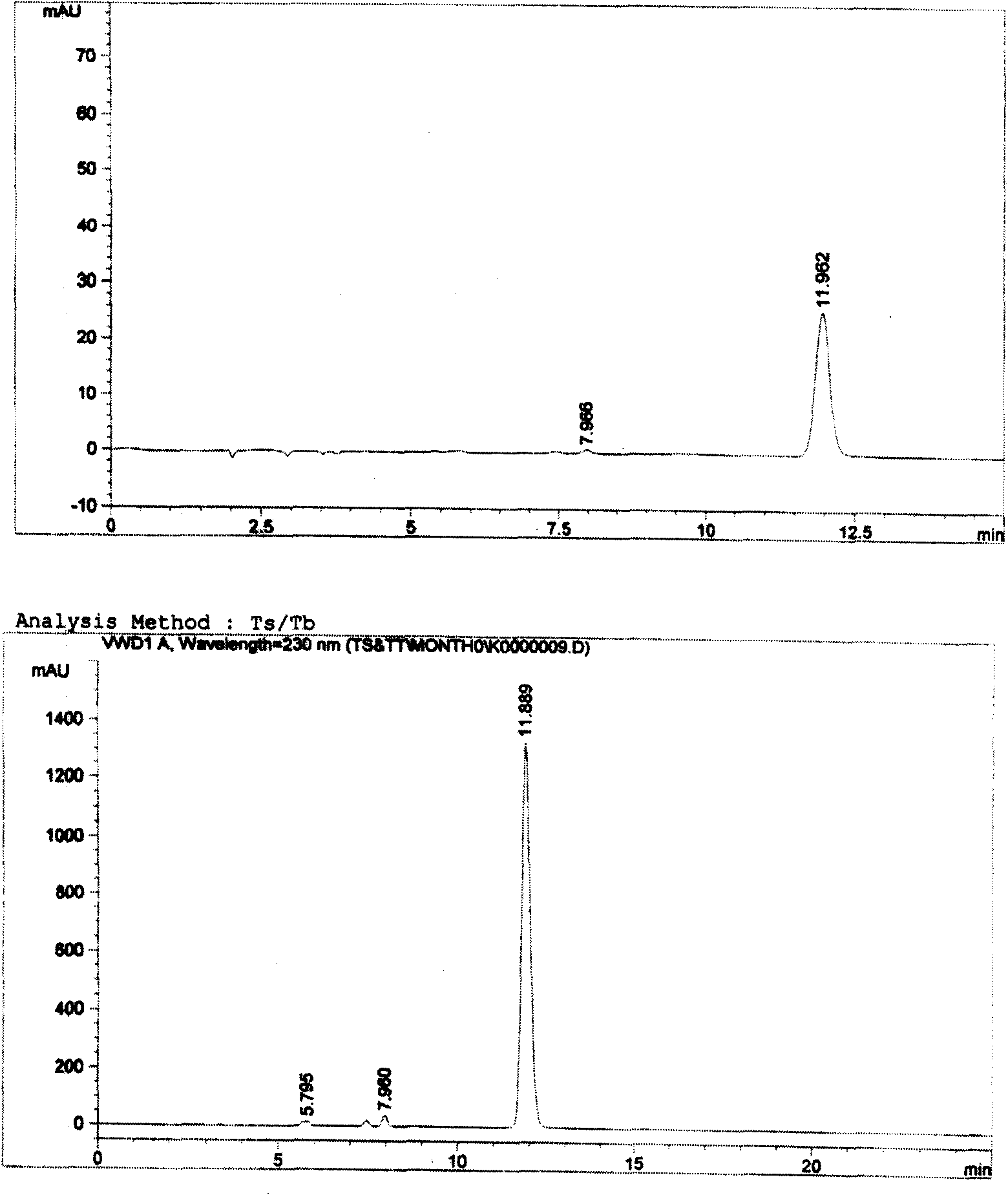
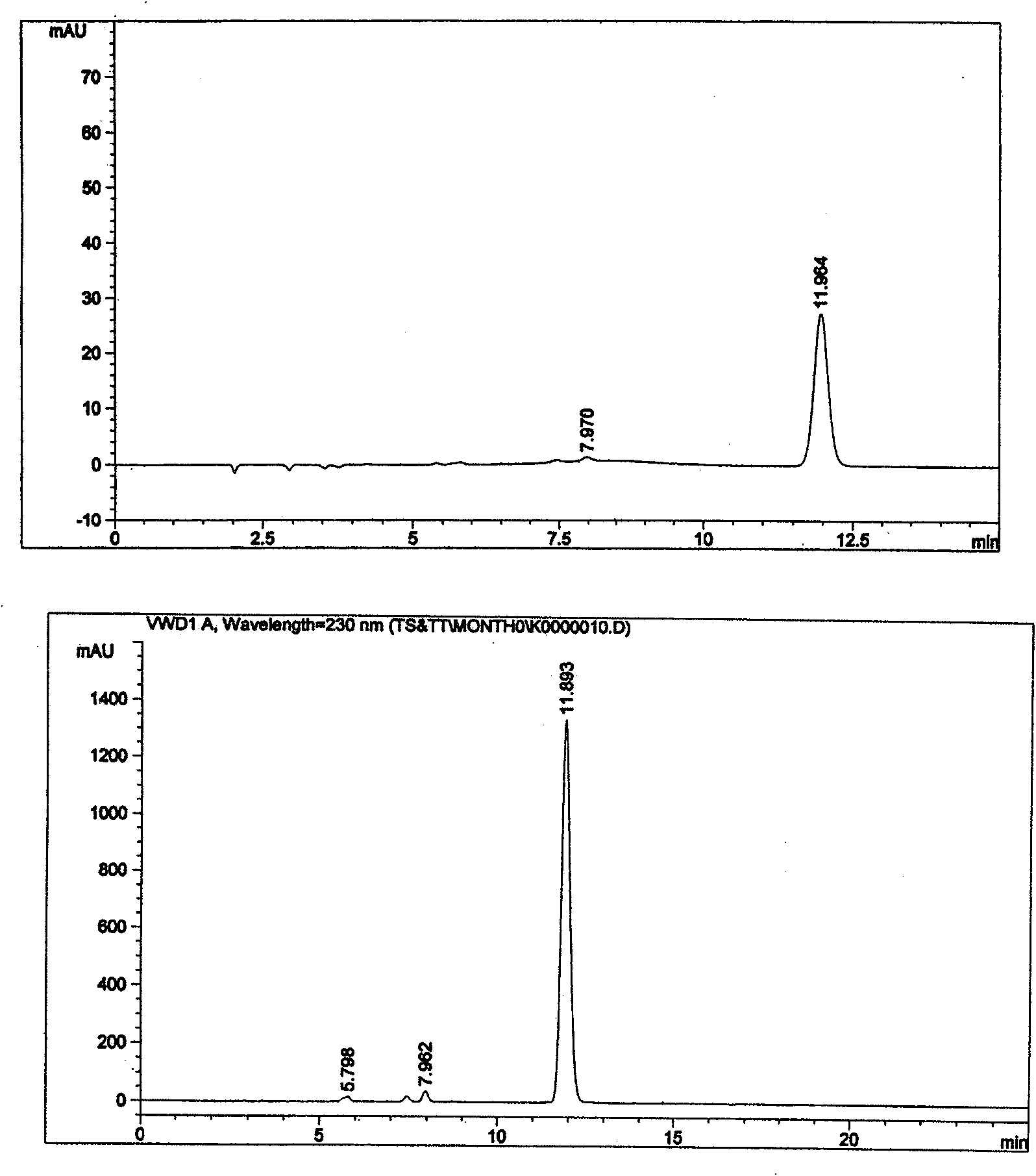
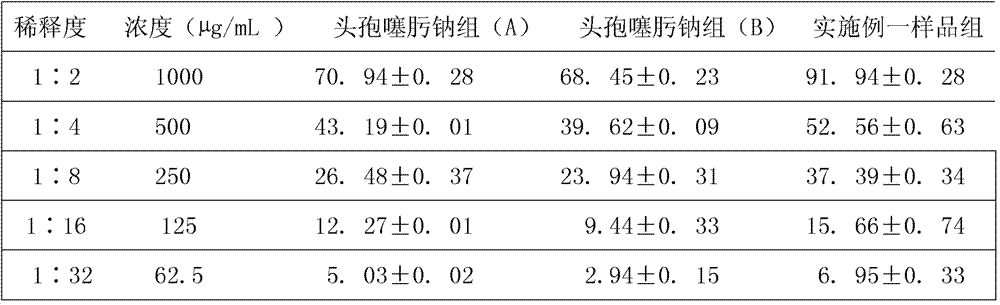
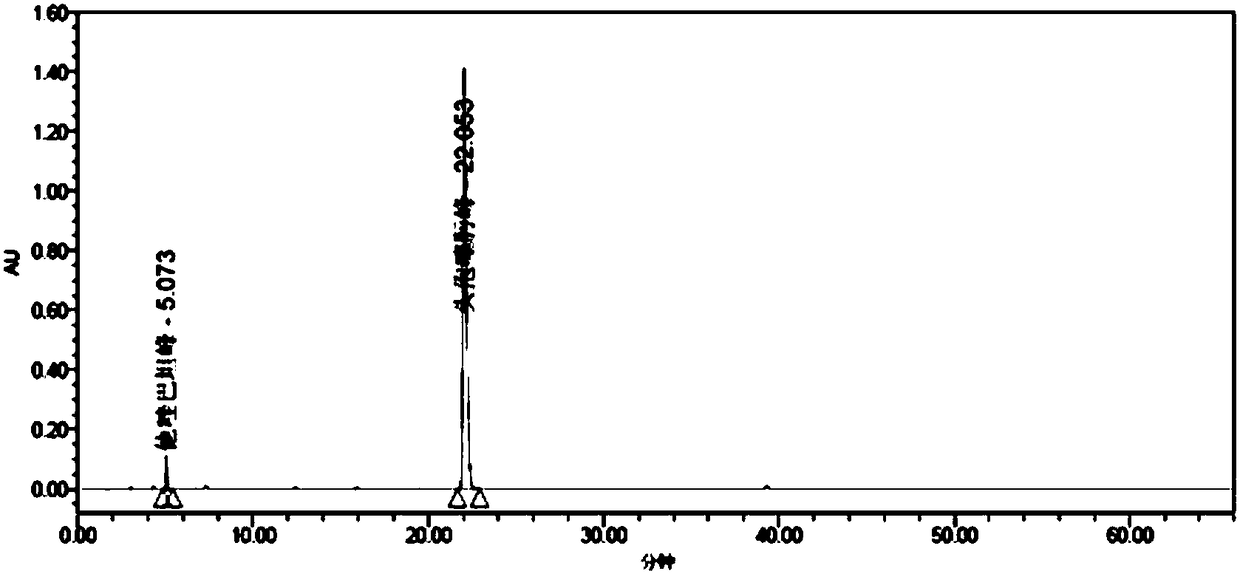
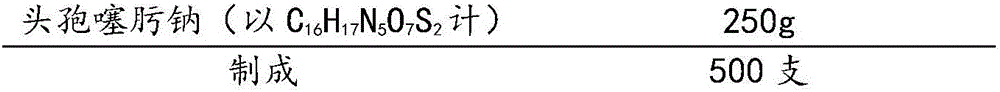Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
89 results about "Cefotaxime Sodium" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
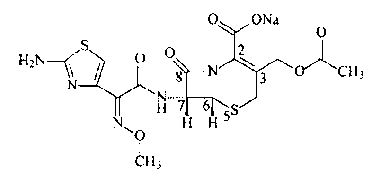
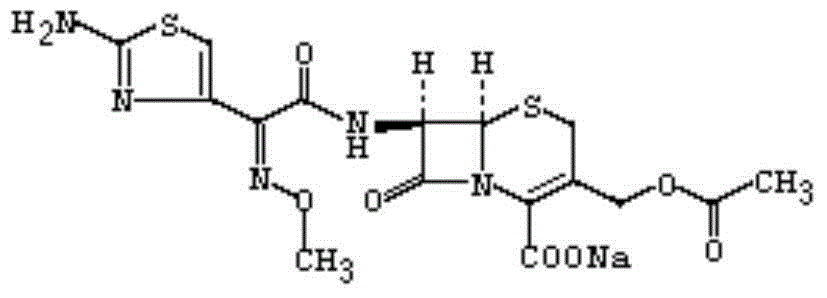
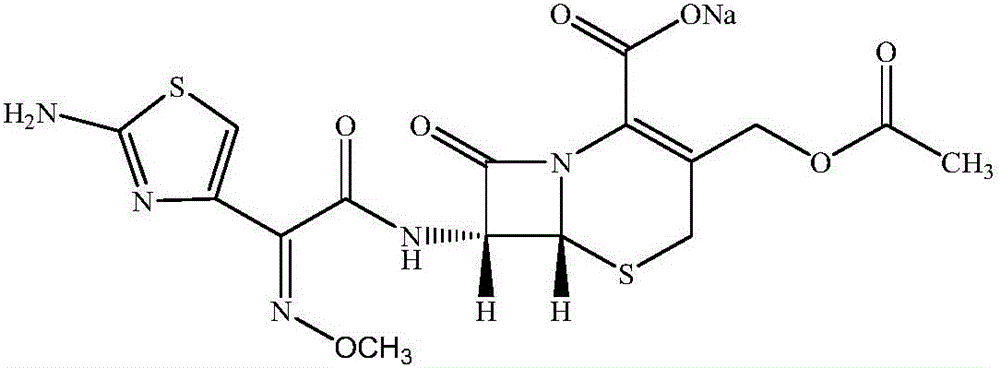
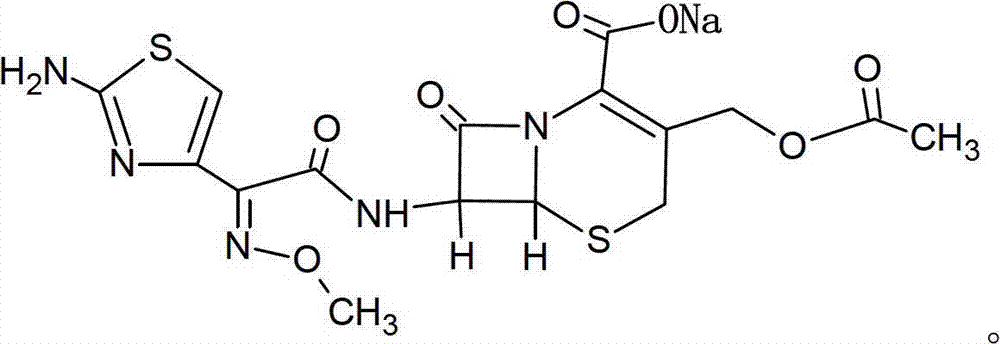
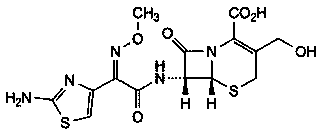
The sodium salt form of cefotaxime, a beta-lactam, third-generation cephalosporin antibiotic with bactericidal activity. Cefotaxime sodium binds to and inactivates penicillin-binding proteins (PBP) located on the inner membrane of the bacterial cell wall. Inactivation of PBPs interferes with the cross-linking of peptidoglycan chains necessary for bacterial cell wall strength and rigidity. This results in the weakening of the bacterial cell wall and causes cell lysis. Compared to the second and first generation cephalosporins, cefotaxime sodium is more active against gram-negative bacteria and less active against gram-positive bacteria.
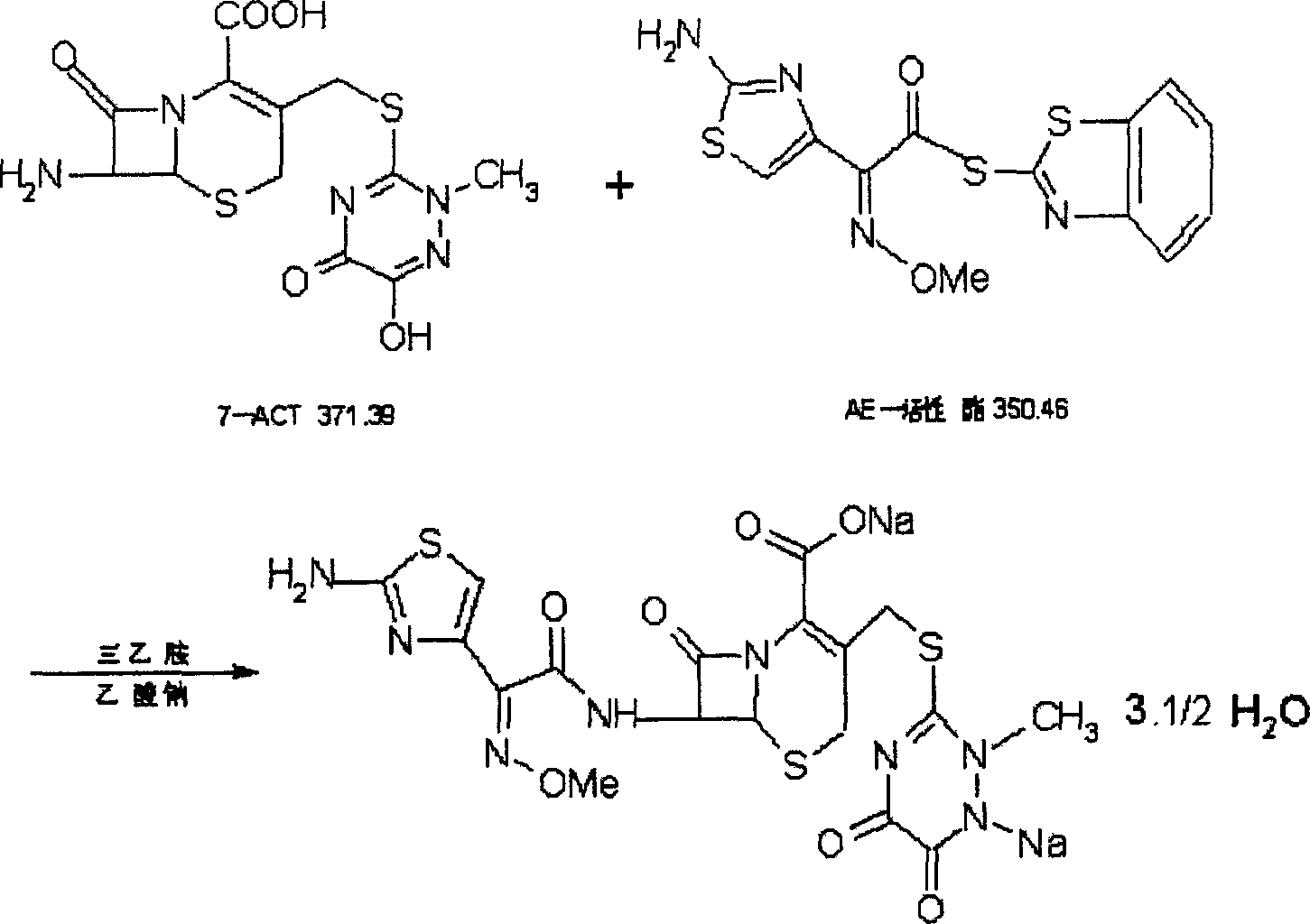
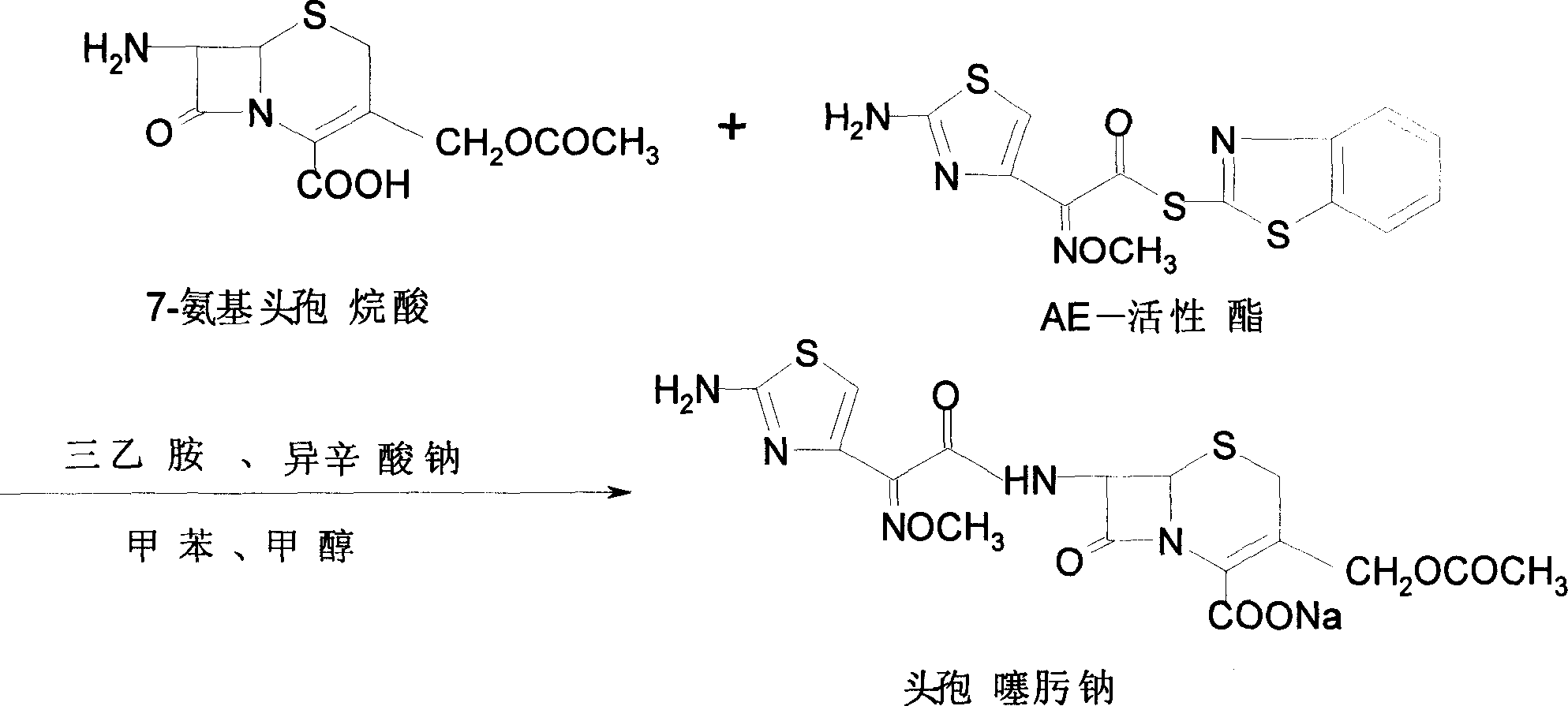
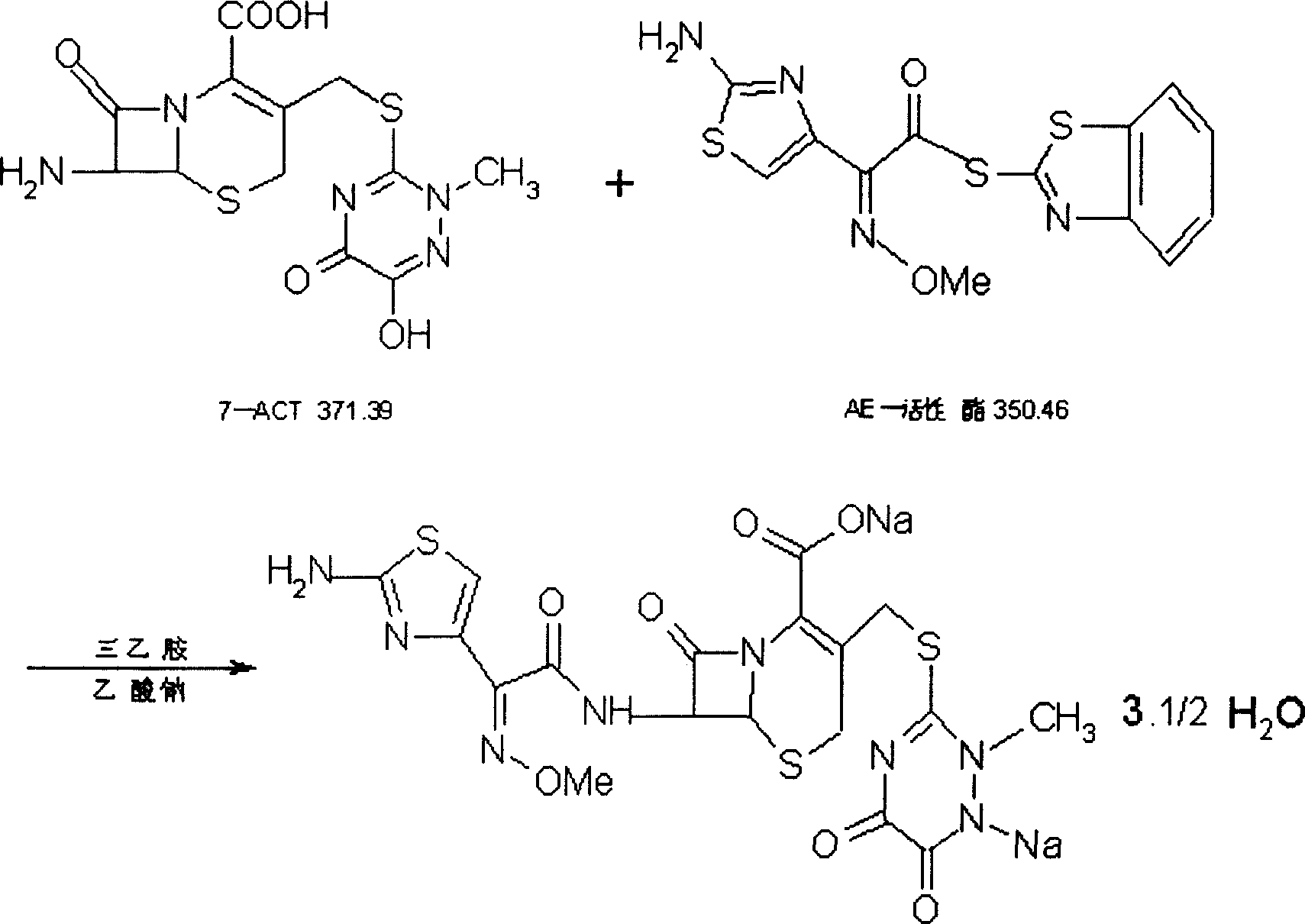
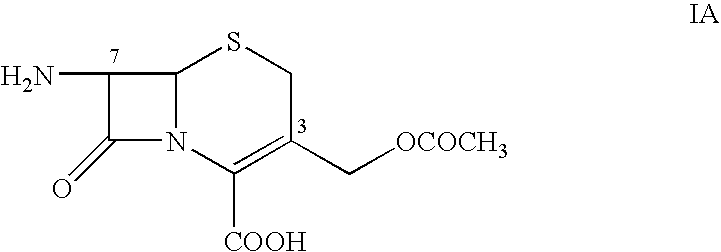
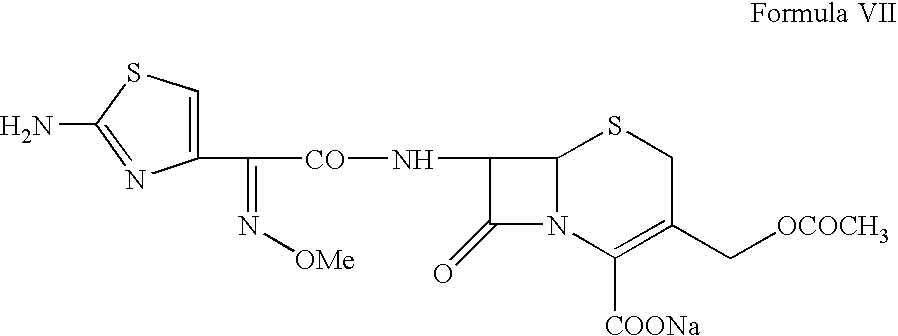
Process for preparing ceftriaxone sodium
Disclosed is a process for preparing ceftriaxone sodium belonging to compound preparation technical field. Protected by nitrogen, 7-ACT3 reacts with AE-active ester under the action of amine intermediate reactant in a solvent. Then a sodium salt forming agent is added in, and the cefotaxime sodium is obtained after reaction and seedout. The solvent is a mixed solvent composed of alkane halocarbon, ethyl acetate, or acetone and alcohol solvent and water. The solvent dosage is small, and the product yield is high.
Owner:REYOUNG PHARMA
Method for converting cefotaxime acid into sodium salt crystal
The invention provides a crystallization method for concerting cefotaxime acid to sodium salt; the method comprises the steps: water, acetone and anhydrous sodium acetate are added into a three-necked bottle; cefotaxime acid is added into the three-necked bottle after the temperature is lowered; then, the mixture in the three-necked bottle is stirred to cause the mixture to dissolve; next, added with active carbon, stirred, filtered and washed with acetone; the filter liquor is combined, added with acetone by dripping and added with crystal seeds, crystallized, stirred, added with acetone by dripping again, cooled, crystallized, filtered, soaked and washed with acetone for twice and dried in vacuum to obtain cefotaxime sodium crystal. The crystallization method has the advantages of simple menstruum, simplicity and feasibility, low cost, good crystal forms, good mobility, easy sub-package, high clarity and good color grade, thus being suitable for large-scale promotion and application and having obvious economic benefits.
Owner:FUJIAN FUKANG PHARMA
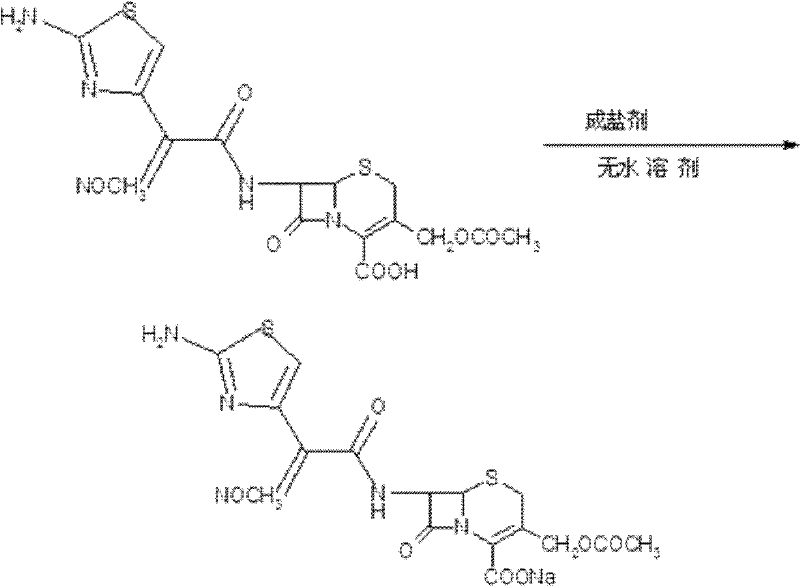
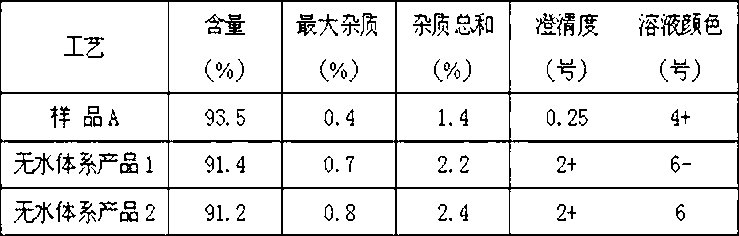
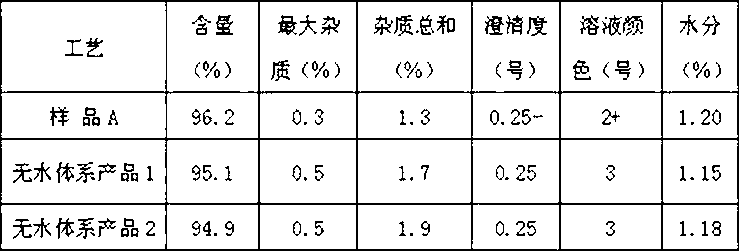
Preparation technology of anhydrous crystal of cefotaxime sodium
InactiveCN102584854ASimple and fast operationShorten the production cycleOrganic chemistryDrug compoundFiltration
The invention relates to a preparation technology of anhydrous crystals of cefotaxime sodium and belongs to the technical field of preparation of medicine compounds. The preparation technology of anhydrous crystals of cefotaxime sodium is characterized in that under the condition of certain temperature, cefotaxime acid and a salt forming agent are added in anhydrous solvent for the salt forming reaction, and after decolorization and fine filtration, crystallization solvent is dripped for precipitating the crystals. The preparation technology has the advantages of simplicity and convenience in operation, short production period and low energy consumption; the crystals are low in moisture content and good in stability, wherein the moisture content is 0.5% to 1.0%; and the obtained crystals are uniform in crystallized grains, good in color, high in purity and stable in quality.
Owner:REYOUNG PHARMA
Crystallization method for cefotaxime sodium
The invention discloses a crystallization method for cefotaxime sodium. According to the method, the cefotaxime sodium is prepared by an organic solvent precipitation method in a water-containing system. The method comprises the following preparation steps: adding cefotaxime acid and a sodium salt into a solvent to perform a salt forming reaction at a certain temperature; decoloring, filtering and washing the salt; merging filtrate; adding the solvent dropwise; growing crystals after adding seed crystals; adding a crystallization solvent dropwise for crystallizing; filtering, washing and drying the crystals in vacuum. The cefotaxime sodium obtained by the method has uniform crystal forms, low specific volume and high fluidity, and is easy to package; the cefotaxime sodium is high in clarity, high in purity, light in solution color, low in impurity content, and high in stability, and is easy to store; the process disclosed by the invention is easy to operate and high in product yield. By utilizing the method, the cefotaxime sodium crystals are prepared in the water-containing system, so that the traditional concept that the quality of the product prepared in the water-containing system is poor is broken.
Owner:NORTH CHINA PHARMA HEBEI HUAMIN PHARMA
Preparation method of cefotaxime sodium crystal
ActiveCN103275101APrevent gelGranular concentrationOrganic chemistrySodium acetateSodium acetrizoate
The invention relates to a preparation method of a cefotaxime sodium crystal. The preparation method comprises the following steps of: dissolving sodium acetate in a mixed solvent of organic solvent and water below 10-40 DEG C, wherein the volume fraction of organic solvent in the mixed solvent is 30-70%; adding cefotaxime acid, and stirring until the cefotaxime acid reacts in the solution; adding a cefotaxime sodium crystal in the solution, then adding elution agents with a feeding time of 2-8 hours, cooling to 5 DEG C below zero to 5 DEG C; and filtering, washing and drying a crystal slurry to obtain the cefotaxime sodium crystal. By the preparation method, the phenomenon of gelatinization frequently seen in the crystallization process of the cefotaxime sodium crystal is avoided, the size distribution of the products is centralized and the major particle size is adjustable from 5mu m to 60my m, the liquidity of the product is good, the process yield is higher than 87%, and the product purity is higher than 95.5%.
Owner:TIANJIN UNIV
Process for preparing cefotaxime sodium
Disclosed is a process for preparing cefotaxime sodium belonging to compound preparation technical field. In a solvent, 7-ACA reacts with AE-active ester under the action of amine intermediate reactant, then a sodium salt forming agent is added in, and the cefotaxime sodium is obtained after reaction and seedout. The invention is characterized in that the solvent is a mixed solvent composed of benzene organic solvent, ethyl acetate, or acetone and alcohol organic solvent. The technological process is simplified, and the product yield is high.
Owner:REYOUNG PHARMA
One-step preparation process of aseptic cefotaxime sodium for injection
The invention relates to a preparation technique for cefotaxime sodium, which comprises: in solvent, reacting between 7-ACA and AE-active ester with amine as intermediate; adding benzothiazole as assisting solvent and sodium salt agent, separating and crystallizing to obtain the product. Wherein, the reaction solvent comprises benzene organic solvent, acetic ester or acetone and alcohol; stirring the 7-ACA and AE-active ester till clear, adding assisting solvent to benzothiazole; degerming, filtering, adding sodium salt agent for reaction; when liquid shows turbid, feeding crystal; separating the crystal by insoluble solvent to cefotaxime sodium, taking crystallization post-treatment and obtaining the product. This invention needs just one-step operation, has good crystallization and short production period, improves product yield, and decreases solvent harm to operation crews.
Owner:REYOUNG PHARMA
Preparation method of cefotaxime sodium spherical crystals
ActiveCN108409753AUniform particle sizeHigh bulk densityOrganic chemistry methodsOrganic solventSeed crystal
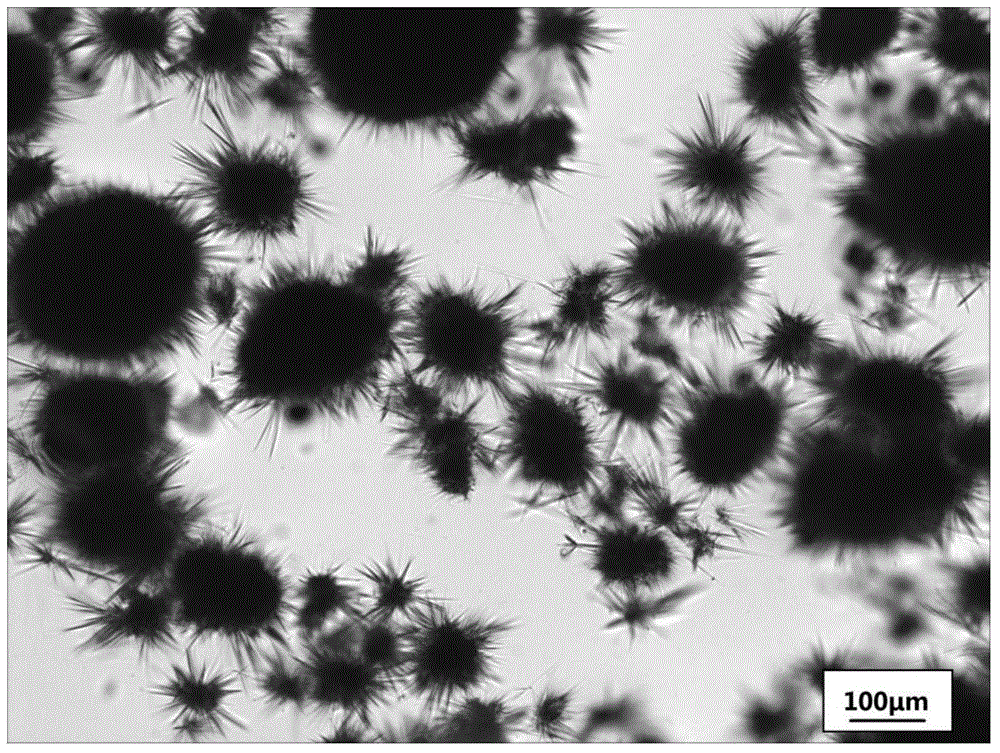
The invention discloses a preparation method of cefotaxime sodium spherical crystals. The preparation method comprises the following steps: preparing a mixed solution of water-organic solvent at 10 to30 DEG C, wherein water and an organic solvent are in the mass ratio of (1 to 1) to (1 to 5); then, preparing a mixed solution of cefotaxime sodium-water-organic solvent with the cefotaxime sodium concentration of 0.1 to 0.3 g / mL; adding a bridging agent, adding cefotaxime sodium seed crystals, and stirring for 5 to 60 minutes; adding the same organic solvent as the organic solvent in the mixed solution dropwise, continuing stirring until crystals separate out, and after the organic solvent is added completely, keeping stirring for 0.1 to 5 hours so that crystals aggregate into spheres; filtering, washing and drying to obtain the cefotaxime sodium spherical crystals. A crystallizing process does not have a gelatinizing phenomenon; the average particle size of a spherical crystal product is about 100 to 200 microns; the crystal particles are mellow and full; the fluidity is high; the tap density is 0.3 to 0.4 g / cm<3>. The product can be subjected to tabletting directly; granulating anddrying processes are omitted; the cost is reduced.
Owner:TIANJIN UNIV
Preparation method of cefotaxime sodium
ActiveCN104086569ALow color levelImprove liquidityOrganic chemistryCeftizoximeCrystallization temperature
The invention discloses a preparation method of cefotaxime sodium. Ceftizoxime acid used as the initial raw material reacts with anhydrous sodium acetate to generate the cefotaxime sodium. In such process, purified water, butanol and acetone are selected as crystallizing solvents to control the mixing speed and crystallization temperature in the crystallization process, so that the finally obtained cefotaxime sodium has favorable flowability and can satisfy the subpackaging requirements in production. Various quality indexes of the obtained cefotaxime sodium conform to the requirements for medicinal standard, and thus, the cefotaxime sodium can satisfy the demands for clinical application.
Owner:石药集团中诺药业(石家庄)有限公司
Novel method for measuring compound cefotaxime sodium sulbactam sodium
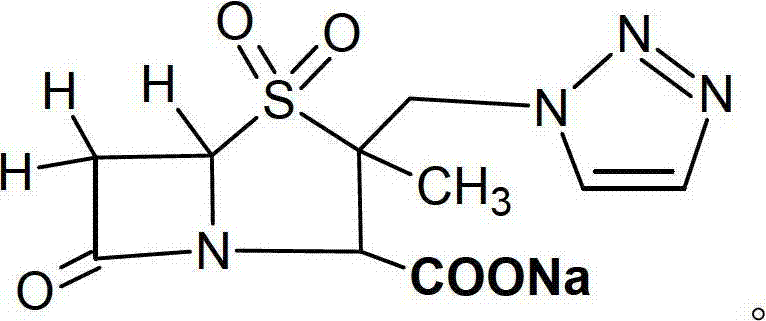
The invention relates to a novel high performance liquid chromatogram (HPLC) method, which can simultaneously detect the content of two single ingredients and relevant impurities in the cefotaxime sodium sulbactam sodium compound. The two ingredients have no interferences or influences. The method has easy operation, strong specificity, high sensitivity, large linear range and good stability, and can be used for detecting a compound preparation and raw materials.
Owner:XIANGBEI WELMAN PHARMA CO LTD
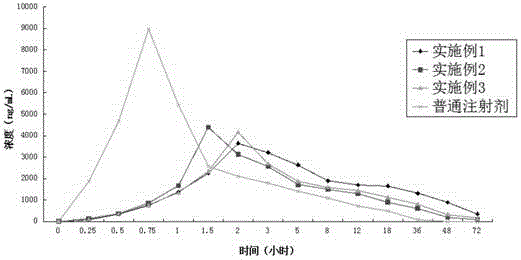
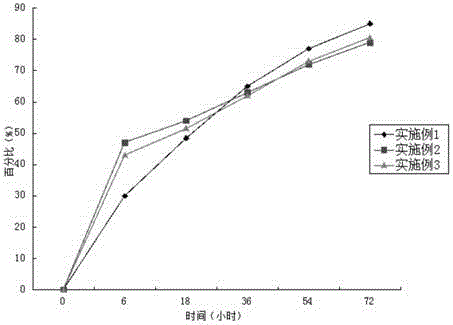
Long-acting cefotaxime sodium injection and preparation method thereof
ActiveCN104644547AImprove efficacyStable blood concentrationAntibacterial agentsOrganic active ingredientsRoom temperatureChitosan succinate
The invention relates to a long-acting cefotaxime sodium injection and a preparation method of the injection. The injection comprises a biodegradable polymer material, wherein the biodegradable polymer material comprises an injectable hydrogel. The preparation method comprises the following steps: mixing the medicines and N-chitosan succinate solution at a certain temperature and stirring rate, and mixing the mixture with carboxymethyl chitosan solution; adding oxidized chondroitin sulfate solution and glutaraldehyde solution in a certain time while stirring, injecting the solution into a circular mold with the bottom diameter of 10mm after stirring, forming drug-loading hydrogel, and performing vacuum drying to constant weight at room temperature.
Owner:BEIJING RED SUN PHARMA
Method for inducing production of tripterygium wilfordii hairy root by agrobacterium rhizogenes
InactiveCN102321664AFill in the gapsVector-based foreign material introductionAngiosperms/flowering plantsRhizobium rhizogenesTriptolide
The invention provides a method for inducing the production of tripterygium wilfordii hairy roots by agrobacterium rhizogenes, which comprises the following steps: (1) obtaining aseptic explants; (2) preparing bacterial liquid of agrobacterium rhizogenes; (3) performing agrobacterium rhizogenes infection to induce hairy roots; (4) establishing a hairy root in-vitro culture system, wherein the establishment of the hairy root in-vitro culture system comprises the following steps: culturing hairy roots in a 1 / 2 MS solid medium containing 500 mg / L cefotaxime sodium under a dark culture condition with a temperature of 27 + / -1 DEG C, performing subculture once every 7 days, reducing the using concentration of cefotaxime sodium gradually during subculture till no bacterium is found, finally putting the completely aseptic hairy roots on the 1 / 2 MS solid medium for subculture and preservation. During the detection of the produced aseptic tripterygium wilfordii hairy roots, a considerable amount of triptolide is found to be contained in the hairy roots, and the content is significantly higher than the content of triptolide in traditional tripterygium wilfordii wild roots and tissue culture roots; the method has good prospects for industrial application. The production of triptolide by hairy root culture fills the gap of traditional Chinese medicine.
Owner:FUJIAN AGRI & FORESTRY UNIV
Novel method for measuring compound cefotaxime sodium tazobactam sodium
The invention relates to a novel high performance liquid chromatogram (HPLC) method, which can simultaneously detect the content of two single ingredients and relevant impurities in the cefotaxime sodium tazobactam sodium compound. The two ingredients have no interferences or influences. The method has easy operation, strong specificity, high sensitivity, large linear range and good stability, and can be used for detecting a compound preparation and raw materials.
Owner:XIANGBEI WELMAN PHARMA CO LTD
Sodium cefetaxime and sodium tazotactam compound preparation for injection
InactiveCN1425376AEnhance clinical antibacterial effectImprove adverse reactionsAntibacterial agentsOrganic active ingredientsSodium tazobactamCefotaxime Sodium
The present invention relates to sodium cefetaxima and sodium tazobactam compound injection preparation. It features that the compound injection preparation consists of sodium cefetaxima and sodium tazobactam in the weight ratio of 5. It has synergetic and accumulated antibacterial effect to drug resisting bacteria strains, strengthened clinical antibacterial effect and no increased negative effect.
Owner:海南国瑞堂制药有限公司
Synthesis method of cefotaxime sodium
The invention relates to a synthesis method of cefotaxime sodium. The method includes the steps that with an acetone-water solution as a solvent, 7-ACA and AE-active ester are subjected to a stirring reaction for 5-15 min; then, a sodium hydroxide solution is added with stirring for a reaction, obtained reaction liquor is filtered and then crystallized with acetone, and the product cefotaxime sodium is obtained. By the adoption of the one-step synthesis method, the reaction yield is high, product quality is stable, and the operation process is simple and convenient; moreover, no amine intermediate reactant or cosolvent is used, so that production safety is high.
Owner:HARBIN HEJIA PHARMA CO LTD
Cefotaxime sodium composition freeze-dried powder for injection
InactiveCN103585117ALarge specific surface areaHigh reactivityAntibacterial agentsOrganic active ingredientsChitosan nanoparticlesMANNITOL/SORBITOL
The invention provides cefotaxime sodium composition freeze-dried powder for injection, relates to the technical field of medicaments and medicament manufacture, and comprises the following raw material components in parts by weight: 7.26-9.17 parts of cefotaxime sodium, 5.78-7.67 parts of chitosan nanoparticles and 81.38-87.10 parts of water for injection. The cefotaxime sodium composition freeze-dried powder for injection disclosed by the invention has the advantages that the chitosan nanoparticles plays a special role in improving the specific surface area and reactivity of the cefotaxime sodium, thus improving the antibacterial effect of the powder; the antibacterial spectrum is widened and the drug resistance is obviously reduced; the medication period of a patient is shortened due to the strengthened activity, and the probability of side reaction caused by accumulated cefotaxime sodium is reduced; and the chitosan nanoparticles can replace mannitol to be used as a freeze-drying skeleton agent for freeze-dried powder, thus eliminating activation of mannitol to a human body.
Owner:HAINAN WEI KANG PHARMA QIANSHAN
Method for simultaneously measuring content of main components and main impurities in cefotaxime sodium tazobactam sodium for injection
InactiveCN108802206AEffective quality controlImprove targetingComponent separationFiller ExcipientPhosphate
The invention belongs to the technical field of the pharmaceutical analysis, and particularly relates to a method for simultaneously measuring content of main components and main impurities in cefotaxime sodium tazobactam sodium for injection. The method comprises the following steps: using octadecyl silane bonded silica gel as filler of a chromatographic column, using mixed solution of phosphoricacid-phosphate buffer solution and methyl alcohol in a certain proportion as a flowing phase, simultaneously detecting the content of two main components and two main impurities in the cefotaxime sodium tazobactam sodium for injection, thereby effectively controlling quality of the cefotaxime sodium tazobactam sodium for injection. In addition, the invention further develops reference solution for positioning the known impurities. The reference solution is suitable for positioning a cefotaxime impurity B and impurity F in an HPLC detecting method, and improving pertinency of impurity detection. The method is strong in specificity, high in precision, good in stability, simple and convenient in operation, and strong in pertinency, an expensive impurity reference substance is prevented frombeing used, and the method has a good application value.
Owner:JIANGSU LINGBAO PHARMA
Method for preparing cefotaxime sodium crystal
ActiveCN104892636AImprove stabilityDiffraction peak intensityOrganic chemistryCefotaxime SodiumRelative humidity
The invention discloses a novel method for preparing a cefotaxime sodium crystal. The method comprises the following steps: adding a cefotaxime sodium solution into an organic solvent the volume of which is 5-15 times that of the cefotaxime sodium solution at 10-30 DEG C, cooling to 0-9 DEG C, filtering, washing and drying to cefotaxime sodium amorphous-form powder; and at 2-40 DEG C, putting the amorphous-form powder into an organic solvent atmosphere or into a water atmosphere with relative humidity of 32-100%, standing and transferring the crystal for 24-48 hours, and washing, filtering and drying to obtain a cefotaxime sodium crystal product. According to the method, a jelling problem in a dissolving-out process is effectively avoided, the operation is simple, and the total molar yield in the process is higher than 80%. The crystal product has a main grain size which can reach 100 microns, is high in content, high in degree of crystallinity and good in stability.
Owner:TIANJIN UNIV
Cefotaxime sodium compound prepared by fluid mechanics principle and preparation of cefotaxime sodium compound
InactiveCN106279208AHigh purityLow impurity contentAntibacterial agentsOrganic active ingredientsX-rayImpurity
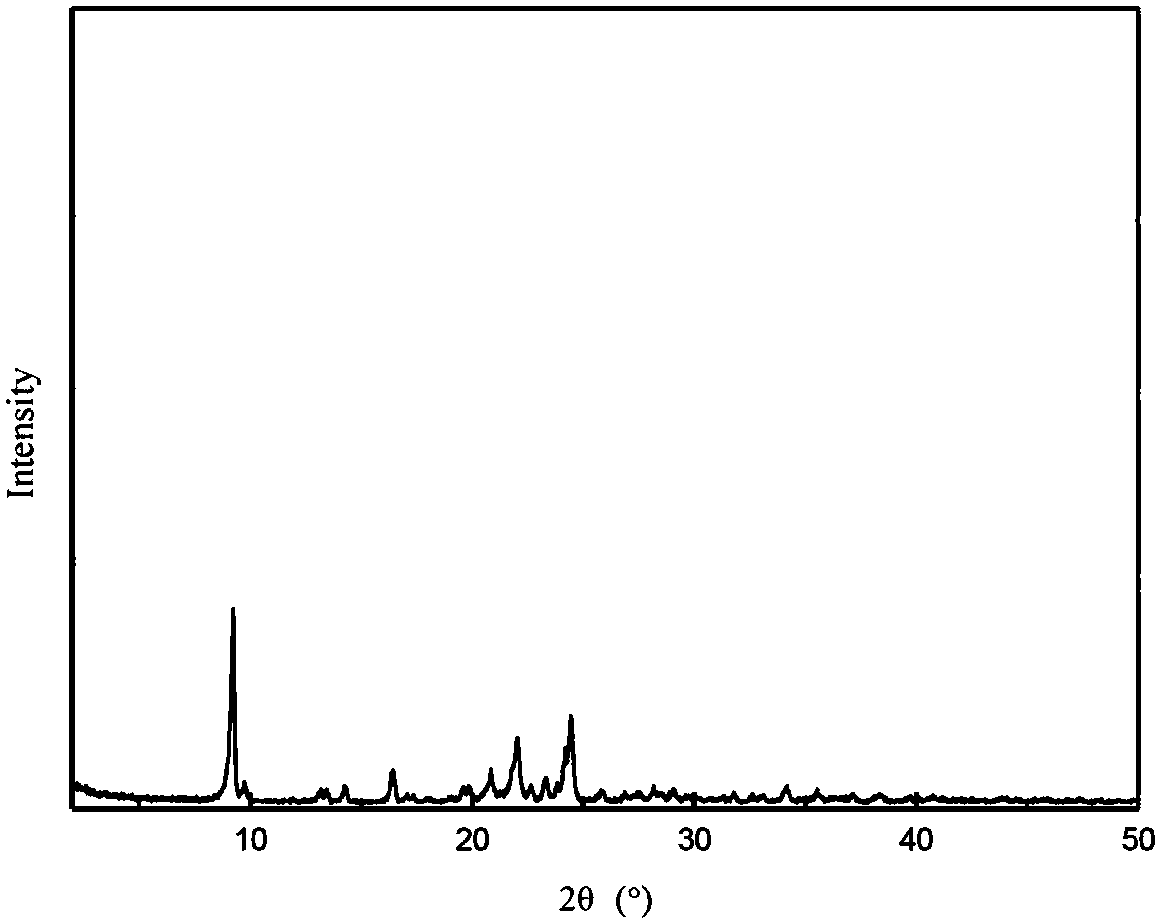
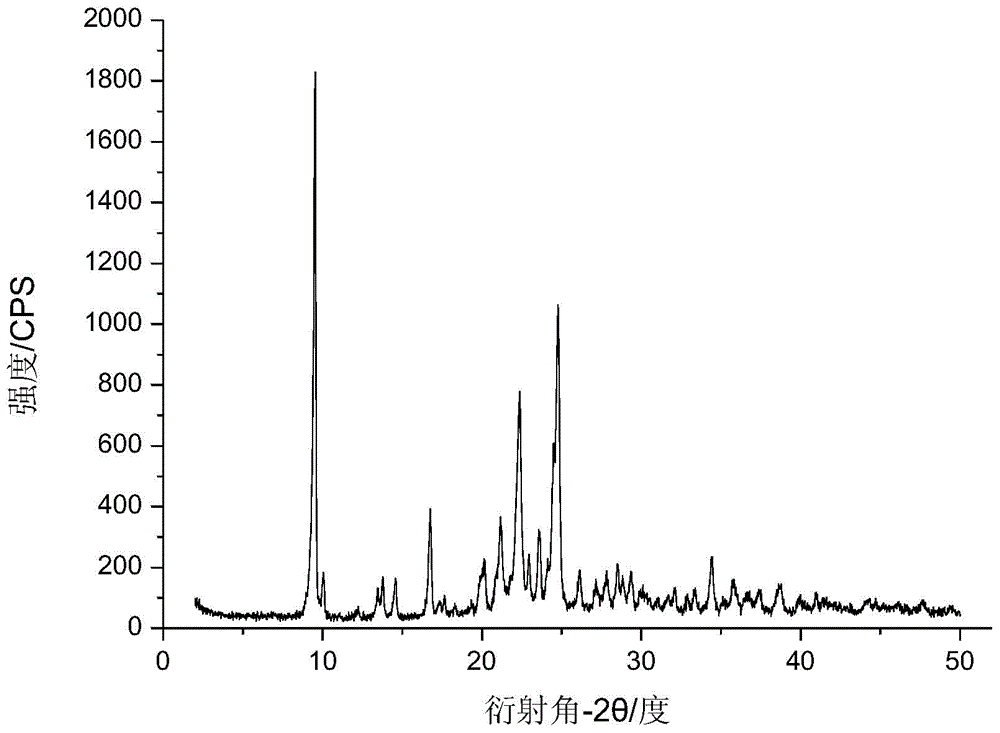
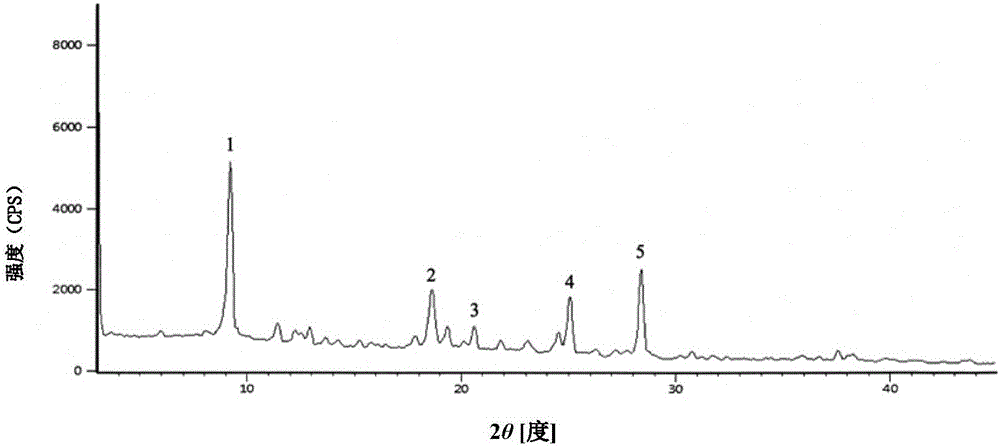
The invention discloses a cefotaxime sodium compound prepared by the fluid mechanics principle and a preparation of the cefotaxime sodium compound, namely efotaxime sodium for injection. A 'research, development and industrialization project of high-end medicine product refining and crystalizing technologies' acquires the second prize of 2015 National Science and Technology Progress Award, and the fluid mechanics principle belongs to one of the high-end medicine product refining and crystalizing technologies. The cefotaxime sodium compound is measured by X-ray powder diffraction, and the main feature peaks indicated by diffraction angles 2theta in the spectrum of the cefotaxime sodium compound is 9.24 degrees, 18.65 degrees, 20.65 degrees, 25.10 degrees and 28.42 degrees. The cefotaxime sodium compound is high in purity, low in impurity content, good in flowability and good in stability.
Owner:陕西顿斯制药有限公司
Semen diluter and preparation method as well as application method thereof
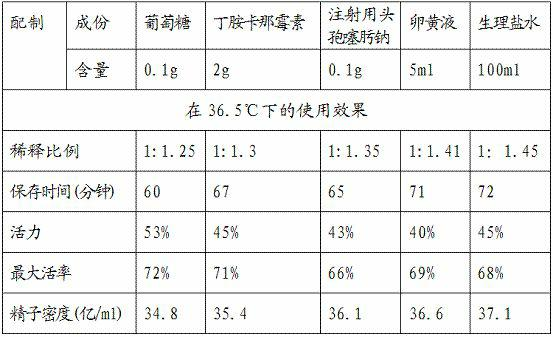
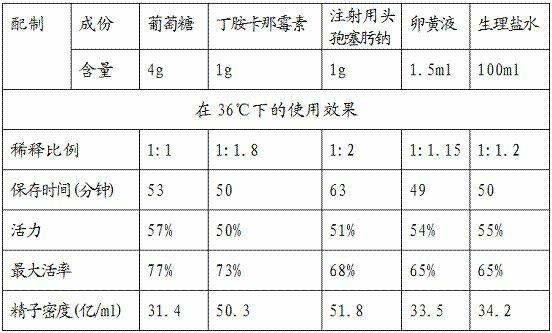
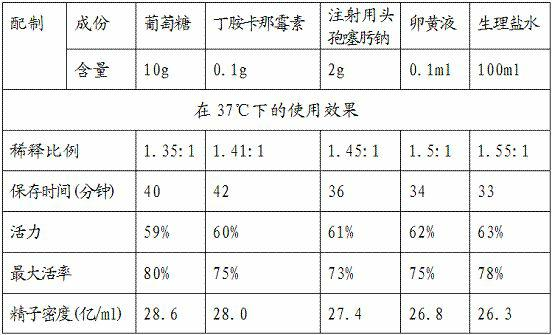
The invention relates to a semen diluter and a preparation method as well as application method thereof. The semen diluter is mainly used for diluting and preserving the semen of breeder cocks. The semen diluter is prepared from the following components: 0.1-10g of glucose, 0.1-2g of amikacini sulfatis for injection, 0.1-2g of cefotaxime sodium for injection, 0.1-5ml of yolk liquid and 100ml of normal saline. The preparation method of the semen diluter comprises the following steps of: (1) respectively dissolving the glucose, the amikacini sulfatis for injection and the cefotaxime sodium for injection in the normal saline; and (2) adding the yolk liquid, and uniformly mixing to prepare the semen diluter. The ratio of the prepared semen diluter to the semen to be diluted is (1:1.5)-(1.5:1). After the semen is diluted by the diluter, the utilization ratio of the semen can be improved, the preservation time of the semen can be prolonged, the semen can be preserved in a wider temperature range, and higher motility and activity of the semen can be kept.
Owner:BEIJING HUADU YUKOU POULTRY
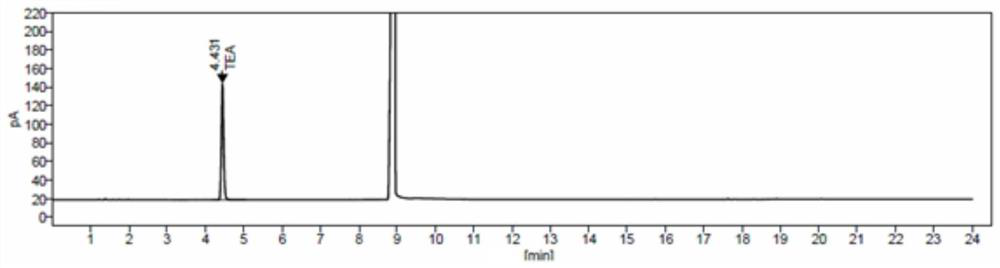
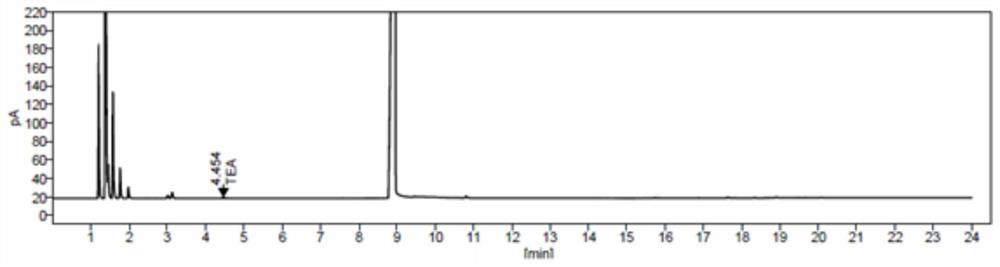
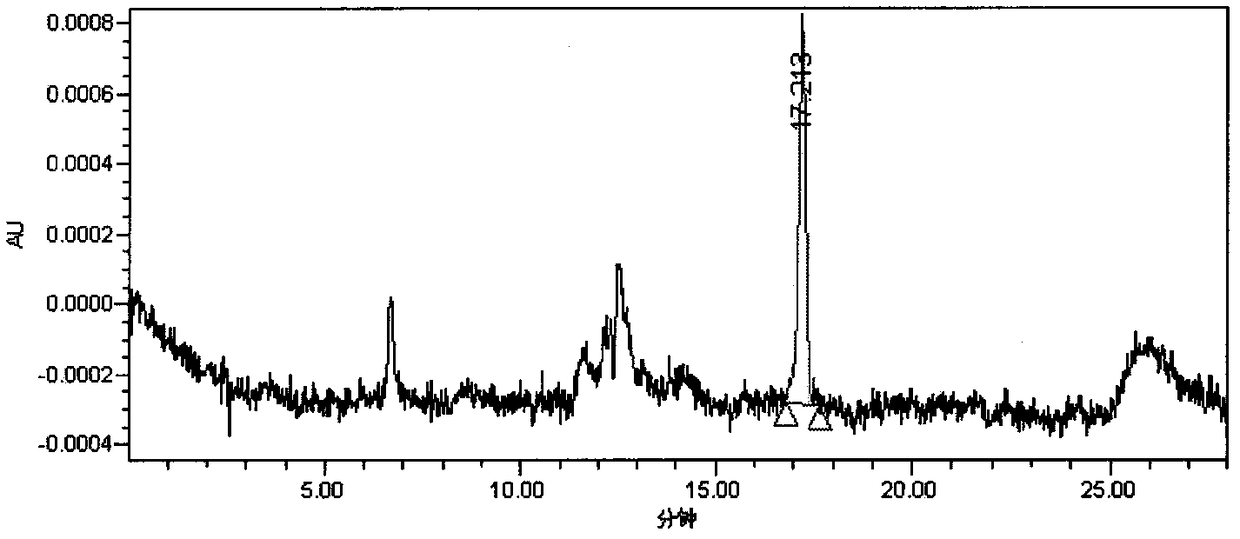
Method for detecting triethylamine in cefotaxime sodium and application of method
InactiveCN111855839AEfficient separationGood peak shapeComponent separationGas liquid chromatographicPhysical chemistry
The invention discloses a method for detecting triethylamine in cefotaxime sodium and application of the method. According to the method for detecting triethylamine in cefotaxime sodium, gas chromatography detection is carried out in a headspace sample injection mode, and the chromatographic column is one of Agilent CP-Sil 8 CB with 30m * 0.32 mm and 1.0 micron or a chromatographic column with equivalent efficiency; the detection process comprises the following steps: injecting a triethylamine reference substance solution and a cefotaxime sodium test solution into a gas chromatograph in a headspace sample injection manner, and performing detecting according to an external standard method. According to the method for detecting residual triethylamine in cefotaxime sodium, to-be-detected triethylamine in cefotaxime sodium can be effectively separated, and the peak shape and the separation degree are good.
Owner:武汉九州钰民医药科技有限公司
Preparation process of cefotaxime sodium powder-injections
InactiveCN107049958AImprove injection safetyAvoid performance degradationAntibacterial agentsPowder deliveryActivated carbonFiltration
The invention discloses a preparation process of cefotaxime sodium powder-injections. The preparation process includes: preparing raw materials, to be more specific, preparing the mixed solvent of ethanol, acetone and water, adding cefotaxime acid into the mixed solvent to perform stirring reaction so as to prepare cefotaxime sodium, adding activated carbon for decoloration, filtering with a sterile membrane to obtain filtrate, adding sterile cefotaxime sodium seed crystals into the filtrate, dropwise adding ethanol under a stirring condition, and performing grain growing, vacuum filtration and drying to obtain the cefotaxime sodium; cleaning soda-lime-glass moulded injection bottles; cleaning rubber plugs; cleaning aluminum covers; performing sterile split charging to obtain the cefotaxime sodium powder-injections. The preparation process has the advantages that the various procedures including the raw material preparation, the bottle cleaning, the plug cleaning and the split charging are strictly controlled, the problem of use effect lowering caused by the performance damage of the cefotaxime sodium during preparation is avoided, product quality is further increased, and the safety of the cefotaxime sodium powder-injections is increased.
Owner:SICHUAN PHARMA
Method for inducing generation of fritillaria cirrhosa embryoids
ActiveCN103461137ASolve the shortage of seedlingsEasy to operateHorticulture methodsPlant tissue cultureFritillaria cirrhosaChemical factor
The invention discloses a method for inducing generation of fritillaria cirrhosa embryoids, belonging to the technical field of biologics. The method comprises the following steps of selecting tender leaf sheath of a fritillaria cirrhosa plant as an explant, sterilizing, subsequently carrying out callus induction, grafting the callus into an MS (Mass Spectrometry)+cefotaxime sodium culture medium, culturing for 50 days to grow embryoids on the surface of the callus, further cutting down the embryoids taking 1-3 embryoids as one group, grafting into a rapid growth culture medium with 0.1-1mg.L<-1> MS+6-BA+100-450mg.L<-1> cefotaxime sodium, culturing for 60 days under the condition that the culture temperature is 15-25 DEG C, the embryoids are shined for 6-20 hours every day, and the shining intensity is 800-2,500lx, so as to grow the embryoids into green small fritillaria cirrhosa plants. According to the method, the fritillaria cirrhosa embryoids can be induced by utilizing antibiotic chemical factors of a certain concentration, and a novel way is provided for breeding of the fritillaria cirrhosa embryoids.
Owner:薛刚
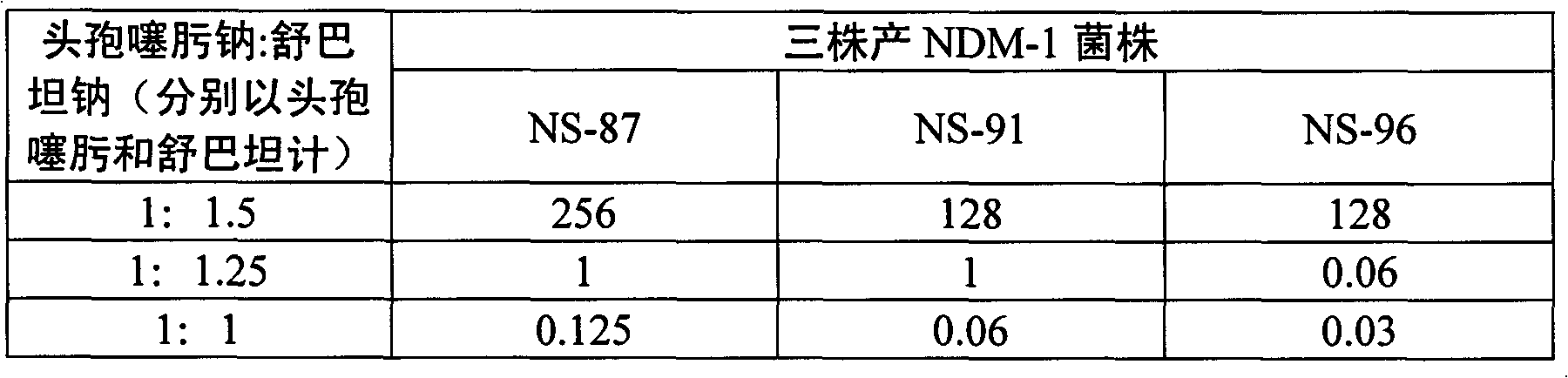
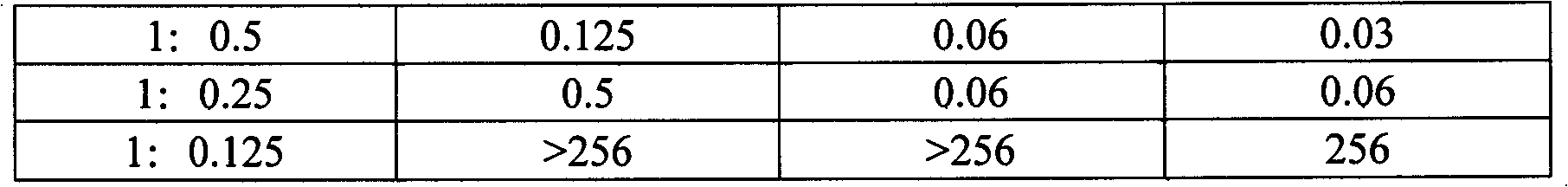
Compound preparation of cefotaxime sodium and sulbactam sodium as well as preparation method and application thereof
ActiveCN102688244AAvoid uneven mixingStable storageAntibacterial agentsPowder deliveryEconomic benefitsDrug product
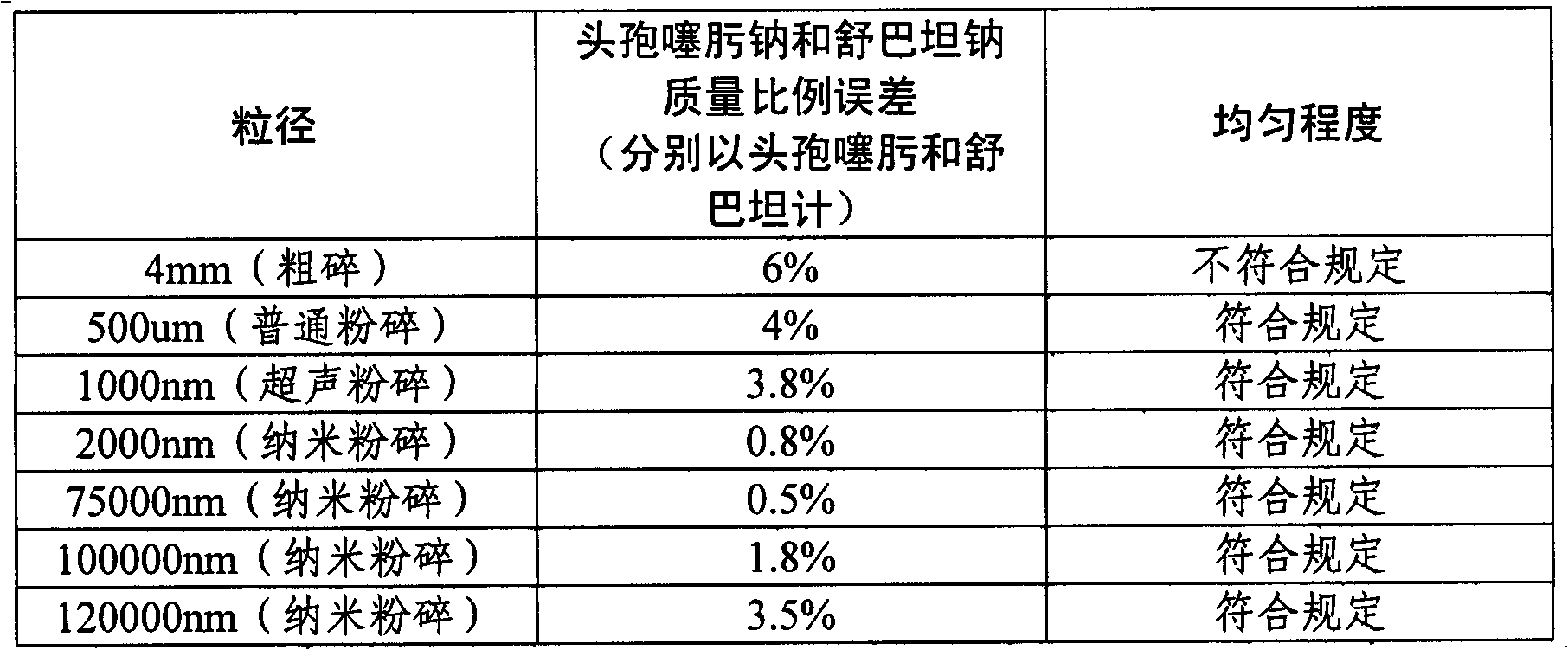
The invention relates to a compound preparation of cefotaxime sodium and sulbactam sodium, as well as a preparation method and application of the compound preparation. The ratio of cefotaxime sodium to sulbactam sodium in the compound preparation is 1: (1.5 to 0.125) in parts by weight of the cefotaxime sodium and the sulbactam sodium; and the grain diameters of cefotaxime sodium and sulbactam sodium are both 2000-100000 nm. According to the invention, a nanometre crushing technology is adopted, and the compound preparation keeps stable at 60 DEG C and has an effective period of 28 months, thus solving the problems of poor high-temperature stability and short effective period, and thus the compound preparation is convenient to transport and store for a long time and is superior to the prior art, and medicine safety and economic benefits are improved.
Owner:XIANGBEI WELMAN PHARMA CO LTD
Cefotaxime sodium and tazobactam sodium preparation for injection and preparing method thereof
ActiveCN102949397AGood content uniformityQuality improvementAntibacterial agentsHeterocyclic compound active ingredientsCefotaxime SodiumTAZOBACTAM SODIUM
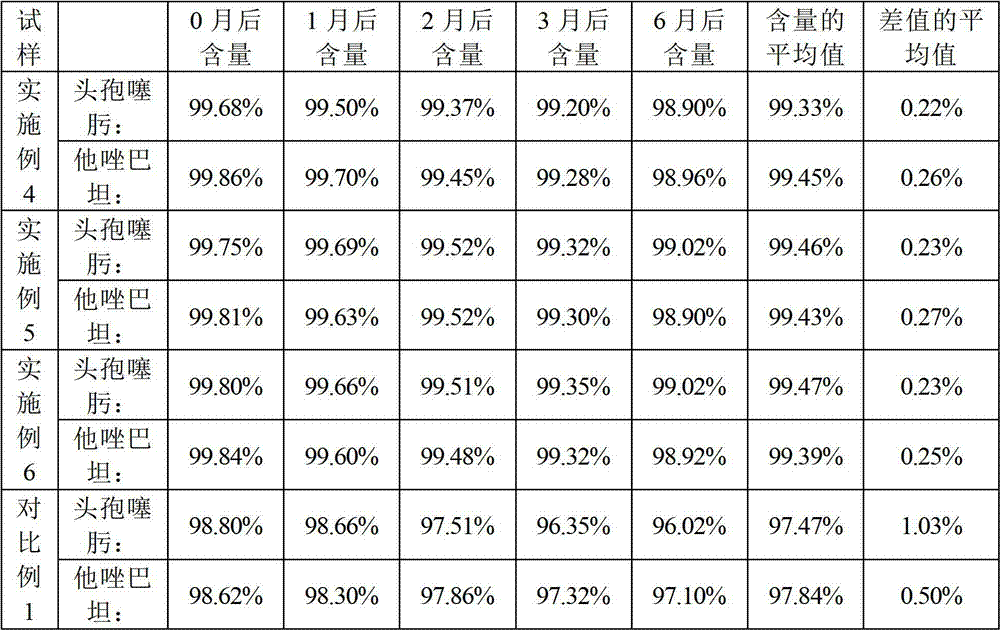
The invention discloses a cefotaxime sodium and tazobactam sodium preparation for injection and a preparing method thereof. The preparing method comprises the step that cefotaxime sodium and tazobactam sodium are mixed uniformly to prepare the cefotaxime sodium and tazobactam sodium preparation for injection, wherein the mass ratio of the cefotaxime sodium to the tazobactam sodium is 1-4:1. A product prepared throughin the preparing method has good uniformity, the operation is simple, no special devices are needed in the production, and the method is suitable for industrial production. The cefotaxime sodium and tazobactam sodium preparation for injection has good content uniformity and stable quality.
Owner:HAINAN HULUWA PHARMA GRP CO LTD
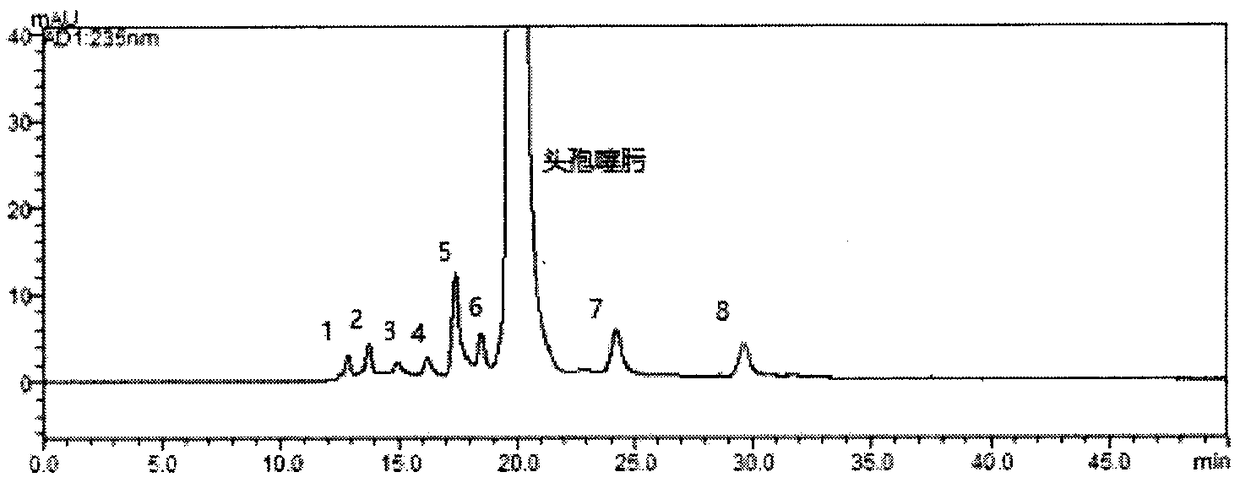
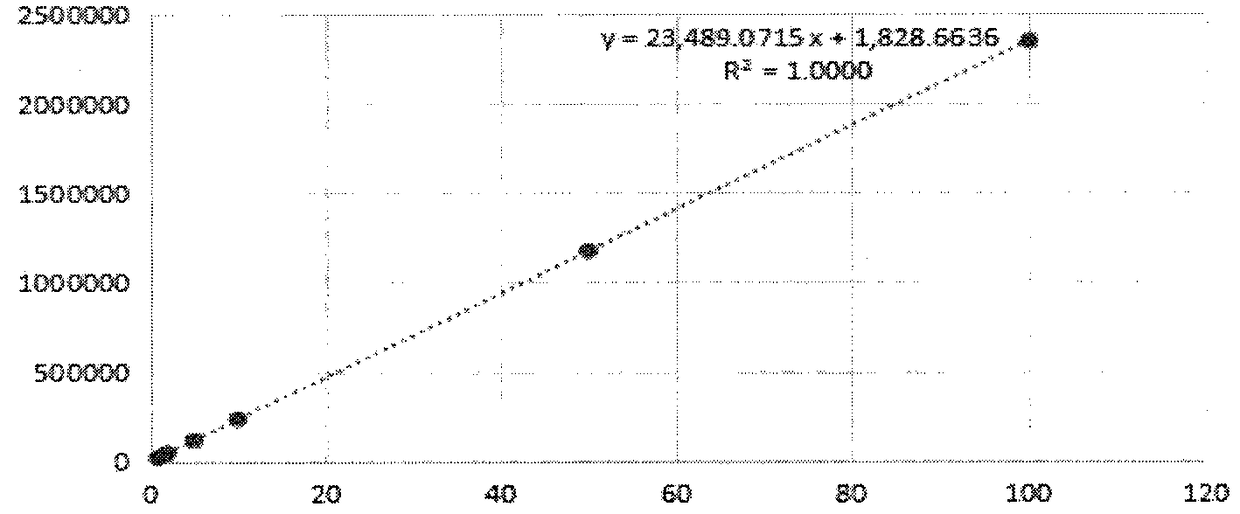
Method for detecting polymers in cefotaxime sodium and injection thereof
InactiveCN108872407AOvercome timeOvercoming separationComponent separationPhosphateColumn temperature
The invention belongs to the field of pharmaceutical analysis, and relates to a method for determining polymers in cefotaxime sodium and an injection thereof. The invention adopts high performance liquid chromatography and an external standard method to determine the content of polymers in cefotaxime sodium-like drugs. The chromatographic conditions are as follows: adopting a chromatographic column with spherical silica gel matrix with the surface bonded with a nano-hydrophilic film is used as a filler, taking 0.1 mol / L phosphate buffer solution [0.1 mol / L disodium hydrogen phosphate-0.1 mol / Lsodium dihydrogen phosphate solution (61:39)] is used as a mobile phase, the flow rate is 0.8 mL / min, the detection wavelength is 235 nm, the injection amount: is 10 muL, and the column temperature:is 35 DEG C. The method has the advantages of a wide linear range, high accuracy, good repeatability and the like, and overcomes the disadvantages of poor reproducibility of the sephadex method, long analysis time, poor separation ability, incapability of accurately determining the content of a single polymer and the like of the sephadex method.
Owner:江苏省食品药品监督检验研究院
One-step preparation process of aseptic ceftriaxone sodium for injection
The invention relates to an improved preparation technique for Ceftriaxone Sodium, which comprises: with nitrogen protection, in solvent, reacting between 7-ACT and AE-active ester with amine as intermediate; adding benzothiazole as assisting solvent and sodium salt agent, separating and crystallizing to obtain the product. Wherein, the reaction solvent comprises halogenated hydrocarbon of alkane, acetic ester or acetone and alcohol and water; stirring the 7-ACT and AE-active ester till clear, adding assisting solvent of benzothiazole and salt agent; extracting, degerming, filtering, adding insoluble solvent for Ceftriaxone Sodium; when liquid shows turbid, feeding crystal; separating the crystal by insoluble solvent to cefotaxime sodium; cleaning, drying treatment to obtain the product for injection. This invention needs just one-step operation, has good crystallization and short production period, improves product yield, and decreases solvent harm to operation crews.
Owner:REYOUNG PHARMA
Preparation method of desacetylcefotaxime
InactiveCN109503630ASynthetic operation is simple and fastEasy to separate and purifyOrganic chemistryChemical synthesisAlkalinity
The invention discloses a preparation method of desacetylcefotaxime. According to the method, D-7-ACA and AE-active ester are taken as reaction materials, and matching control is performed on raw material ratio, reaction temperature and system acidity or alkalinity, so that a desacetylcefotaxime finished product is formed. The invention provides a technical method for preparing desacetylcefotaximethrough a chemical synthesis means, can obtain desacetylcefotaxime standard substance with high yield, purity and convenience, and further improves the production quality of cefotaxime sodium.
Owner:河北合佳医药科技集团股份有限公司
Process for the production of cefotaxime sodium
A process for the production of 7-[2-(2-amino-4-thiazolyl)-2-syn-methoxyimino-acetamido]-3-acetoxymethyl-3-cephem-4-carboxylic acid (Cefotaxime) in aqueous isopropyl alcohol is provided. The synthesis provides the product in greater than 99 % HPLC purity.
Owner:WOCKHARDT LTD
Inducing method and proliferation method of red clover hairy roots
InactiveCN110972954AImprove conversion rateImprove survival rateHorticulture methodsPlant tissue cultureBiotechnologyRed Clover
The invention discloses an inducing method and proliferation method of red clover hairy roots, and belongs to the technical field of biology. The inducing method comprises the following steps of firstly, performing disinfection and cleaning treatment on red clover seeds, placing the treated red clover seeds in MS liquid culture mediums, and performing culture so as to obtain red clover bacteria-free seedlings; with the red clover bacteria-free seedlings as infestation materials, and injecting agrobacterium rhizogenes infestation liquid into the infestation materials; and then inoculating the MS culture mediums containing acetosyringone, with the infestation materials to which the agrobacterium rhizogenes infestation liquid is injected, performing co-culture treatment, then transferring theco-cultured MS culture mediums into MS culture mediums containing cefotaxime sodium, and performing bacteriostasis culture treatment so as to obtain induced converted hairy roots. According to the inducing method disclosed by the invention, through screening appropriate explants and improving a bacterial liquid infestation manner, besides, through controlling the addition concentration of the acetosyringone and the cefotaxime sodium and controlling co-culture time, the inducing conversion rate and the survival rate of the hairy roots can be greatly increased.
Owner:INNER MONGOLIA UNIV FOR THE NATITIES
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com