Establishment method of fingerprint spectrum of traditional Chinese medicine composition
A technology of fingerprints and establishment methods, which is applied in the field of establishment of Kechuanshun pills and fingerprints, can solve the problems of few Kechuanshun pills and cannot fully reflect the effective chemical information of Kechuanshun pills, so as to ensure drug safety, The effect of guaranteeing the effect of treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0085] Example 1 Establishment of Chromatographic Conditions
[0086] Preparation of the test article:
[0087] Take 3g of the sample of batch number FT1641, pulverize it, accurately weigh it, place it in a 50ml Erlenmeyer flask, add 25ml of methanol precisely, ultrasonicate for 30min, let it cool to room temperature, and obtain it.
[0088] Chromatographic conditions:
[0089] Chromatographic instrument: Agilent 1200 high performance liquid chromatograph, chromatographic column: Agilent SB-C18 chromatographic column, column temperature 30°C, injection volume 10 μl, flow rate: 1ml / min.
[0090] 1.1 Determination of UV detection wavelength
[0091] The mobile phase is acetonitrile-0.1% phosphoric acid / water, using gradient elution, the elution time is 120 minutes, the volume percentage of acetonitrile is from 5% to 95%, and the volume percentage of 0.1% phosphoric acid solution is from 95% to 5%; respectively examine 225nm and 254nm , 283nm, 300nm, and 320nm wavelength ch...
Embodiment 2
[0112] Example 2 Examination of sample preparation methods
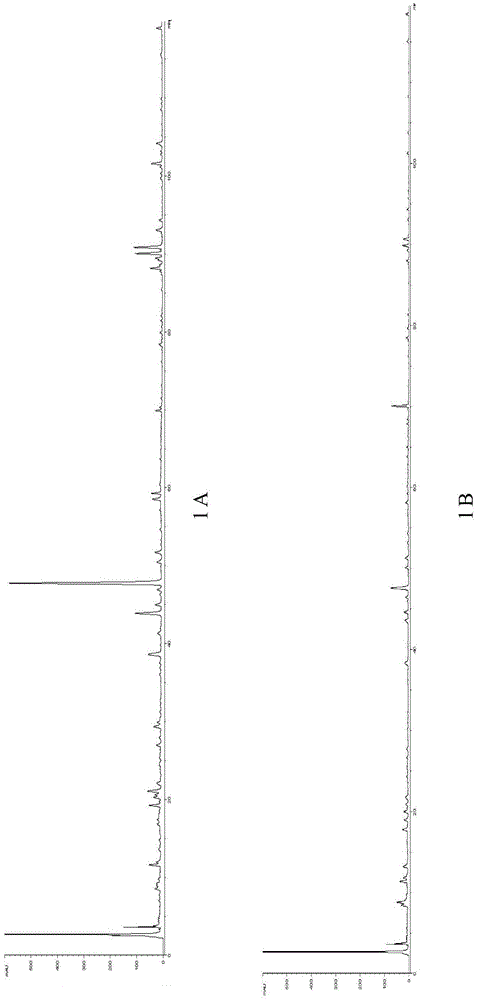
[0113] Under the chromatographic conditions established in Example 1, the chromatograms of the Kechuanshun Pill test solution prepared by ultrasonic extraction, reflux extraction, and cold extraction were investigated respectively.
[0114] Ultrasonic extraction: Take 3 g of Kechuanshun pills with batch number FT1641, pulverize them, accurately weigh them, place them in a 50ml Erlenmeyer flask, add 25ml of methanol precisely, and after ultrasonication for 30min, let cool to room temperature to obtain the obtained product.
[0115] Reflux extraction: Take 3g of Kechuanshun pills with batch number FT1641, pulverize them, accurately weigh them, put them in a 50ml Erlenmeyer flask, add 25ml of methanol precisely, heat to reflux for 2 hours, let cool to room temperature, and obtain the obtained product.
[0116] Cold soaking extraction: Take 3g of Kechuanshun pills with batch number FT1641, pulverize them, accurately we...
Embodiment 3
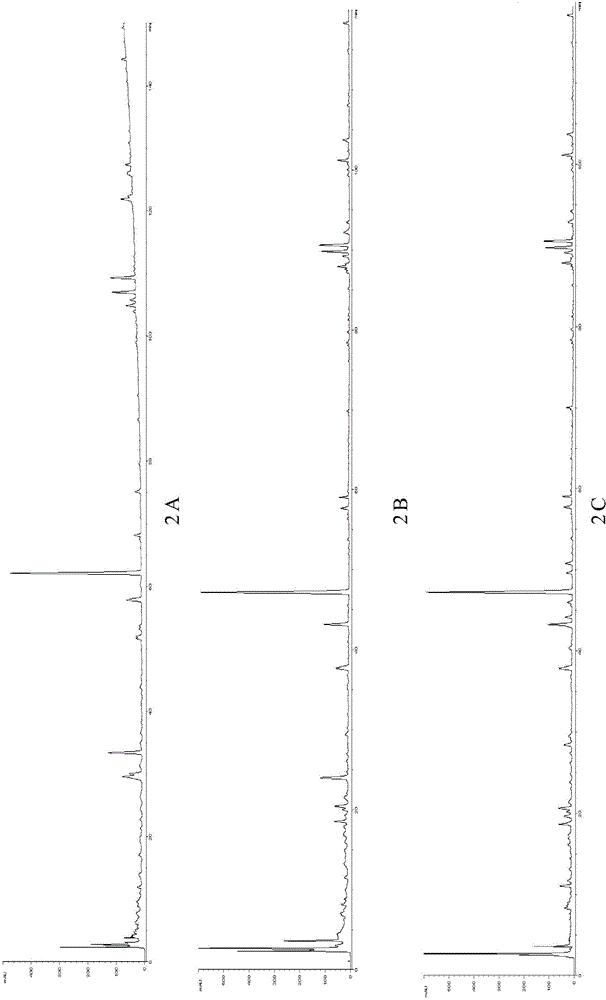
[0119] Example 3 Composition testing of Kechuanshun pills
[0120] 1. Chromatographic column: model is Agilent SB-C18 (5μm, 250×4.6mm ser#:880952-708);
[0121] 2. Mobile phase: use acetonitrile-0.1% phosphoric acid / water solution as the mobile phase, and carry out gradient elution according to the regulations in Table 2-4;
[0122] 3. Flow rate: 1.0mL / min;
[0123] 4. Detection wavelength: 225nm;
[0124] 5. The column temperature is 30°C;
[0125] 6. Preparation of the test solution: prepared according to the method described in the "ultrasonic extraction" item in Example 2;
[0126] 7. Determination method: Precisely draw 10 μl of the test solution, inject it into a liquid chromatograph, measure, and record the chromatogram for 120 minutes.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com