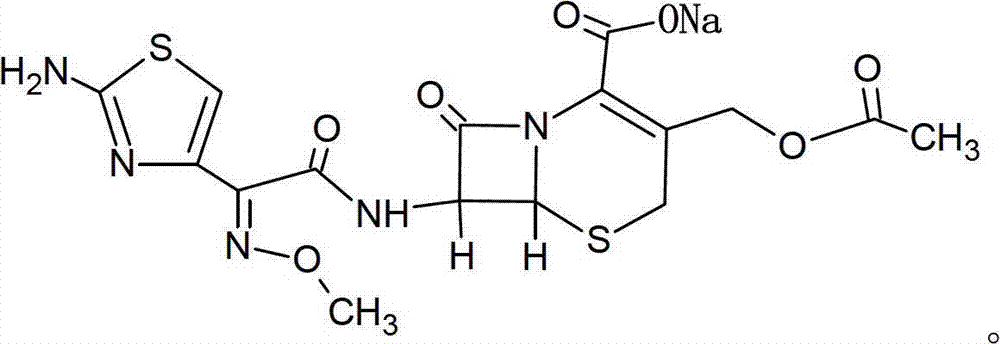
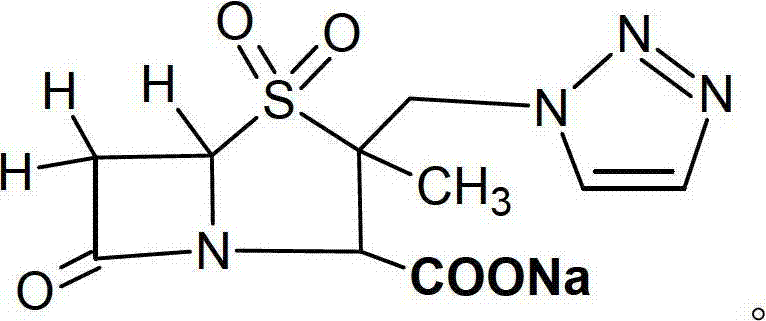
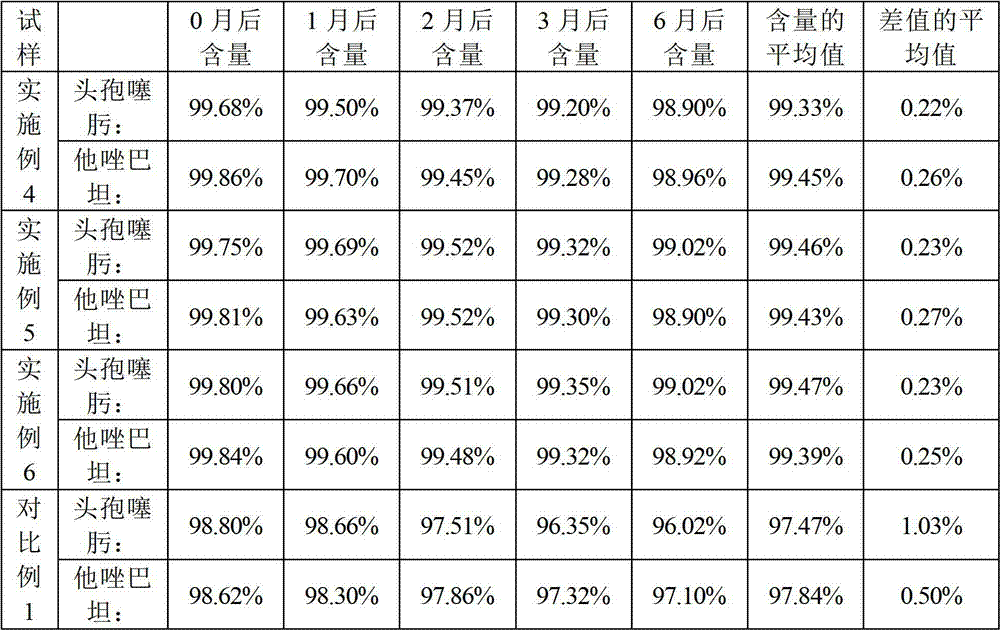
Cefotaxime sodium and tazobactam sodium preparation for injection and preparing method thereof
A technology of tazobactam sodium and cefotaxime sodium, applied in the field of medicine, can solve problems such as discoloration and poor content uniformity, achieve good sterilization effect, avoid uneven mixing, and simple operation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Preparation of cefotaxime sodium and tazobactam sodium preparations for injection
[0034] The mass ratio of cefotaxime sodium to tazobactam sodium is 3.9:1.
[0035] Put 279.5g of cefotaxime sodium in the mixer and mix for 10 minutes, add 107.3g of newly prepared lyophilized powder of tazobactam sodium, mix for 5 minutes while passing compressed air, then add 559g of cefotaxime sodium and mix for 10 minutes , add 107.3g freshly prepared lyophilized powder of tazobactam sodium, mix for 5 minutes while feeding compressed air, discharge, sub-package to prepare cefotaxime sodium for injection and tazobactam sodium preparations.
Embodiment 2
[0037] Preparation of cefotaxime sodium and tazobactam sodium preparations for injection
[0038] The mass ratio of cefotaxime sodium to tazobactam sodium is 3.95:1.
[0039] Put 279.5g of cefotaxime sodium in the mixer and mix for 10 minutes, add 106.2g of freshly prepared lyophilized powder of tazobactam sodium, mix for 5 minutes while passing compressed air, then add 559g of cefotaxime sodium and mix for 10 minutes , add 106.2g freshly prepared lyophilized powder of tazobactam sodium, mix for 5 minutes while feeding compressed air, discharge, sub-package to prepare cefotaxime sodium for injection and tazobactam sodium preparations.
Embodiment 3
[0041] Preparation of cefotaxime sodium and tazobactam sodium preparations for injection
[0042] The mass ratio of cefotaxime sodium to tazobactam sodium is 3.98:1.
[0043] Put 279.5g of cefotaxime sodium in the mixer and mix for 10 minutes, add 105.3g of newly prepared lyophilized powder of tazobactam sodium, mix for 5 minutes while passing compressed air, then add 559g of cefotaxime sodium and mix for 10 minutes , add 105.3g freshly prepared lyophilized powder of tazobactam sodium, mix for 5 minutes while feeding compressed air, discharge, sub-package to prepare cefotaxime sodium for injection and tazobactam sodium preparations.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com