Method for simultaneously measuring content of main components and main impurities in cefotaxime sodium tazobactam sodium for injection
A technology of cefotaxime sodium and tazobactam sodium is applied in the field of drug analysis to achieve the effects of high accuracy, strong pertinence and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Embodiment one: determination of assay method.
[0047] 1. Determination of chromatographic conditions:
[0048] Use the following chromatographic conditions to screen for the best detection method:
[0049] Chromatographic conditions (1):
[0050] Refer to the chromatographic condition test under the related substances of cefotaxime sodium in the 7th edition of the European Pharmacopoeia, wherein:
[0051] Chromatographic column: Waters XBridge Shield C18 (5μm, 250mm×4.6mm) chromatographic column;
[0052] Mobile phase: mixed phosphate buffer (take 3.5g potassium dihydrogen phosphate and 11.6g disodium hydrogen phosphate, add 1000mL water to dissolve, adjust the pH value to 7.0 with phosphoric acid)-methanol (100:18v / v);
[0053] Elution method: isocratic elution;
[0054] Mobile phase flow rate: 1.0mL / min;
[0055] Injection volume: 10μL;
[0056] Detection wavelength: 235nm.
[0057] Solution preparation (1):
[0058] Weigh about 10 mg of cefotaxime reference...
Embodiment 2
[0139] Embodiment 2: methodological validation of the assay method.
[0140] 1. Validation of impurity analysis method:
[0141] The establishment process of the assay method is described in Example 1, and the following experiments in this example are used to illustrate the advantages of the assay method.
[0142] ●Exclusiveness:
[0143] (1) Acid damage:
[0144] Sample acid destruction solution: Weigh about 50 mg of cefotaxime sodium and tazobactam sodium for injection (batch number: 20110102), accurately weigh it, place it in a 50 mL volumetric flask, add 1 mL of hydrochloric acid solution (0.1 mol / L), and store at room temperature Stand for 30 minutes, add an equal amount of sodium hydroxide solution (0.1mol / L) to neutralize, then add a solvent to make up the volume, shake well, and you get it.
[0145] Acid destruction solution of cefotaxime reference substance: Weigh about 42mg of cefotaxime reference substance, weigh it accurately, place it in a 50mL volumetric flask...
Embodiment 3
[0294] Embodiment 3: Determination of sample measurement results and limits.
[0295] 1. Content determination of main impurities:
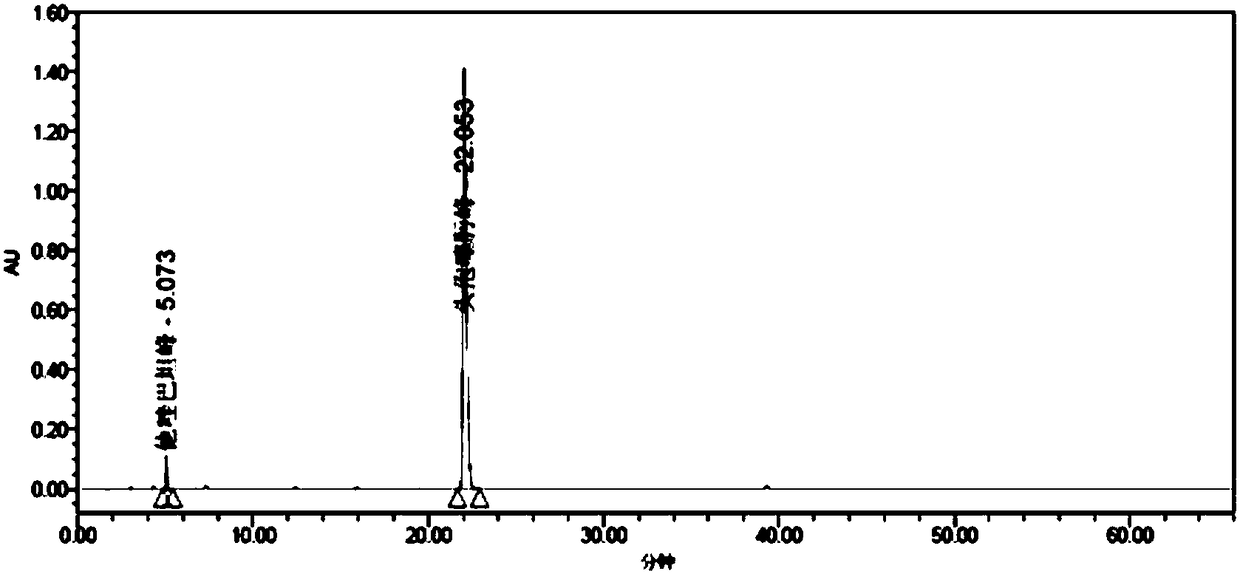
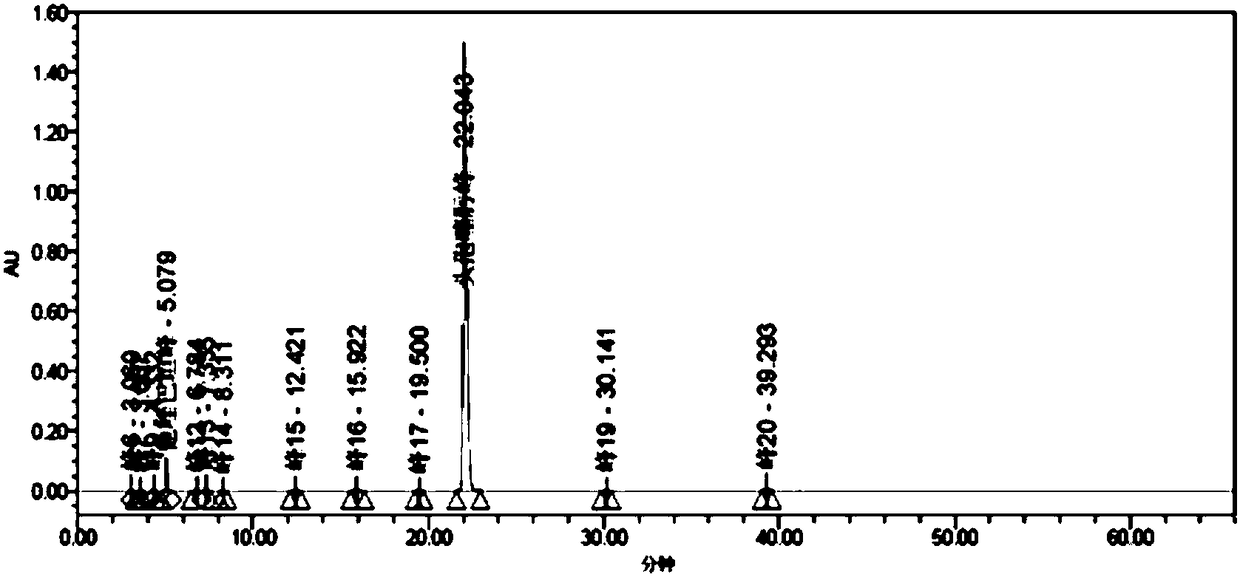
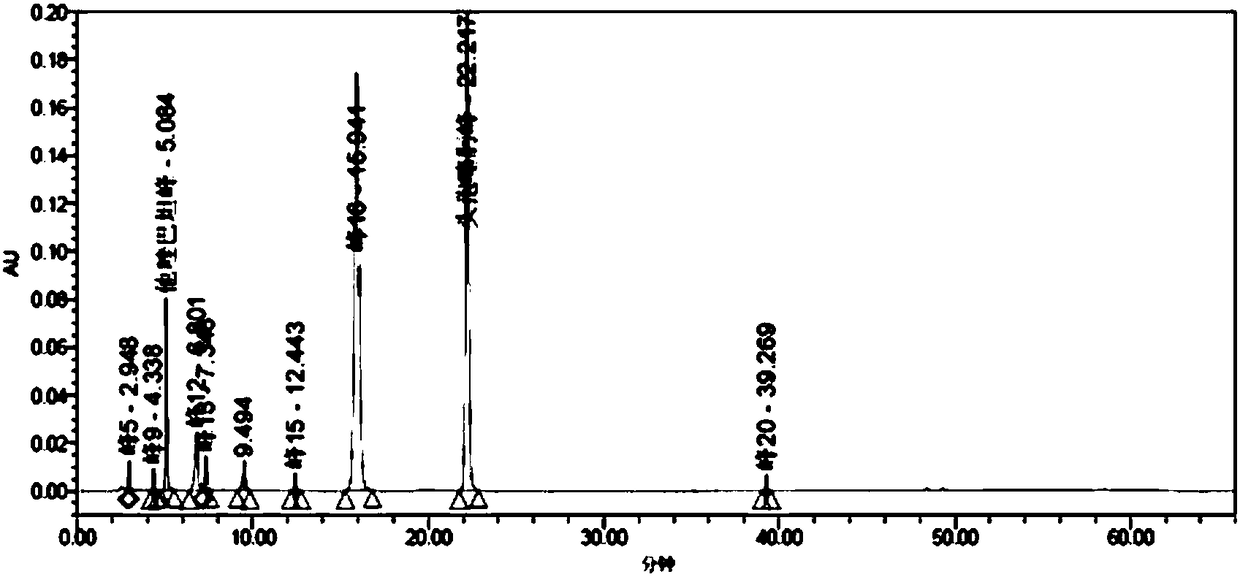
[0296] A series of methodological investigations were carried out on the impurity analysis method, and the results proved that the method has good system applicability and can effectively detect the two main impurities in cefotaxime sodium and tazobactam sodium for injection. The results of the determination of the impurity content in the 3 batches of samples are shown in Table 37.
[0297] Table 37. Determination results of main impurities
[0298]
[0299] According to the above-mentioned inspection results, combined with the cefotaxime sodium for injection in the 2010 edition of the Chinese Pharmacopoeia and the cefotaxime sodium impurity limit requirements and stability test results in the 8th edition of the European Pharmacopoeia, the impurity content limit is formulated: in the test solution In the chromatogram, deducting the blank sol...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com