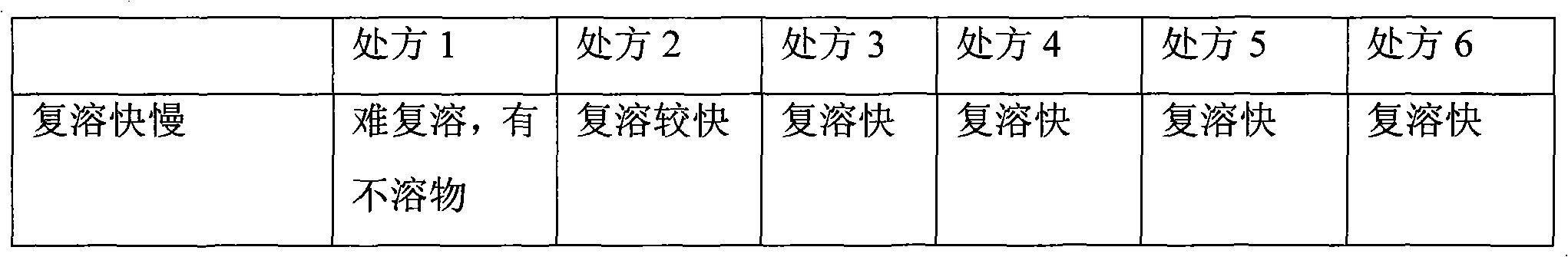
The invention provides high safety polyene phosphatidyl choline for injection and a preparation method thereof. Each injection contains the polyene phosphatidyl choline, vitamin B6, vitamin B12, nicotinamide, sodium pantothenate, a solubilizer and cryoprotectant. The injection is characterized in that the weight ratio of the solubilizer to the polyene phosphatidyl choline is 0.4-0.6:1, and is 0.5:1 preferably. The dosage of the solubilizer is reduced, the potential safety problem is reduced, the problem of re-dissolution is solved by optimizing the formula and the process, and the excellent re-dissolution is maintained on the premise of reducing the dosage of the solubilizer.
Owner:北京中海康医药科技发展有限公司
Bacteriostat-free water-soluble vitamin freeze-dried preparation for injection
ActiveCN101007018AThoroughly sterilizedUniform and thorough continuous sterilizationOrganic active ingredientsPowder deliveryGlycineVitamin B12
Disclosed is an water soluble vitamin freeze-drying preparation containing no bacteria inhibitor, wherein each bottle of the preparation comprises constituents of aneurine mononitrate 2.79-3.41mg, riboflavin sodium phosphate 4.41-5.39mg, nicotinamide 36-44mg, chlorhydric pyridoxine 4.41-5.39mg, sodium pantothenate 14.85-18.15mg, vitamin C sodium 101.7-124.3mg, biotin 54-66mug, folic acid 0.36-0.44mg, vitamin B12 4.5-5.5mug, glycine 270-330mg, and dium ethylenediamine tetraacetate 0.45-0.55mg.
Owner:费森尤斯卡比华瑞制药有限公司
Water-soluble vitamin freeze-dried preparation for injection and preparation method thereof
ActiveCN103110656AImprove stabilityGuarantee drug safetyPowder deliveryMetabolism disorderFreeze-dryingSodium ascorbate
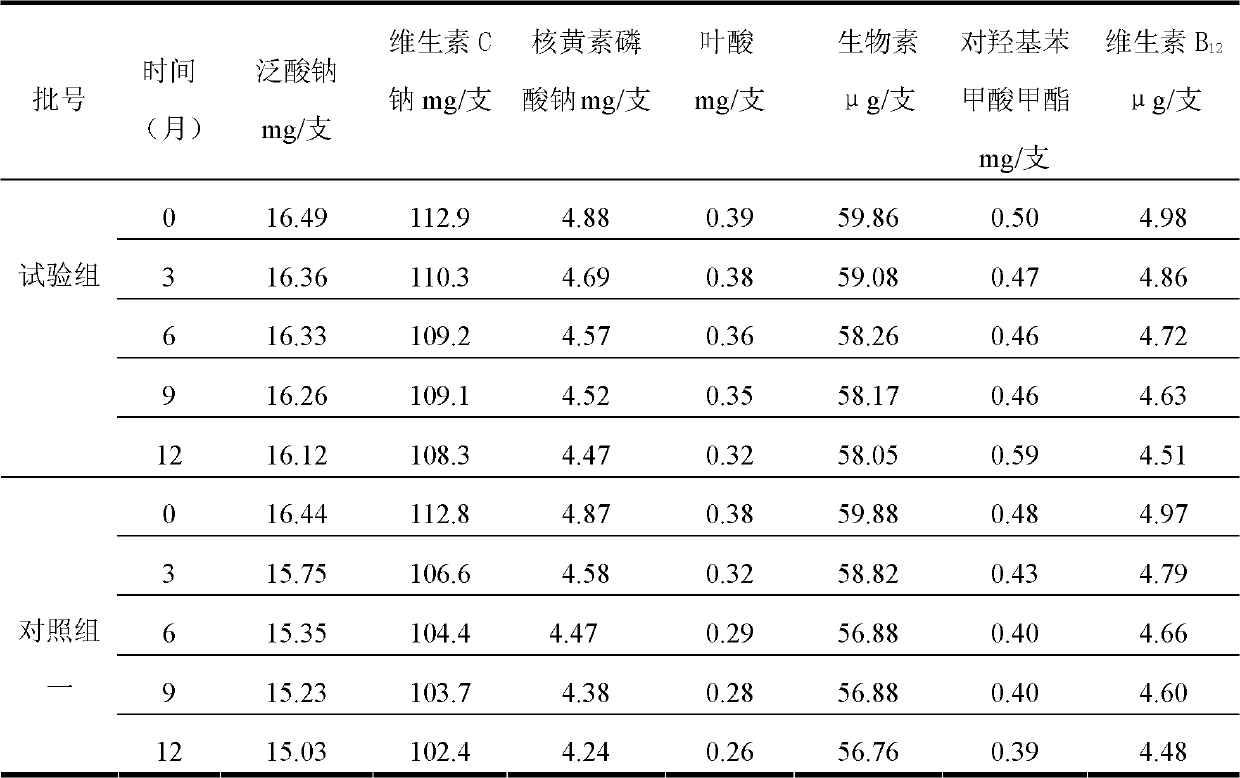
The invention discloses a water-soluble vitamin freeze-dried preparation for injection. 1000 bottles of freeze-dried preparations are prepared from the following raw materials: 2.8-3.4g of thiamine mononitrate, 36-44g of nicotinamide, 4.4-5.4g of pyridoxine hydrochloride, 14.8-18.1g of sodium pantothenate compound, 4.4-5.4g of riboflavin sodium phosphate, 102-124g of sodium ascorbate, 54-66mg of biotin, 0.36-0.44g of folic acid, 4.5-6.0mg of vitamin B12, 0.4-0.6g of methyl p-hydroxybenzoate, 280-320g of glycine, 0.4-0.6g of ethylene diamine tetraacetic acid disodium salt and 2000-3000ml of water for injection. The stability of existing sodium pantothenate is obviously improved by the obtained sodium pantothenate compound, thus ensuring that the sodium pantothenate containing water-soluble vitamin freeze-dried preparation for injection has ideal stability and curative effect and further ensuring medication safety of the patients.
Owner:SHANXI PUDE PHARMA CO LTD
Industrial preparation method of high-purity sodium pantothenate
ActiveCN104592053AInhibition of dissolutionAvoid oilyOrganic compound preparationCarboxylic acid amide separation/purificationSolubilityDistillation
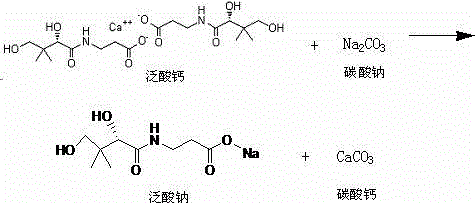
The invention discloses an industrial preparation method of high-purity sodium pantothenate. The method comprises the following steps: adding calcium pantothenate into purified water and dissolving to obtain calcium pantothenate solutions; adding sodium carbonate solutions into sodium pantothenate solutions, performing reaction, filtering and collecting filter liquid; adding active carbon into the filter liquid to be filtered and then filtered by a film; concentrating the liquid filtered by the film and adding ethanol; adding concentrated liquid into which ethanol is added into acetone; growing grains and drying to obtain sodium pantothenate. According to the industrial preparation method, high-temperature drying by distillation and multi-time repeated concentration are not needed, so that the working hours are shortened, and the side reaction is reduced; a special crystallizing condition is not needed, so that the problems that ethanol crystals are difficult to dissolve, easy in oil outflow and difficult to separate out are solved; because the process is simple, the working hours can be shortened by 1-2 times, and the production cost can be reduced by more than 50 percent. The used solvent is low in cost, the defects of high solubility of sodium pantothenate in ethanol and low yield are overcome, and the quality and the yield of the product are guaranteed; moreover, the purity of the product reaches 99.8 percent, and the yield is increased to 90 percent; therefore, the method is more suitable for industrial production.
Owner:HEBEI YIPIN PHARMA
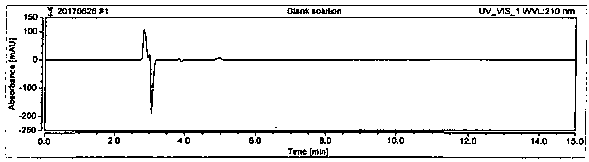
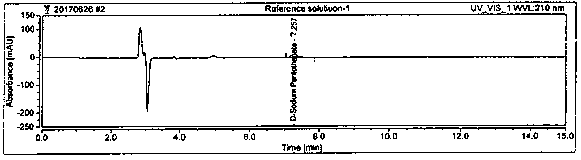
Method for detecting D-calcium pantothenate impurities
InactiveCN110082448AControl contentQuality improvementComponent separationAcetonitrileSodium pantothenate
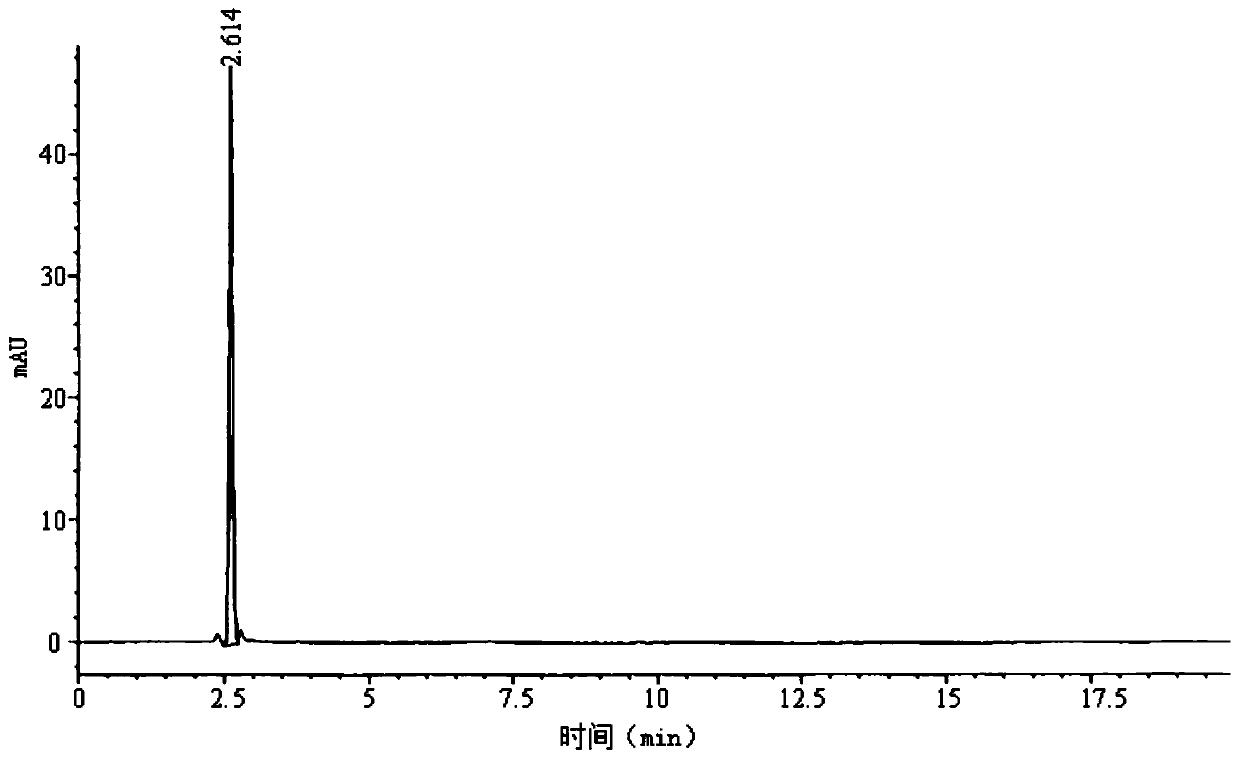
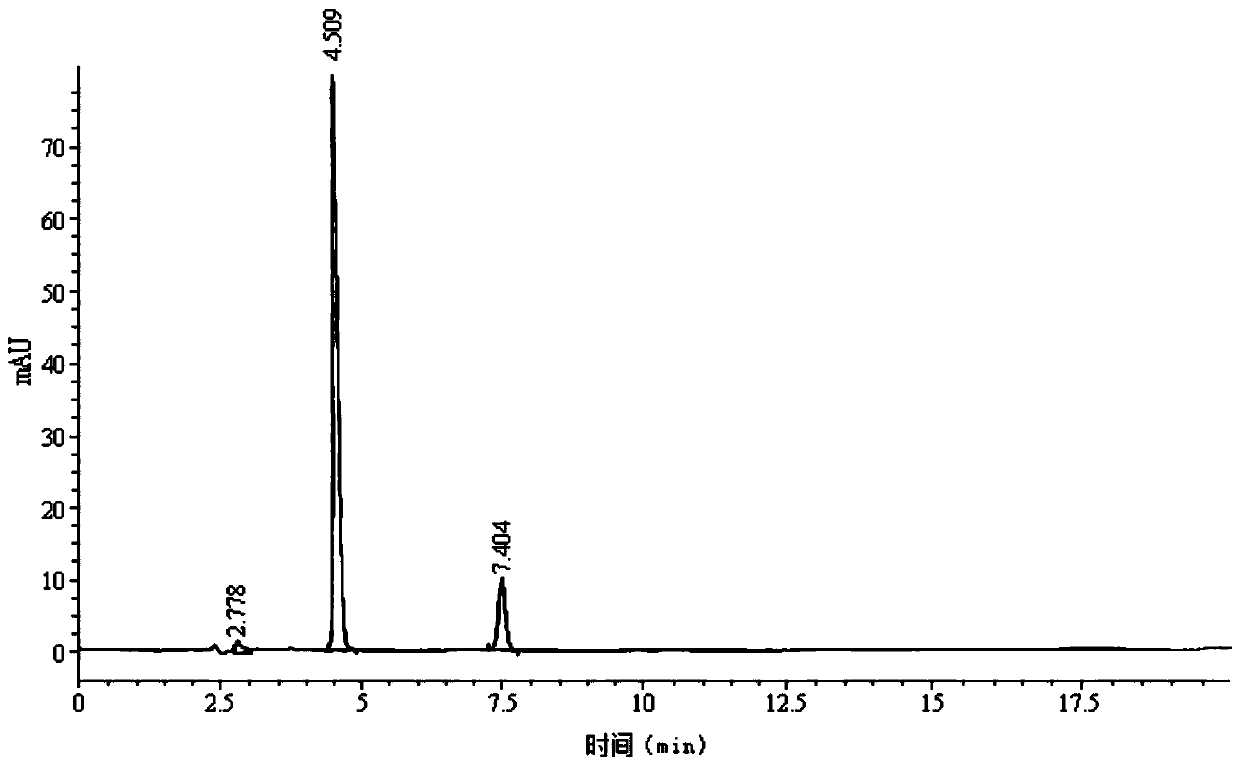
The invention belongs to the technical field of chromatographic columns, and particularly relates to a method for detecting D-calcium pantothenate impurities. The impurities comprise beta-alanine, D-pantolactone and sodium pantothenate. The detection method is a high performance liquid chromatography method; a flowing phase is a mixed solution of a phosphate buffer solution and acetonitrile with avolume ratio of 8.5-9.5 : 1. The impurities are key impurities affecting the stability of finished D-calcium pantothenate; the high performance liquid chromatography method adopted by the method canenable a chromatographic peak of the D-calcium pantothenate and various impurity peaks to be well separated, and the content of the impurities in a to-be-detected sample can be accurately determined at the same time; and the method is high in detection sensitivity, low in detection limit and suitable for detection of the impurities in a production process of the D-calcium pantothenate.
Owner:内蒙古精晶生物科技有限公司
Water-soluble vitamin combination medicament
InactiveCN103656611APyrogenic reaction noEliminate reactivityMetabolism disorderDigestive systemSodium pantothenatePharmacology
The invention provides a water-soluble vitamin combination medicament which is characterized by being prepared from the medical components in parts by weight: 2.0-4.0 parts of thiamine hydrochloride, 3.0-5.0 parts of riboflavin sodium phosphate, 3.5-7.0 parts of pyridoxine hydrochloride, 0.002-0.008 part of cobamamide, 100-150 parts of vitamin C sodium, 30-50 parts of nicotinamide, 0.3-0.7 part of folic acid, 0.04-0.08 part of biotin, 10-20 parts of sodium pantothenate, 30-80 parts of sodium glutamate, 0.02-0.04 part of reduced glutathione and 0.2-0.8 part ethylenediamine tetraacetic acid disodium. The invention also provides a preparation method for the combination medicament. All the aspects of safety, stability and treating effect of the water-soluble vitamin combination medicament disclosed by the invention are higher than those of the conventional water-soluble vitamin medicament prepared in the prior art.
Owner:吴赣英
A kind of industrialized preparation method of high-purity sodium pantothenate
ActiveCN104592053BOptimize production process stepsSave man hoursOrganic compound preparationCarboxylic acid amide separation/purificationSolubilityDistillation
The invention discloses an industrial preparation method of high-purity sodium pantothenate. The method comprises the following steps: adding calcium pantothenate into purified water and dissolving to obtain calcium pantothenate solutions; adding sodium carbonate solutions into sodium pantothenate solutions, performing reaction, filtering and collecting filter liquid; adding active carbon into the filter liquid to be filtered and then filtered by a film; concentrating the liquid filtered by the film and adding ethanol; adding concentrated liquid into which ethanol is added into acetone; growing grains and drying to obtain sodium pantothenate. According to the industrial preparation method, high-temperature drying by distillation and multi-time repeated concentration are not needed, so that the working hours are shortened, and the side reaction is reduced; a special crystallizing condition is not needed, so that the problems that ethanol crystals are difficult to dissolve, easy in oil outflow and difficult to separate out are solved; because the process is simple, the working hours can be shortened by 1-2 times, and the production cost can be reduced by more than 50 percent. The used solvent is low in cost, the defects of high solubility of sodium pantothenate in ethanol and low yield are overcome, and the quality and the yield of the product are guaranteed; moreover, the purity of the product reaches 99.8 percent, and the yield is increased to 90 percent; therefore, the method is more suitable for industrial production.
Owner:HEBEI YIPIN PHARMA
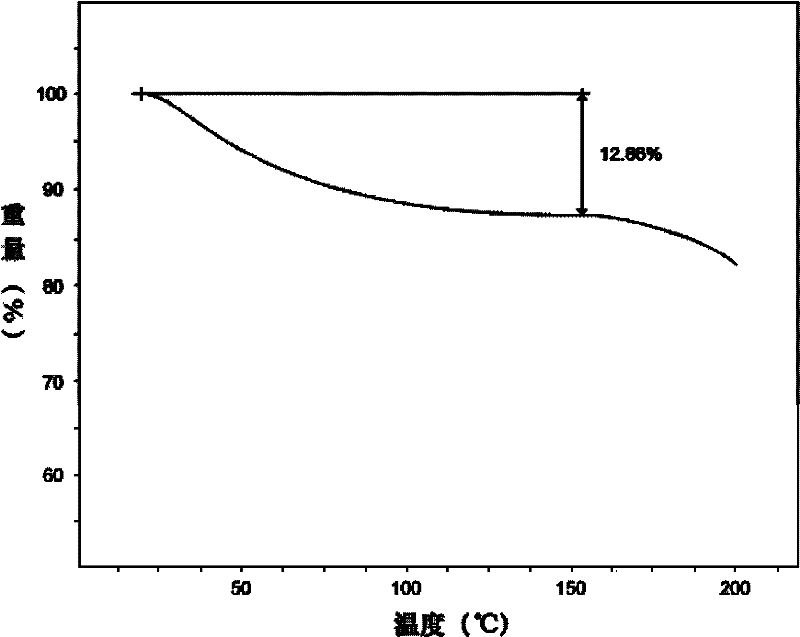
Sodium pantothenate compound, and composition preparation containing it
ActiveCN103145579BImprove stabilityGuarantee drug safetyOrganic active ingredientsPowder deliveryX-raySodium pantothenate
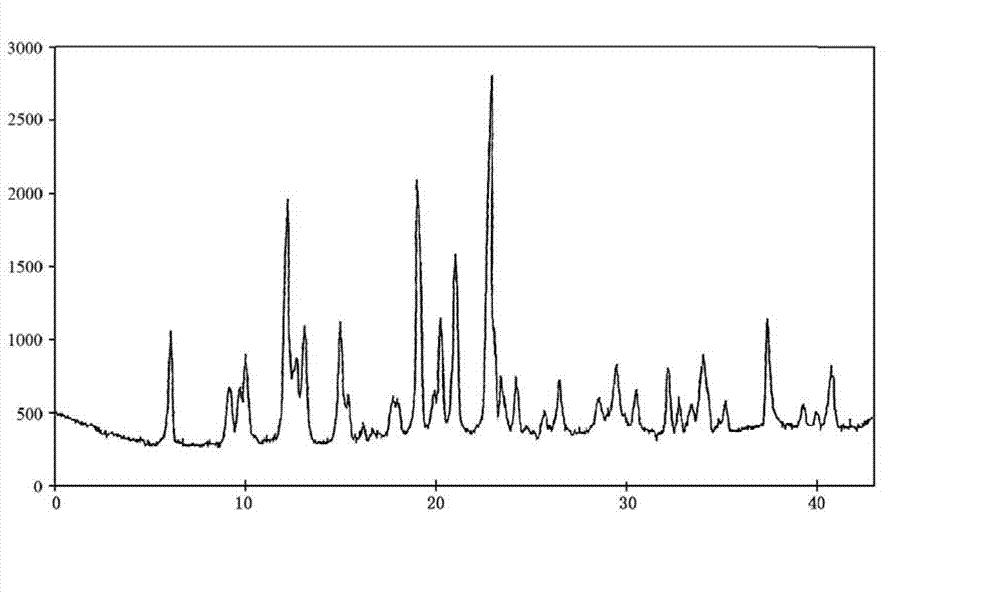
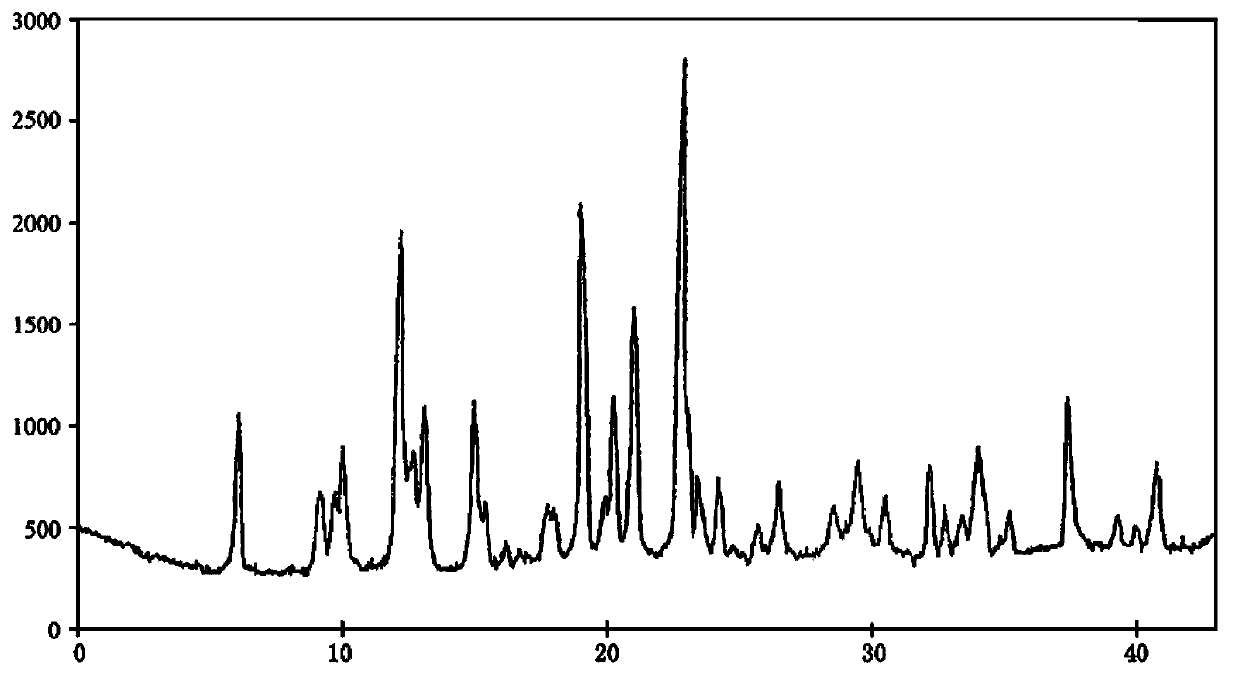
The invention discloses a sodium pantothenate compound. Sodium pantothenate is determined through a powder X-ray diffraction assay method, and characteristic diffraction peaks of an X-ray powder diffraction pattern appear at 2theta+ / -0.2DEG diffraction angles of 6.02DEG, 9.08DEG, 9.92DEG, 12.16DEG, 12.63DEG, 13.01DEG, 14.93DEG, 18.95DEG, 20.20DEG, 20.96DEG, 22.81DEG, 23.40DEG, 24.16DEG, 26.41DEG, 29.39DEG, 32.11DEG, 33.93DEG, 37.28DEG and 40.72DEG. The sodium pantothenate compound and its preparation have substantially better advantages than present sodium pantothenate compounds in stability tests. The sodium pantothenate compound disclosed in the invention can substantially improve the stability of the preparation, so the medicine safety of patients can be guaranteed.
Owner:SHANXI PUDE PHARMA CO LTD
Preparation method of high-purity sodium pantothenate
ActiveCN110105235AUniform particle sizeImprove liquidityOrganic compound preparationCarboxylic acid amide separation/purificationSodium pantothenateSolvent
The invention discloses a preparation method of high-purity sodium pantoate. According to the method, beta-alanine and an alkali are adopted as raw materials for a reaction in a low-level alkanol solvent to obtain sodium beta-alaninate, the sodium beta-alaninate reacts with D-pantolactone in a low-level alkanol solvent to obtain a crude sodium pantothenate product, and the crude sodium pantothenate product is refined in 95% ethanol and crystallized to obtain sodium pantothenate. Sodium pantoate prepared by means of the method has the advantages that being uniform in particle size, good in fluidity, low in wettability, easy to operate, environmentally friendly, high in yield and purity and suitable for industrial production.
Owner:珠海润都制药股份有限公司
Water-soluble vitamin pharmaceutical composition for injection
ActiveCN104382865AQuality assuranceImprove efficiencyPowder deliveryMetabolism disorderBenzoic acidSodium lactate
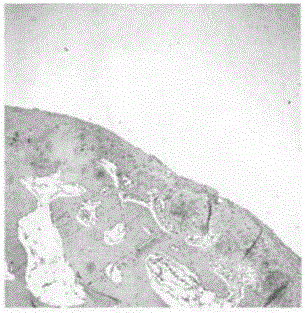
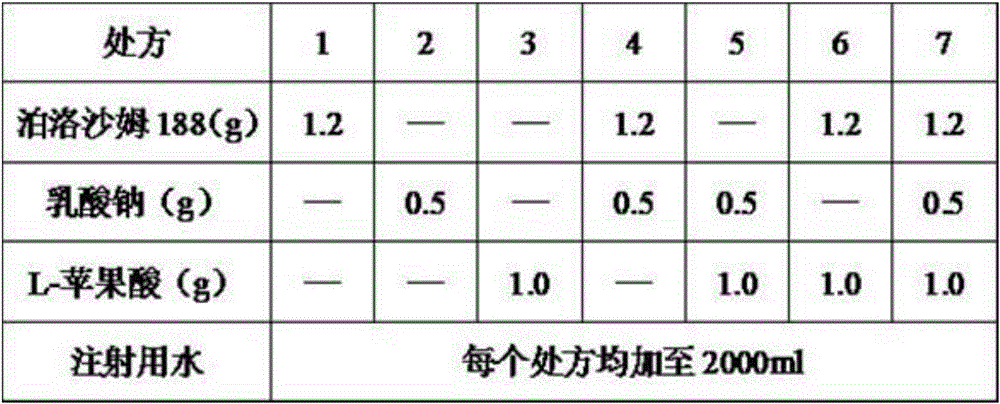
The invention relates to a water-soluble vitamin pharmaceutical composition for injection. The water-soluble vitamin pharmaceutical composition for injection is prepared from 3.1g of thiamine mononitrate, 40g of nicotinamide, 16.5g of sodium pantothenate, 60mg of biotin, 5.0mg of vitamin B12, 0.5g of disodium ethylene diamine tetraacetate, 4.9g of riboflavin sodium phosphate, 4.9g of pyridoxine hydrochloride, 113g of sodium vitamin C, 0.4g of folic acid, 300g of glycine, 0.5g of methyl parahydroxybenzoate, 1.2g of poloxamer 188, 0.5g of sodium lactate, 1.0g of L-malic acid and water for injection, wherein the water for injection is added until the total volume is 2000ml. The water-soluble vitamin pharmaceutical composition for injection, disclosed by the invention, is good in stability and greatly improved in medication safety.
Owner:湖北美林药业有限公司
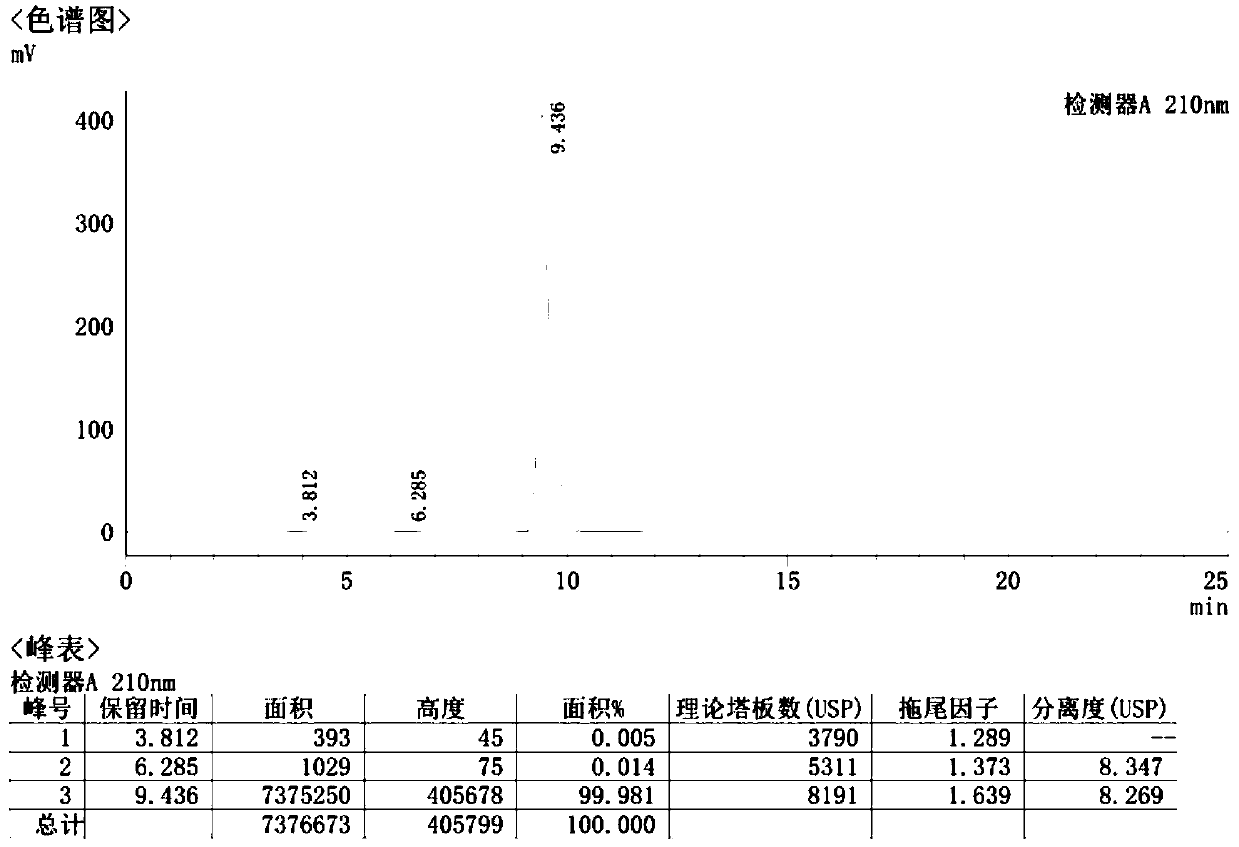
Method for detecting sodium pantothenate isomers
The invention aims to provide a method for detecting sodium pantothenate isomers. The method comprises the following steps: performing pre-column derivatization on an isomer sodium L-pantothenate in sodium D-pantothenate by adopting a reagent amylase-tris(3,5-dimethylphenyl carbamate) derivative, thereby realizing separation and determination of the isomer sodium L-pantothenate in the sodium D-pantothenate. Therefore, the method completely accords with the guiding principle of validation of the Chinese pharmacopoeia method in aspects such as system suitability, specificity, precision degree, detection limit, limit of quantitation, linearity and range, accuracy, durability and the like, and can be used for quality control of sodium pantothenate bulk drugs.
Owner:珠海润都制药股份有限公司
Chondrocyte induction substrate for microfracture surgery and preparation method
ActiveCN105255814APromote differentiationEasy to operateVertebrate cellsArtificial cell constructsInsulin-like growth factorVitamin C
The invention provides a chondrocyte induction substrate for microfracture surgery and a preparation method. The chondrocyte induction substrate comprises gel and additives, the gel comprises fibrous protein, collagen, hyaluronic acid and thrombin, and the culture medium final comprises the additives of 233 micromoles-287 micromoles of vitamin C, 3.7 micromoles-8.9 micromoles of linoleic acid, 9 micromoles-17 micromoles of cholesterol, 6 nanomoles-15 nanomoles of dexamethasone, 43 micromoles-58 micromoles of acetylcysteine, 18 micrograms / mL-32 micrograms / mL of transferrin, 22 nanomoles-52 nanomoles of sodium selenite, 13 micromoles-24 micromoles of sodium pantothenate, 28 micromoles-43 micromoles of biotin, 7 micrograms / mL-18 micrograms / mL of insulin-like growth factors, 2 nanograms / mL-9 nanograms / mL of epidermal growth factors, 2 nanograms / mL-9 nanograms / mL of fibroblast growth factors, 2 nanograms / mL-9 nanograms / mL of platelet-derived growth factors, 30 nanograms / ml-70 nanograms / ml of transforming growth factors and 1% of insulin iron-selenium transducin.
Owner:SHANGHAI BIOMED UNION BIOTECHNOLOGY CO LTD
Preparation and detection method of high-purity high-melting-point sodium pantothenate
ActiveCN111072512AHigh purityHigh melting pointOrganic compound preparationComponent separationPropanoic acidOrganic synthesis
The invention discloses a preparation and detection method of high-purity high-melting-point sodium pantothenate, and relates to the technical field of organic synthesis. The preparation and detectionmethod comprises the steps that calcium pantothenate and anhydrous sodium carbonate are used for preparing sodium pantothenate, and then the impurity beta-aminopropionic acid in the sodium pantothenate is detected; according to the invention, a method for rapidly detecting amino acids is used, and strong chromophoric group fluorenylmethoxycarbonyl or benzyloxycarbonyl is introduced, so that the content of the sodium pantothenate hydrolysis impurity beta-aminopropionic acid can be rapidly detected through a liquid phase; meanwhile, the high-purity and high-melting-point sodium pantothenate isprepared by controlling the concentration temperature, the water consumption and the pH value of the filtrate, so that the sodium pantothenate reaches the medicinal level.
Owner:HEFEI HUAFANG PHARMA SCI & TECH
Optimum matching multiple water-soluble vitamin medicinal composition containing high-efficiency bacteriostat and preparation thereof
InactiveCN101259278AReduce dosageHigh activityMetabolism disorderPharmaceutical non-active ingredientsPotential toxicityVitamin C
The invention relates to a drug combination of water-soluble multivitamins with the best proportion containing the new efficient bacteriostatic agent and a preparation method thereof. The drug combination includes, for example, the preferable weight portion ratio is: 0.1 portion of compound distributed phydroxy sodium formate, 3.1 portions of vitamin B1, 4.9 portions of vitamin B2, 40 portions of nicotinamide, 4.9 portions of vitamin B6, 16.5 portions of sodium pantothenate, 113 portions of sodium vitamin C, 0.06 portion of biotin, 0.4 portion of folic acid and 0.005 portion of vitamin B12. Used for treating the lack of vitamins or preventing the deficiency of vitamins, the drug combination contains the new efficient bacteriostatic agent in very low amount, eliminates the side effects of the bacteriostatic agent on human bodies and ensures the safety of the bacteriostatic agent. Simultaneously, the vitamin content ratios of the components are all very low, thus solving the adverse reactions caused by the excess water-soluble multivitamins or the generated potential toxicity. The drug combination of the invention can be made into injections or lyophilized agents, produced through the conventional process and equipment and has the advantages of easy industrialization, stable quality and low cost.
Owner:南京丰恺思药物研发有限公司
Water-soluble vitamin composition for injection and quality control method thereof
The invention relates to a water-soluble vitamin composition for injection and a quality control method thereof. Specifically, the invention relates to the method for quality control of the water-soluble vitamin freeze-dried powder injection composition for injection; the composition contains the following components by weight: 2.8-3.4 mg of thiamine nitrate, 36-44 mg of nicotinamide, 4.4-5.4 mg of pyridoxine hydrochloride, 14.8-18.1 mg of sodium pantothenate, 4.4-5.4 mg of riboflavin sodium phosphate, 102-124 mg of sodium vitamin C, 54-66 [mu]g of biotin, 0.36-0.44 mg of folic acid, 4.5-6.0 [mu]g of vitamin B12, 0.4-0.6 mg of methyl p-hydroxybenzoate, 250-350 mg of glycine, and 0.3-0.7 mg of ethylenediaminetetraacetic acid disodium salt. The invention also relates to the composition. The method provided by the invention has excellent effects as described in the specification.
Owner:CHENGDU TIANTAISHAN PHARMA
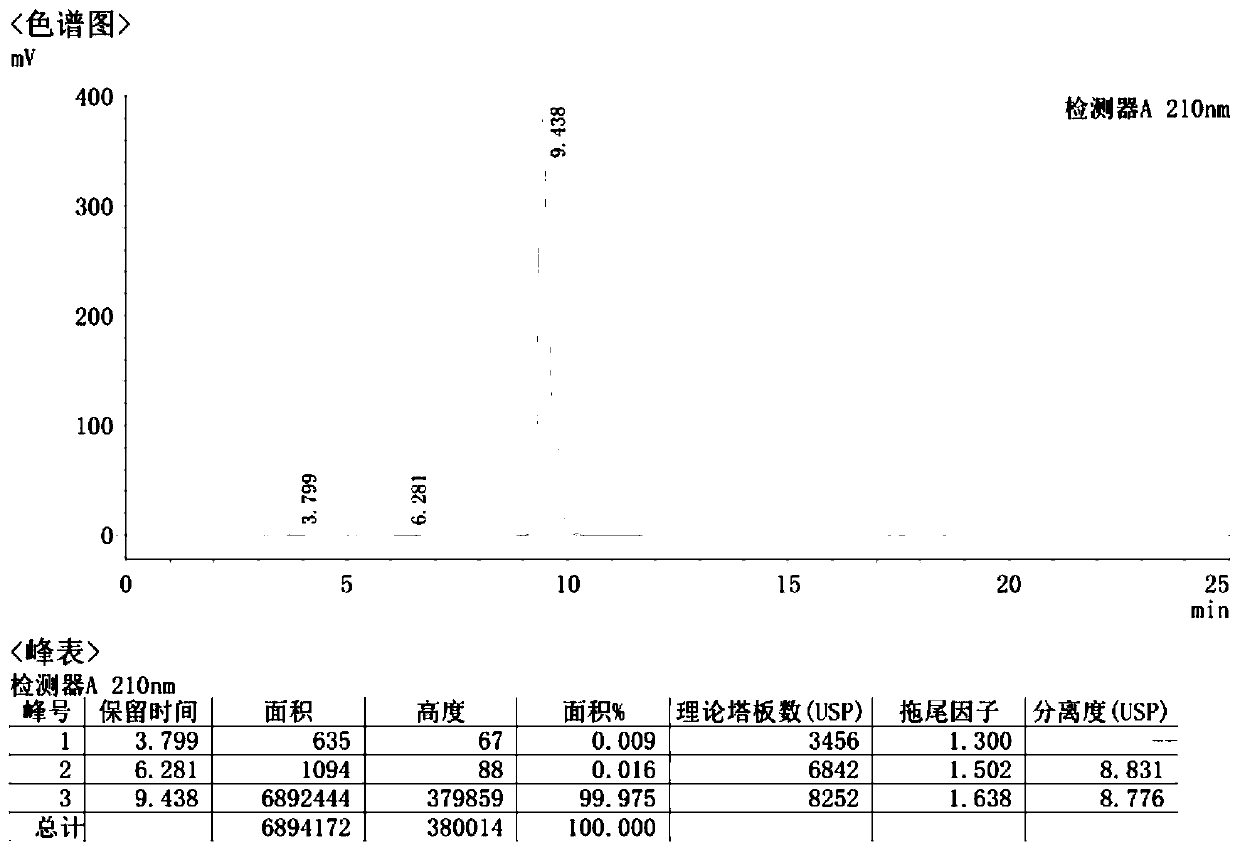
A kind of detection method of sodium pantothenate isomer
The invention aims to provide a method for detecting sodium pantothenate isomers. The method comprises the following steps: performing pre-column derivatization on an isomer sodium L-pantothenate in sodium D-pantothenate by adopting a reagent amylase-tris(3,5-dimethylphenyl carbamate) derivative, thereby realizing separation and determination of the isomer sodium L-pantothenate in the sodium D-pantothenate. Therefore, the method completely accords with the guiding principle of validation of the Chinese pharmacopoeia method in aspects such as system suitability, specificity, precision degree, detection limit, limit of quantitation, linearity and range, accuracy, durability and the like, and can be used for quality control of sodium pantothenate bulk drugs.
Owner:珠海润都制药股份有限公司
Bacteriostat-free water-soluble vitamin freeze-dried preparation for injection
The invention relates to a freeze-dried preparation of water-soluble vitamin composition for injection without bacteriostatic agent. According to the technical scheme provided by the present invention, the components of this preparation are that each bottle contains: 2.79-3.41 mg of thiamine nitrate, 4.41-5.39 mg of riboflavin sodium phosphate, 36-44 mg of nicotinamide, 4.41-5.39 mg of pyridoxine hydrochloride mg, sodium pantothenate 14.85-18.15mg, sodium vitamin C 101.7-124.3mg, biotin 54-66μg, folic acid 0.36-0.44mg, vitamin B124.5-5.5μg, glycine 270-330mg, edetate disodium 0.45 -0.55 mg. The invention can fundamentally eliminate clinical adverse reactions such as allergy induced by antibacterial agents to users, and improve product safety.
Owner:费森尤斯卡比华瑞制药有限公司
Medicinal composition for treating viral hepatitis, hepatocirrhosis and congestive cardiac failure
InactiveCN1679614AImprove clinical symptomsSigns improvedDigestive systemAntiviralsVitamin CActive component
A composite medicine for treating viral hepatitis, hepatocirrhosis, and congestive heart failure is prepared proportionally from 10 active components including juvabe 300, bepella, pyridoxinium hydrochloride, sodium pantothenate, etc.
Owner:FUKANGREN BIO PHARMA
Compound vitamin B injection and preparation method thereof
ActiveCN110251461BIncrease the amount of feedLow costMetabolism disorderPharmaceutical delivery mechanismBiotechnologyVITAMIN B COMPLEX INJECTION
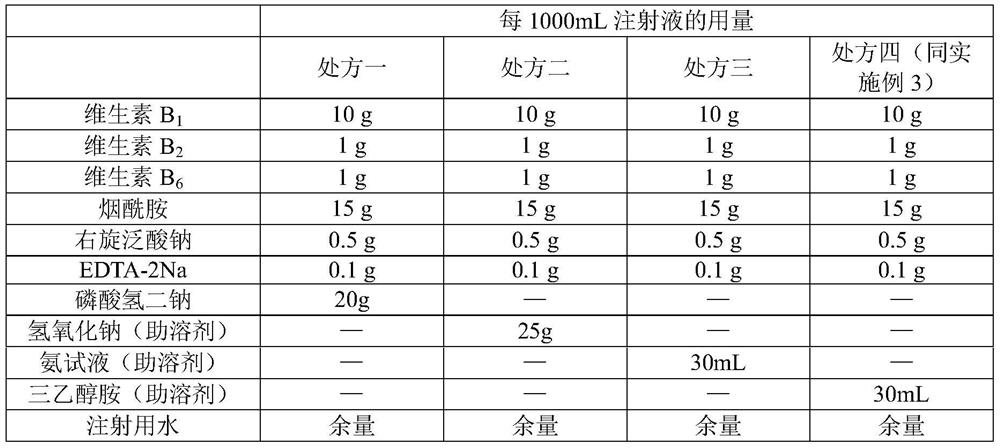
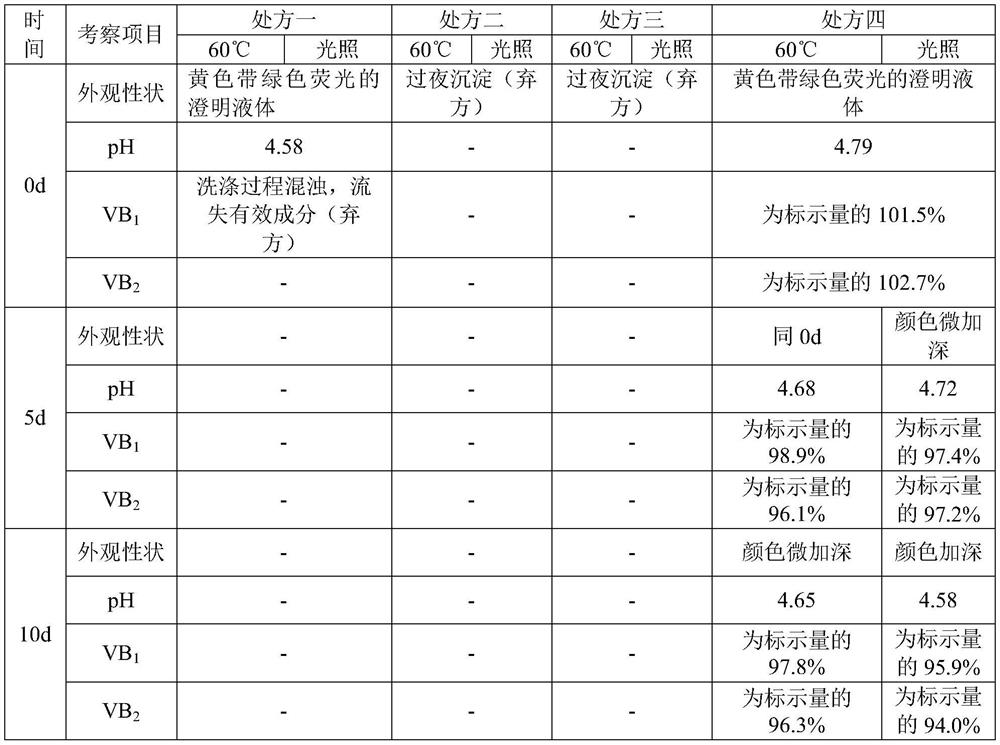
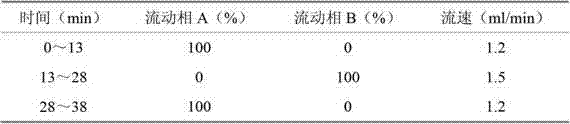
The compound vitamin B injection of the present invention is composed of 9.8-10.02g vitamin B per 1000mL 1 , 0.98~1.02g Vitamin B 2 , 0.98~1.02g Vitamin B 6 , 14.7~15.3g nicotinamide, 0.49~0.51g sodium d-pantothenate, 0.05~0.15g EDTA-2Na, 20~40mL triethanolamine, and the balance is water for injection. Specifically, add triethanolamine to 60% of the prescription water for injection, add vitamin B 2 , to get solution one; turn vitamin B 1 , Vitamin B 6 , Niacinamide, and D-pantothenate sodium are added to 20% of the prescribed amount of water for injection to obtain solution 2; mix solution 2 and solution 1 and adjust the pH value to 3.8-5.2, and add the remaining amount of water for injection; filter, fill, Sterilize. The compound vitamin B injection prepared by the invention has low raw material cost, high efficiency, stable quality and meets the quality standard of veterinary drugs.
Owner:河南益华动物药业有限公司
A kind of preparation method of high-purity sodium pantothenate
ActiveCN110105235BUniform particle sizeImprove liquidityOrganic compound preparationCarboxylic acid amide separation/purificationMicrobiologySodium pantothenate
Owner:珠海润都制药股份有限公司
Water-soluble vitamin freeze-dried preparation for injection and preparation method thereof
ActiveCN103110656BImprove stabilityShort freeze-drying process timePowder deliveryMetabolism disorderFreeze-dryingSodium ascorbate
Owner:SHANXI PUDE PHARMA CO LTD
Optimum matching multiple water-soluble vitamin medicinal composition containing high-efficiency bacteriostat and preparation thereof
InactiveCN101259278BReduce dosageHigh activityMetabolism disorderPharmaceutical non-active ingredientsPotential toxicitySide effect
The invention relates to a drug combination of water-soluble multivitamins with the best proportion containing the new efficient bacteriostatic agent and a preparation method thereof. The drug combination includes, for example, the preferable weight portion ratio is: 0.1 portion of compound distributed phydroxy sodium formate, 3.1 portions of vitamin B1, 4.9 portions of vitamin B2, 40 portions ofnicotinamide, 4.9 portions of vitamin B6, 16.5 portions of sodium pantothenate, 113 portions of sodium vitamin C, 0.06 portion of biotin, 0.4 portion of folic acid and 0.005 portion of vitamin B12. Used for treating the lack of vitamins or preventing the deficiency of vitamins, the drug combination contains the new efficient bacteriostatic agent in very low amount, eliminates the side effects of the bacteriostatic agent on human bodies and ensures the safety of the bacteriostatic agent. Simultaneously, the vitamin content ratios of the components are all very low, thus solving the adverse reactions caused by the excess water-soluble multivitamins or the generated potential toxicity. The drug combination of the invention can be made into injections or lyophilized agents, produced through the conventional process and equipment and has the advantages of easy industrialization, stable quality and low cost.
Owner:南京丰恺思药物研发有限公司
High-grade chipping-resistant water-based ink and preparation method thereof
The invention discloses high-grade chipping-resistant water-based ink and a preparation method thereof. The high-grade chipping-resistant water-based ink is prepared from mineral oil, pigments, and the like, wherein cellulose resin is lac resin, hydroxypropyl cellulose, hydroxypropyl methyl cellulose, carboxymethyl cellulose, methyl cellulose, microcrystalline cellulose, gelatin, casein, and the like; a conductive agent is sodium lactate, sodium acetate or sodium pantothenate; the high-grade chipping-resistant water-based ink is prepared from 1-30 parts of charcoal powder, 1-30 parts of lac resin, 0.1-20 parts of cellulose resin, 40-90 parts of more than 60% (v / v) ethanol, 3-20 parts of water and 0.1-2 parts of conductive agent by weight. The water-based ink and the preparation method have the beneficial effects that by adopting the preparation method comprising the steps of weighing the materials according to weight and parts by weight, preparing a first solvent, preparing a dispersion, preparing a lac solution and preparing the high-grade chipping-resistant water-based ink after mixture, the prepared water-based ink does not easily undergo chipping, is convenient to use, has good safety performance, is low in maintenance cost and has longer service life.
Owner:HANGZHOU TALENT DECORATION PAPER
Water-soluble vitamin composition freeze-drying preparation for injection
ActiveCN101904862BGranularity adjustableConcentrated particle size distributionPowder deliveryMetabolism disorderFreeze-dryingDissolution
The invention discloses a water-soluble vitamin composition freeze-drying preparation for injection. The freeze-drying preparation comprises the following raw materials and is prepared into 1,000 bottles: 2.8 to 3.4 grams of thiamine mononitrate, 36 to 44 grams of nicotinamide, 4.4 to 5.4 grams of pyridoxine hydrochloride, 14.8 to 18.1 grams of sodium pantothenate, 4.4 to 5.4 grams of riboflavin sodium phosphate, 102 to 124 grams of sodium vitamin C, 54 to 66 milligrams of biotin, 0.36 to 0.44 gram of folic acid, 124.5 to 6.0 milligrams of vitamin B, and 0.4 to 0.6 gram of methyl-p-hydroxy benzoate, wherein the nicotinamide is nicotinamide hydrate, and the sodium pantothenate is sodium pantothenate hydrate. The water-soluble vitamin for injection has adjustable grain size, concentrated grain size distribution, glabrous surface, high product fluidity, greatly improved stability and high dissolution rate, and a preparation process of the preparation is simple and is favorable for popularization and use.
Owner:SHANDONG YUXIN PHARMA CO LTD
A preparation method of cartilage inductive matrix for microfracture surgery
ActiveCN105255814BPromote differentiationEasy to operateVertebrate cellsArtificial cell constructsInsulin-like growth factorVitamin C
The invention provides a cartilage-inducing matrix for microfracture surgery and a preparation method thereof. The cartilage-inducing matrix includes gel and additives, the gel is fibrin, collagen, hyaluronic acid, and thrombin; the additive is in the culture medium The final concentration of vitamin C: 233μM-287μM; linoleic acid: 3.7μM-8.9μM; cholesterol: 9μM-17μM; dexamethasone: 6nM-15nM; acetylcysteine: 43μM-58μM; transferrin: 18μg / mL~32μg / mL; sodium selenite: 22nM~52nM; sodium pantothenate: 13μM~24μM; biotin: 28μM~43μM; insulin-like growth factor: 7μg / mL~18μg / mL; epidermal growth factor: 2ng / mL~9ng / mL; fibroblast growth factor: 2ng / mL~9ng / mL; platelet-derived growth factor: 2ng / mL~9ng / mL; transforming growth factor: 30ng / ml~70ng / ml; 1%.
Owner:SHANGHAI BIOMED UNION BIOTECHNOLOGY CO LTD
Water-soluble vitamin pharmaceutical composition for injection
ActiveCN104382865BQuality assuranceImprove efficiencyPowder deliveryMetabolism disorderSodium lactateVitamin B12
The invention relates to a water-soluble vitamin pharmaceutical composition for injection. The water-soluble vitamin pharmaceutical composition for injection is prepared from 3.1g of thiamine mononitrate, 40g of nicotinamide, 16.5g of sodium pantothenate, 60mg of biotin, 5.0mg of vitamin B12, 0.5g of disodium ethylene diamine tetraacetate, 4.9g of riboflavin sodium phosphate, 4.9g of pyridoxine hydrochloride, 113g of sodium vitamin C, 0.4g of folic acid, 300g of glycine, 0.5g of methyl parahydroxybenzoate, 1.2g of poloxamer 188, 0.5g of sodium lactate, 1.0g of L-malic acid and water for injection, wherein the water for injection is added until the total volume is 2000ml. The water-soluble vitamin pharmaceutical composition for injection, disclosed by the invention, is good in stability and greatly improved in medication safety.
Owner:湖北美林药业有限公司
Method for preparing D-sodium pantothenate from D-calcium pantothenate mother liquor
InactiveCN112321447ALow priceHigh purityOrganic compound preparationCarboxylic acid amide separation/purificationBiotechnologyActivated carbon filtration
The invention discloses a method for preparing sodium D-pantothenate from calcium D-pantothenate mother liquor. The method comprises the following steps: carrying out reflux concentration on the calcium pantothenate mother liquor, adding water, stirring, adding sodium carbonate, reacting, filtering, adding dilute sulfuric acid into the filtrate to regulate the pH value, adding activated carbon, filtering, concentrating the filtrate, adding methanol, cooling, crystallizing, filtering, and drying to obtain sodium D-pantothenate. According to the invention, 10%-20% of calcium D-pantothenate stillremains in the crystallization mother liquor of calcium D-pantothenate to prepare sodium pantothenate. A solvent used in the reaction is low in price, a mother solution needing to be recycled is usedas a raw material to prepare a new product, the cost has huge advantages, the reaction is environmentally friendly, the safety is high, the reaction time is short, the solvent can be recycled, the steps are simple, and the purity of the obtained sodium D-pantothenate product is high.
Owner:安徽泰格生物科技有限公司
Methods for preparing beta-alanine, beta-alanine salt and pantothenate
PendingUS20220185769A1Avoid problemsReduce contentCarbon-nitrogen lyasesOrganic compound preparationPtru catalystSodium pantothenate
Provided is a method for preparing β-alanine, the method comprising: preparing a β-alanine product from a reactant containing fumaric acid and aqueous ammonia in the presence of a catalyst, wherein the catalyst contains a catalyst composition containing aspartase and L-aspartic acid-α-decarboxylase, and adding fumaric acid during the reaction, wherein the total moles of the fumaric acid added is equal to the initial moles of the aqueous ammonia in the reactant minus the initial moles of the fumaric acid in the reactant. Also provided are methods for preparing a β-alanine salt (in particular calcium β-alanine, sodium β-alanine, and potassium β-alanine) and a pantothenate (in particular calcium pantothenate, sodium pantothenate, and potassium pantothenate).
Owner:GUANG AN MOJIA BIOTECHNOLOGY CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com