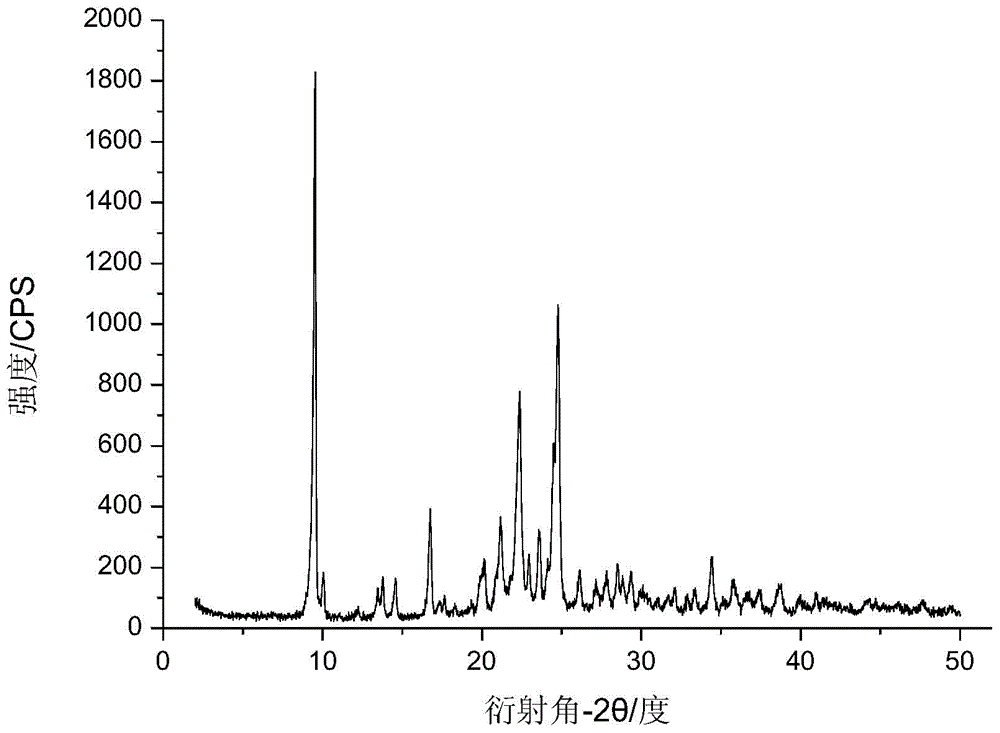
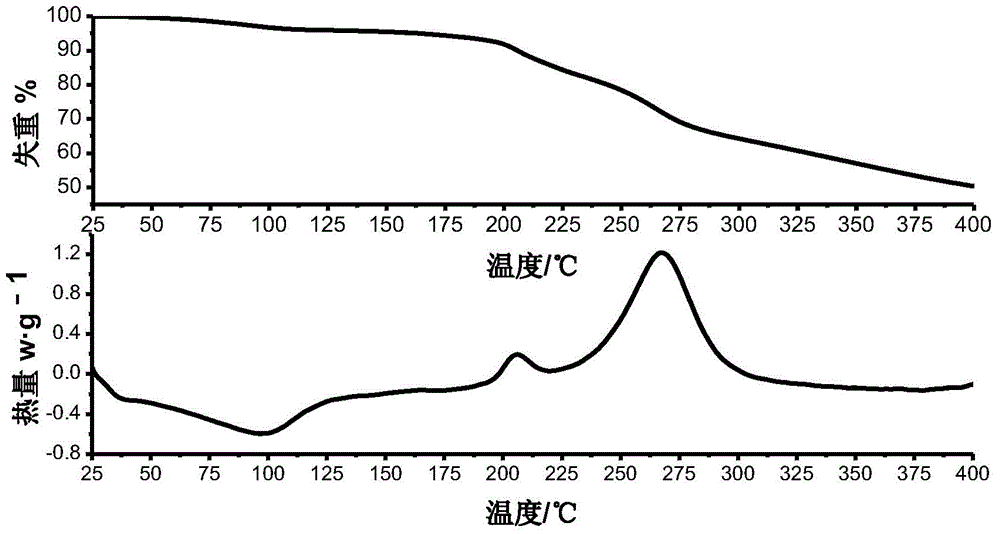
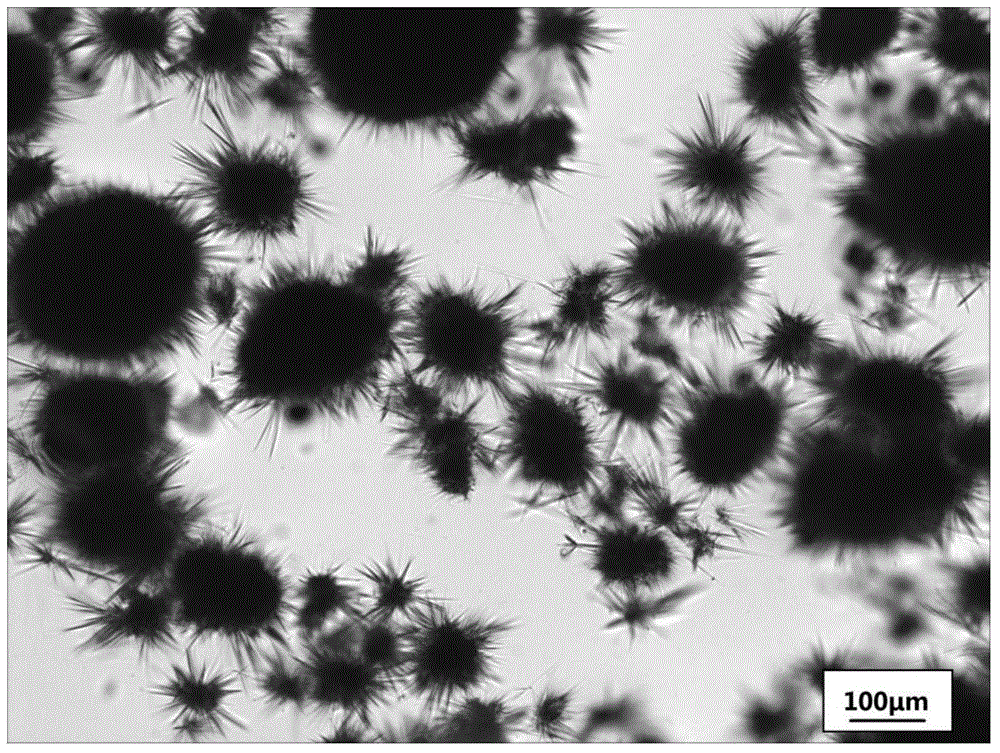
Method for preparing cefotaxime sodium crystal
A technology for cefotaxime sodium and crystals is applied in the field of preparation of cefotaxime sodium crystals, which can solve the problems of reducing heat transfer, mass transfer efficiency, adding a large amount of elution agent, and unavoidable gel formation, and achieves easy gelation. The effect of condensation, large crystal size and concentrated distribution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Prepare the formamide solution of 0.029g / mL sodium acetate, add cefotaxime acid, the mol ratio of sodium acetate and cefotaxime acid is 1.2:1, add active carbon decolorization after stirring reaction, filter to obtain clarified cefotaxime sodium solution, The concentration is 0.14g / mL. Take 30mL of cefotaxime sodium solution and add it dropwise to 300mL of methanol at 20°C within 100min. After the dropwise addition, the temperature was lowered to 5° C. at a cooling rate of 1° C. / min, filtered, washed with methanol, and dried at -70° C. under an absolute pressure of 36 Pa for 24 hours to obtain amorphous cefotaxime sodium powder with a molar yield of 97%. The obtained amorphous powder was left standing in a water atmosphere with a relative humidity of 32% at 30°C for 24h, washed and filtered, and then dried under reduced pressure at 40°C for 36h to obtain a crystalline product with a molar yield of 84%. The total yield of the process is 81.5%, and the crystal product co...
Embodiment 2
[0028]Prepare 0.13g / mL sodium acetate N-N dimethylformamide solution, add cefotaxime acid, the molar ratio of sodium acetate to cefotaxime acid is 1.1:1, add activated carbon to decolorize after stirring the reaction, filter to obtain clarified cefotaxime Sodium oxime solution, the concentration is 0.70g / mL. Take 30mL of cefotaxime sodium solution and add dropwise to 300mL of ethanol and ethyl acetate mixed solvent at 20°C within 10min. After the dropwise addition, the temperature was lowered to 5°C at a cooling rate of 1°C / min, filtered and washed with ethyl acetate. Dry at -60° C. for 12 hours under an absolute pressure of 100 Pa to obtain amorphous cefotaxime sodium powder with a molar yield of 96%. The obtained amorphous powder was left standing in 2° C. saturated water vapor for 48 hours, washed and filtered, and then dried under reduced pressure at 40° C. for 28 hours to obtain a crystalline product with a molar yield of 83.6%. The total molar yield of the process is 8...
Embodiment 3
[0031] Prepare the aqueous solution of sodium acetate of 0.048g / mL, add cefotaxime acid, the mol ratio of sodium acetate and cefotaxime acid is 1:1, add activated carbon decolorization after stirring reaction, filter and obtain clear cefotaxime sodium solution, concentration 0.28g / mL. Take 30 mL of cefotaxime sodium solution and add dropwise to 150 mL of ethyl acetate at 10°C within 20 min. After the dropwise addition, the temperature was lowered to 0°C at a cooling rate of 0.2°C / min, filtered, washed with ethyl acetate, and dried at -60°C under an absolute pressure of 100 Pa for 36 hours to obtain the amorphous powder of cefotaxime sodium. The molar yield was 98%. The obtained amorphous powder was left standing in saturated water vapor at 20°C for 24h, washed and filtered, and then dried under reduced pressure at 40°C for 24h to obtain a crystalline product with a molar yield of 85.5%. The total molar yield of the process is 83.8%, and the crystal product content is 95.8%. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Granularity | aaaaa | aaaaa |
| Decomposition temperature | aaaaa | aaaaa |
| Thermal decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com