Novel method for measuring compound cefotaxime sodium tazobactam sodium
A technology of cefotaxime sodium and tazobactam sodium, which is applied in the detection field of the new compound cefotaxime sodium and tazobactam sodium, can solve the problems of not being affected by another component, instability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0005] Example 1. Preliminary detection of compound raw materials and preparations
[0006] The experiment uses "cefotaxime sodium tazobactam sodium for injection", the ratio is 6:1, and the specification is 1.0g. Due to the simple loading changes of other formulations and specifications, the source of raw materials and the preparation process have no Significant differences. Therefore, the proportion and specification samples were used for research.
[0007] The test sample is white, off-white or slightly yellowish white powder, and the following various tests are carried out.
[0008] 1. Identification test:
[0009] The two components, tazobactam and cefotaxime, exist in the form of sodium salt, and should show a flame reaction of sodium salt when burned. Take platinum wire, moisten it with hydrochloric acid, dip it in this product, burn it in a colorless flame, and the flame will appear bright yellow.
[0010] The two components, tazobactam sodium and cefotaxime sodium...
Embodiment 2 3
[0011] 2. Detection test
[0012] Test sample acidity inspection: get the test sample and add water for injection to make a solution containing 0.1 g of cefotaxime per 1 ml, and measure it with an acidity meter. As a result, the pH of the test sample is between 4.5-6.5, which is a qualified product.
[0013] Clarity inspection of the test sample solution: take 5 bottles of the test sample, add water to make a solution containing about 0.1g of this product per 1ml, compare it with No. 1 turbidity standard solution, and check the clarity. Results The test sample was a clear liquid, which did not exceed No. 1 turbidity standard solution, and the clarity met the relevant regulations, so it was a qualified sample.
[0014] Test sample solution color inspection: take 5 bottles of test sample, add water to dissolve, place in a 25ml Nessler colorimetric tube, add water to dilute to 10ml, and compare with the yellow hue standard colorimetric solution. Results The test sample was a cle...
Embodiment 2
[0017] Embodiment two, the analysis and quality control method of compound raw material and preparation
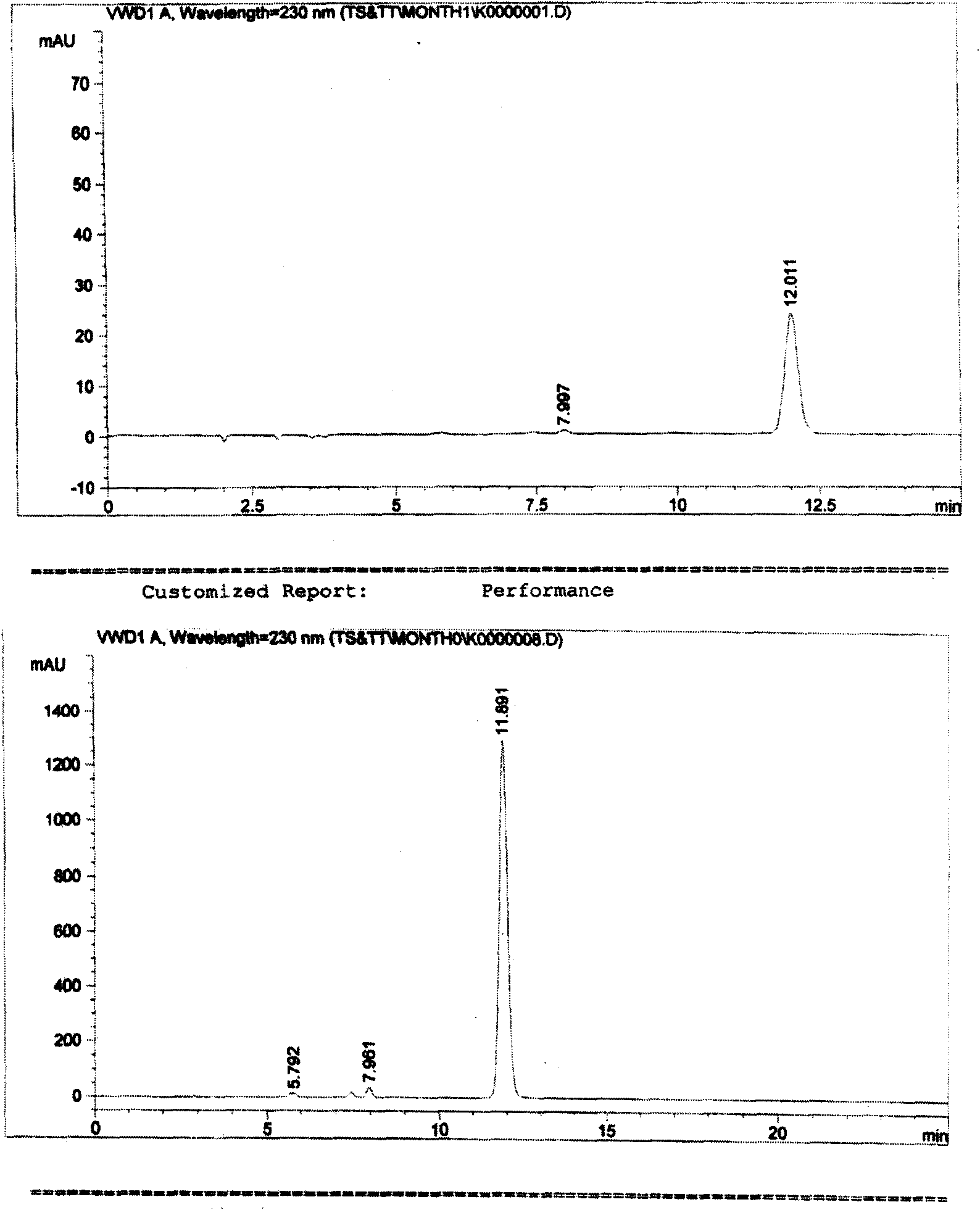
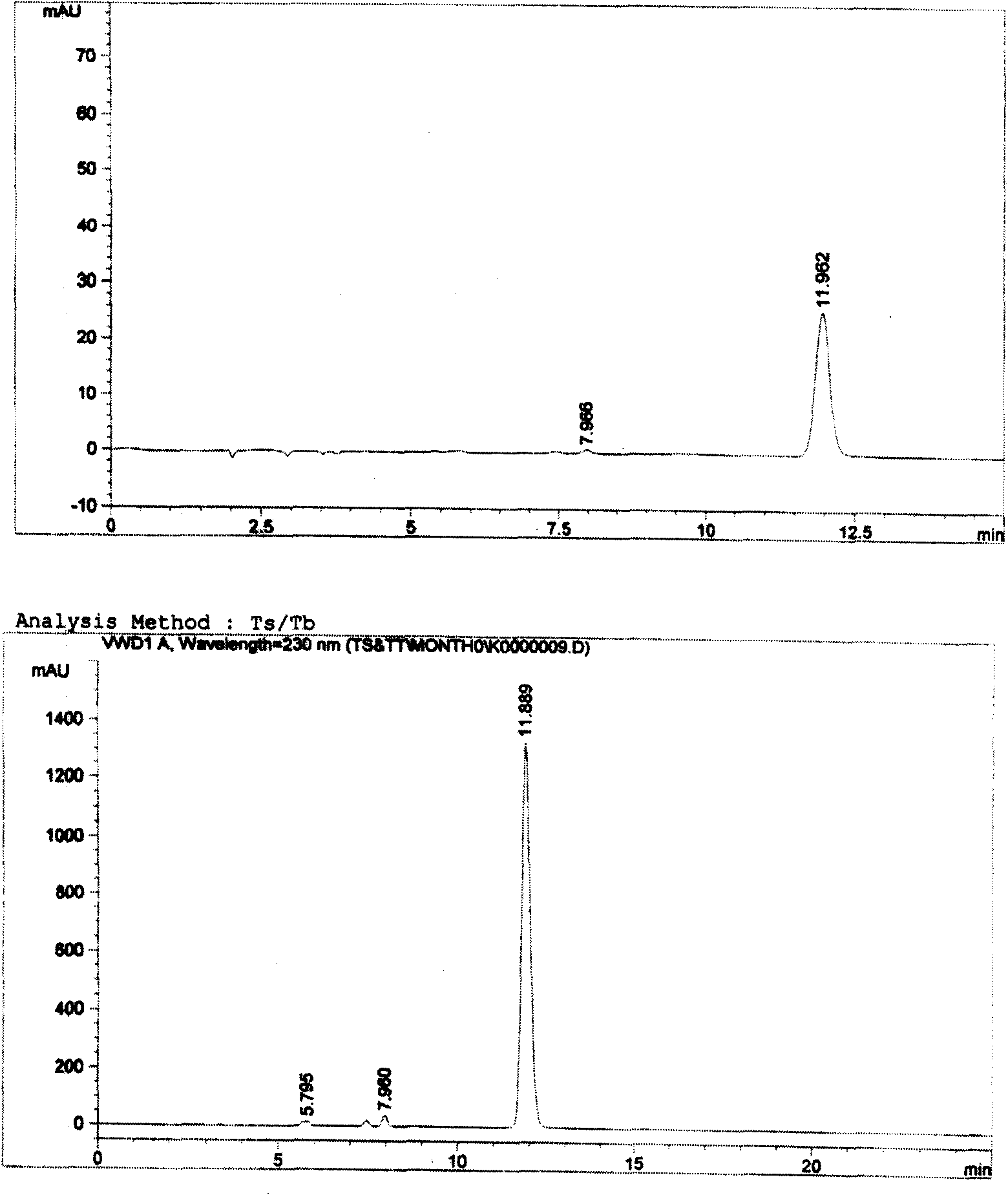
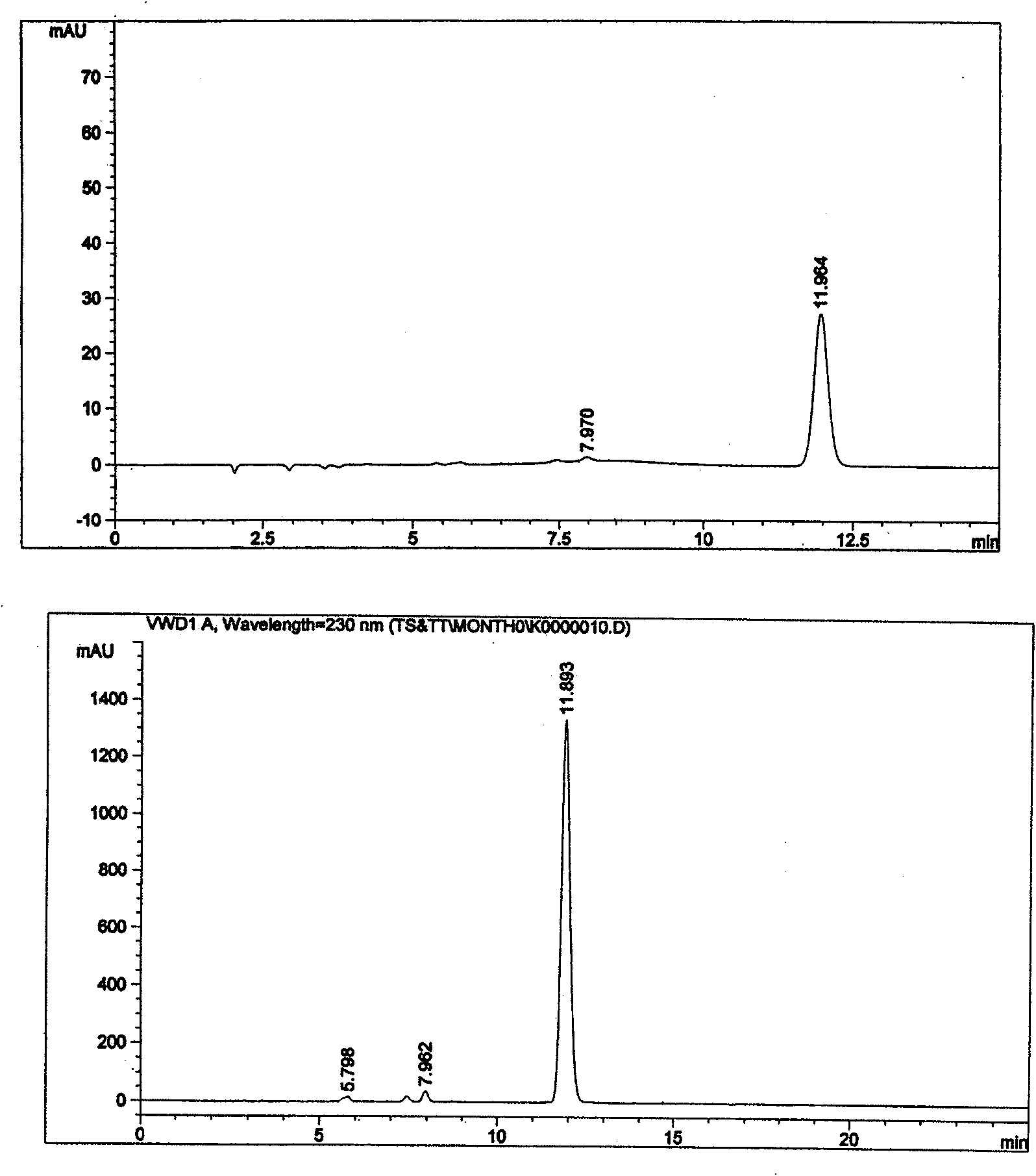
[0018] In order to control the quality of the product, it is necessary to analyze and study the possible impurities in the product. Because there is no previous experience of mixing the two substances together, the existing information only has a test method for the relevant impurities in a single cefotaxime sodium (or tazobactam sodium).
[0019] Although such an inspection method is effective for the inspection of a single substance, it is not clear whether it can inspect the relevant impurities in the mixture, because whether the two main components will affect each other's detection after being prepared into a mixed preparation, and whether the two impurities will also affect the detection of the other. There are many uncertain factors in the detection of the other party, and a large number of experiments are required to verify. Therefore, we have established a corres...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com