Method for detecting sodium pantothenate isomers
A detection method, the technology of sodium pantothenate, applied in the direction of measuring devices, instruments, scientific instruments, etc., to achieve the effects of high precision, good stability, and high system applicability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] (1) Experimental materials and instrument conditions
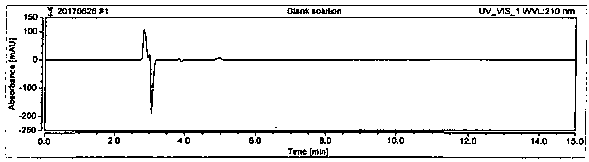
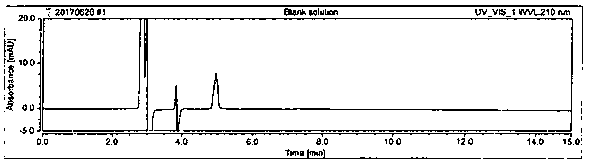
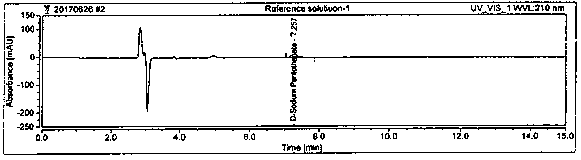
[0046] Instrument: High performance liquid chromatography: Thermo; Chromatographic column: Chiralpak AD-H, 5 μm, 4.6×250 mm; Flow rate: 1.0mL / min; Column temperature: 25°C; Injection volume: 10 μl; Running time: 15min; Detection Wavelength: 210nm. Inject the blank solution, system suitability solution and test solution into the liquid chromatograph respectively, record the chromatogram, and the chromatographic conditions are as follows: Chromatographic column: use amylose-tris(3,5-xylylcarbamate) derivative As filler; flow rate 1.0mL / min; column temperature: 25°C; injection volume: 10μl; running time: 15min; detection wavelength: 210nm; mobile phase: n-hexane-isopropanol-trifluoroacetic acid, gradient elution , and its gradient is shown in Table 1.
[0047] Table 1 - Gradient Elution Conditions
[0048] time (min)
mobile phase A
mobile phase B
mobile phase C
0
85
15
0.2
5 ...
Embodiment 2
[0063] Embodiment 2 detection method system applicability test of the present invention
[0064] System suitability is achieved by measuring the RSD of the peak area of D-sodium pantothenate in the 5-pin reference solution and the resolution of the peaks of D-sodium pantothenate and L-sodium pantothenate in the system suitability solution. The RSD of the peak area of D-sodium pantothenate is ≤2.0%, and the resolution of the peaks of D-sodium pantothenate and L-sodium pantothenate in the system suitability solution is ≥1.5. Prepare blank solution, reference solution, and system suitability solution as described in Example 1. Under the chromatographic conditions described in Example 1, enter 1 needle of blank solution, 5 needles of reference solution, and 1 needle of system suitability solution to obtain a chromatogram Figure, such as figure 1 , figure 2 , image 3 , Figure 4 , Figure 5 and Image 6 , according to the formula conversion results are shown in the tabl...
Embodiment 3
[0068] Embodiment 3 Detection method specificity test of the present invention
[0069] The specificity of the method is realized by studying the peak identification and selectivity, and it is required that the blank solvent does not interfere with the detection; the D-sodium pantothenate positioning solution has no interference with the detection of L-sodium pantothenate; The resolution of adjacent peaks should be ≥1.5, and the recovery rate of sodium L-pantothenate should be between 90.0% and 110.0%. Prepare blank solution, DL-sodium pantothenate stock solution, L-sodium pantothenate fixing solution (system suitability solution), D-sodium pantothenate fixing solution and selective solution as described in Example 1. After the system is balanced, inject 1 needle of blank solution, 3 needles of L-sodium pantothenate positioning solution, 3 needles of selective solution, and 1 needle of D-sodium pantothenate positioning solution, and record the chromatogram, as figure 1 , fi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| separation | aaaaa | aaaaa |
| separation | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com